Abstract
Human natural killer (NK) cell, which is an important lymphocyte for immune surveillance, is highly sensitive to heat, but the nature of its response to and its mechanistic regulation by heat remain unclear. Here we determined the effect of in vitro heat shock and in vivo hyperthermia on human NK cell cytotoxicity. Human peripheral blood mononuclear cells (PBMC) obtained from healthy volunteers were subjected to heat shock in vitro (42°C, 1 h). PBMC from cancer patients receiving intentional hyperthermia (42°C, 1 h) for cancer therapy were also obtained. NK cytolytic activity was determined in these samples. NK cell cytotoxicity was down-regulated by heat shock in vitro at 5 h, but at 24 h after heat shock, the NK cytotoxicity was comparable to that with its respective control. Furthermore, we observed that the mRNA and protein expression levels of perforin, which is the cytolytic granule of NK cells, were regulated by heat shock in a similar manner as NK cytotoxicity at 5 h and at 24 h after heat shock. Heat regulation involved the perforin protein in CD56dim but not in CD56bright NK cell subset. Heat shock neither induced cell death nor altered the expression of some NK activating receptors and adhesion molecules. Moreover, whole-body hyperthermia at 42°C for 1 h of cancer patients also suppressed the cytotoxicity of NK cells but recovered to basal level 1 week after hyperthermia. Heat shock in vitro and in vivo temporarily represses the cytotoxicity of human NK cells.
Introduction
Hyperthermia is used in conjunction with other established cancer therapy for the treatment of malignant disease Citation[1]. With increased accordance on the close connection between cancer and immune system, many studies have been focused on the understanding of hyperthermia-induced immune responses (reviewed in Citation[2]). In this context, substantial attention has been given to natural killer (NK) cell, a large lymphocyte that functions as important innate immune defense against viral-infected and tumor cells. Human NK cells can be divided into two subsets based on the cell-surface density of CD56: the CD56dim and CD56bright NK subset. These two subsets exhibit distinct phenotypic and functional properties. CD56dim cells, which are the majority of human NK cells (∼90%), contain more cytolytic granules, such as perforin, and are naturally more cytotoxic than CD56bright NK cells but produce negligible amount of cytokines Citation[3], Citation[4]. On the other hand, CD56bright NK cell is an immunoregulatory subset that functions as major producer of NK-derived cytokines Citation[3].
Although NK cells have been demonstrated to be highly sensitive to heat Citation[5], Citation[6], and that thermal stress in vitro reduces NK cell lytic function Citation[7], Citation[8], the actual mechanism underlying heat-regulation of NK cells remain ambiguous. We have previously demonstrated that the murine NK cell cytotoxicity was suppressed after heat shock through the down-regulation of perforin Citation[9]. However, considering that human and mouse NK cells are different from each other in terms of their functional subsets Citation[10], we considered it relevant and necessary for clinical applications to investigate the effect of heat shock on human NK cells. Here we demonstrate that heat shock continuously suppressed cytotoxicity of human NK cells until 24 h by down-regulating perforin mRNA and protein expression. Heat shock regulation of perforin protein selectively occurs in CD56dim NK cell population. The temporary suppression of NK cell activity was also observed in tumor patients receiving hyperthermia. Our findings clarify to a certain extent one of the possible mechanisms of hyperthermia regulation of human NK cell function.
Materials and methods
Cell lines
The erythroleukaemia cell line, K562, was obtained from RIKEN Cell Bank (Tsukuba, Japan). EGFP-transfected K562 (EGFP-K562) cells were established as described previously Citation[11]. The cells were cultured in RPMI1640 medium (Sigma, St. Louis, MO) supplemented with 10% FBS (Biowest, France), 50 IU/ml penicillin and 50 µg/ml streptomycin (GIBCO). Cells were maintained at 37°C in 5% CO2.
Isolation and culture of peripheral blood mononuclear cells and NK cells
Human peripheral blood samples were collected from adult donors after informed consent was obtained. Investigations were carried out in accordance to ethical standards authorized by the ethics committee of the Kumamoto University Graduate School of Medical Sciences or the ethics committee of Luke Hospital (Nakano, Tokyo). Peripheral blood mononuclear cells (PBMC) were isolated using lymphocyte separation medium (LSM, MP Biomedicals, OH). NK cells were isolated from freshly prepared PBMC by CD56 MicroBeads using magnetic cell-sorting technique (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. The binding of CD56 monoclonal antibody has been shown not to affect NK cell cytotoxicity Citation[4].
Hyperthermia
In vitro hyperthermia was performed by immersing the cells in 42°C water bath for 1 h. Cells were immediately cooled down in an ice bath before the medium was changed, and were re-incubated at 37°C. Control cells were left in the incubator at 37°C.
For whole-body hyperthermia, patients were anaesthetized during the procedure, which utilized a far-infrared radiation heat device (RHD2002 and RHS7500; Enthermics Medical Systems Inc., WI) Citation[12]. The temperature was maintained at 42–43°C locally in the hyperthermic chamber and 41.5–42°C systemically (rectal temperature) for 1 h as described previously Citation[13]. Peripheral blood samples were collected before, and at 3 h and 1 week after treatment from eight patients receiving intentional hyperthermia therapy for malignant tumors.
Flow cytometry
For cell surface expression analysis, 1 × 106 cells were stained with the following monoclonal antibodies: flourescein isothiocyanate (FITC)-conjugated anti-CD2 CD94, CD58 (BD PharMingen, San Diego, CA), PE-conjugated anti-NKG2D, NKp46 (Beckman Coulter, Fullerton, CA), CD3 (Caltag, Burlingame, CA), APC-conjugated anti-CD56 (Beckman Coulter), PECy7-conjugated anti-CD3 (eBioscience, San Diego, CA). Cells were incubated on ice for 30 min, washed twice and resuspended in staining medium. For staining of intracellular perforin, cells were fixed with 1% paraformaldehyde for 10 min at room temperature after staining of cell surface molecules, permeabilized with 0.1% saponin for 10 min at room temperature and stained with anti-perforin-FITC (eBioscience) for 30 min on ice. Cells were washed twice with permeabilization buffer and once with staining medium. Data analysis was performed using FACSCalibur flow cytometer and Cell Quest Software (Becton Dickinson).
Cytolytic assay
Using freshly prepared PBMCs isolated from healthy donors, the NK activity in PBMC against EGFP-K562 cells was measured by flow cytometry with propidium iodide (PI) as described before Citation[11] with some modifications. Specifically, non-heat shock control cells were kept at 37°C while heat shocked samples were placed in water bath maintained at 42°C for 60 min. Heat shock-treated cells were then transferred to 37°C incubator. After 5 h or 24 h, cells were used for cytolytic assay. For the assay, mixtures of effector-target cells at effector-to-target (E:T) ratios of 160 : 1 and 40 : 1 were incubated at 37°C for 4 h. Total of 50 µl of 2 µg/ml PI was added with 15 min incubation. GFP and PI were measured by flow cytometry. Cytolytic activity was calculated as described previously Citation[11], that is: % Lysis = A/(A + B) × 100 − C (%), where A is the percentage of PI+EGFP+ cells; B is the percentage of PI−GFP+ cells at each E/T ratio; C is the percentage of spontaneous PI+ cells without effector cells (A/(A + B) × 100 (%) at E/T ratio = 0).
Cell death assay
Detection of apoptotic cells was performed using flow cytometric analysis of Annexin V (Annexin V-PE; BD Biosciences) stained cells according to the manufacturer's instructions. Briefly, cells were first stained with anti-CD3-PC7 (Caltag) and anti-CD56-APC (Beckman Coulter) for 30 min on ice. Cells were washed with cold PBS and resuspended in Annexin V binding buffer (0.01 M Hepes, 0.14 M NaCl, 2.5 mM CaCl2). Annexin V-PE and Viaprobe (7-AAD; BD Biosciences) were added into 1 × 105 cell suspension and incubated for 15 min at room temperature. Fluorescence intensity of Annexin V and Viaprobe was analyzed in CD3−CD56+ cells.
Real time quantitative-polymerase chain reaction (Q-PCR)
NK cells were purified from PBMC using magnetic beads (Miltenyi Biotec, Auburn, CA). This positive selection with anti-CD56 microbeads has no experimental problems at least for the real time Q-PCR experiment, because it is well-known that CD56 does not contribute cellular function in human NK cells Citation[14]. Total RNA was isolated from NK cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcription was performed using iScript cDNA Synthesis kit (Bio-Rad Laboratories) and real-time quantitative PCR was performed using iQ SYBER GreenSupermix (Bio-Rad Laboratories) according to the manufacturer's instructions. Real time PCR was carried out at 95°C for 3 min, then 40 cycles of denaturation (95°C for 10 s) and annealing/extension (60°C, 1 min). The sequences of perforin primers were described previously Citation[15] and GAPDH (internal control) primers were as follows: GAPDH (up) 5′-CGGGAAGCTTGTGATCAATGG3′ and GAPDH (down) 5′-GGCAGTGATGGCATGGACTG-3′.
Statistical analysis
The different groups were tested for statistical significance using paired two-tailed Student's t test. Value of p < 0.05 was considered to be statistically significant.
Results
Heat shock continuously suppresses cytotoxicity of human NK cells in vitro until 24 h
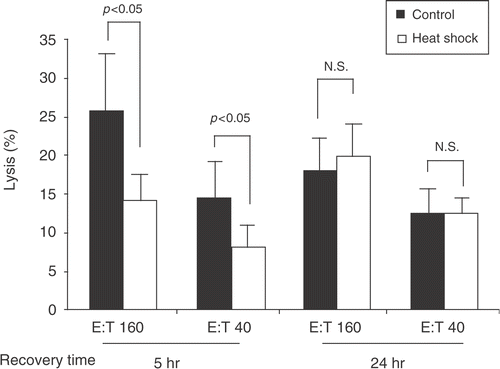
Heat-treated (1 h, 42°C) and control (37°C) PBMC from three healthy donors were recovered at indicated times and the cytotoxicity against a highly susceptible NK target, the K562 cell line, was determined by flow cytometric analysis. We observed that at 5 h after heat shock, NK cytotoxicity was dramatically reduced in heat-treated human primary PBMC compared with nonheat shock control. However at 24 h, NK cytotoxicity in heat-treated PBMC was comparable to the control group (). These results suggest that heat shock suppresses NK cytotoxicity and that this suppression is temporary and reversible.
Figure 1. Heat shock temporarily suppresses cytotoxicity of human NK cells in vitro. PBMC obtained from healthy donors were heat shock treated at 42°C for 1 h or maintained at 37°C (control). Five or twenty-four hours after heat shock, the heat-shocked and control PBMC were each co-cultured with EGFP-transduced K562 cells at 37°C for 4 h followed by 15 min incubation with PI to assess for the cytolytic activity of PBMC. Effector : Target (E:T) ratios were 160 : 1 and 40 : 1. After the incubation, the percentages of PI+ cells (dead cells) within EGFP+ cells were analyzed with flow cytometry, and cytotoxicity was calculated as described in the Section, ‘Methods’. Percentage means of specific lysis ± S.E. from three donors are displayed. P was assessed by paired t-test. N.S. = not significant.
Heat shock regulates perforin expression selectively in CD56dim NK cells
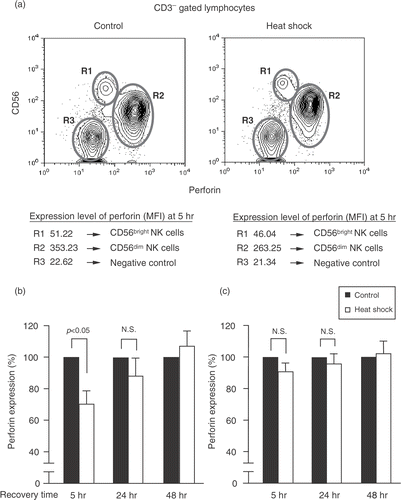
Because perforin is the key mediator of NK-mediated cell killing Citation[16], Citation[17], we investigated using PBMC from three healthy donors, the effect of heat shock on perforin expression in the two subsets of NK cells, CD56dim and CD56bright NK cells, at various recovery times. Heat-treated and control NK cells were tested for the expression of intracellular perforin by flow cytometry. Interestingly, heat shock predominantly affected perforin expression in CD56dim NK cells, which is a highly cytotoxic subset (). Perforin expression in CD56dim NK cells treated with heat shock was significantly reduced compared with control at 5 h after heat shock. At 24 h, the expression level of perforin in heat shock-treated cells was slightly but not significantly lower than that of the control cells () and at 48 h after heat shock, a complete recovery of perforin expression was observed in the treated cells. On the other hand, there was no significant difference in the perforin expression of CD56bright NK cells between control and heat shock-treated cells at various time points (). Therefore, heat shock seemed to modulate perforin protein in CD56dim but not in CD56bright NK cell subset.
Figure 2. Heat shock down-regulates perforin expression on CD56dim NK cells. (a) PBMC were maintained at 37°C or heat shock treated at 42°C for 1 h and were recovered at 5, 24, and 48 h. Cells were stained with anti-CD56-APC and anti-CD3-PE. Then cells were fixed, permeabilized, and stained with anti-perforin-FITC. Lymphocytes were gated for CD3− population and perforin expression in different lymphocyte subpopulations was determined by flow cytometry. CD3−CD56− populations (B cells) were used as negative controls. Non-specific binding was not observed with isotype control mAbs (data not shown). Percentage means of perforin expression ± S.E. (n = 3) in heat-treated and control of (b) CD56dim NK cells and (c) CD56bright NK cells at the indicated times are presented. P was assessed by paired t-test. N.S. = not significant. MFI = Mean fluorescence intensity.
Heat shock regulates perforin mRNA expression in NK cells
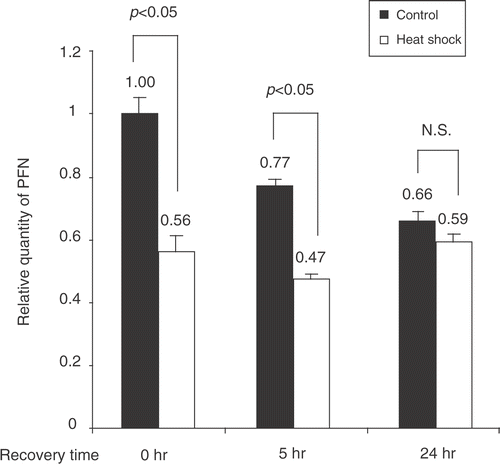
We determined whether the down-regulation of perforin by heat shock was at the transcriptional level. As shown in , the relative quantity of perforin mRNA in heat-treated purified NK cells was significantly suppressed immediately (0 h) and at 5 h after heat shock compared with their respective controls, indicating that heat shock affects the transcription of perforin in NK cells. When the recovery time was extended to 24 h, the quantity of perforin mRNA in NK cells subjected to heat shock was comparable to that of the control group (), consistent with the findings on perforin protein expression and cytolytic activity.
Figure 3. Heat shock regulates perforin mRNA expression in NK cells. Primary NK cells were heat-shock treated or kept at 37°C (control). Total RNA was recovered from control and treated cells at 0, 5, and 24 h. RNA extracts were subjected to real time Q-PCR using perforin and GAPDH primers. Quantity of perforin mRNA normalized to GAPDH is shown as mean ± S.E. (n = 3).
Heat shock does not induce cell death and down-regulation of surface molecules
We next asked whether the profound decrease of NK cell activity and suppression of perforin could be due to cell death. We performed cell death analysis by flow cytometry with Annexin V and 7-AAD staining on untreated and heat-treated PBMC from four donors. At 5 h after heat shock, we did not observe a significant difference in the percentage of apoptotic NK cells between the control and heat-treated groups (). Moreover, both groups were undergoing cell death at spontaneous level. These data suggest that heat shock at 42°C for 1 h did not induce cell death in NK cells.
Figure 4. Heat shock does not induce cell death and down-regulation of surface molecules. (a) PBMC were recovered at 5 h after heat shock, stained with anti-CD56-APC and anti-CD3-PECy7, Annexin V-PE (for apoptotic cells) and 7-AAMD (for dead cells). NK (CD3−CD56+) cell population was analyzed by the fluorescence intensities of Annexin V and 7-AAMD. Numbers at the right side of each panel indicate percentages of each fraction. Representative data from four donors are shown. (b) Heat-treated NK cells in (a) were analyzed for the expression of indicated surface molecules with FITC-or PE-conjugated mAbs. Thick lines indicate the cell surface receptor expression with or without heat shock and thin lines indicate the isotype-matched controls. Representative data was obtained from three donors.
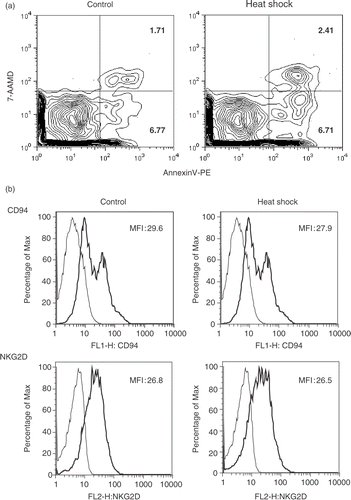
The balance of signal transmitted from cell surface activating and inhibitory receptors could determine NK cell activity Citation[8]. Thus, we investigated the potential relationship between heat shock and expression of NK cell receptors and adhesion molecules. Heat-treated and control PBMC from three donors were stained with anti-CD56, anti-CD3, and the indicated antibodies, and were analyzed by flow cytometry. However, we observed that at 5 h after heat shock, expression levels of CD94, activating receptors (NKG2D, NKp46) and adhesion molecules (CD58, CD2) in heat-treated cells did not significantly differ from those of the control group (), suggesting that heat shock did not significantly affect the expression of these cell surface receptors in NK cells.
Whole-body hyperthermia temporarily suppresses cytotoxicity of NK cells
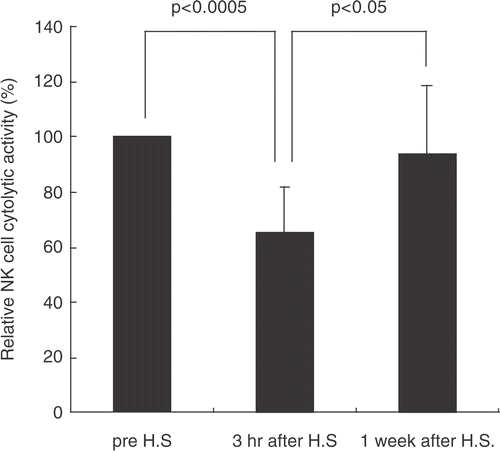
The cytotoxicity of NK cells before and after whole-body hyperthermia was evaluated in blood samples from eight cancer patients undergoing hyperthermia therapy at 41.5–42°C for 1 h. There was a significant decrease of NK cell cytotoxicity after 3 h treatment but it reverted to basal level after 1 week (). These results reflected those of in vitro heat shock () and support our observations that NK cell activity is temporarily down-regulated by heat shock.
Figure 5. Whole-body hyperthermia temporarily suppresses cytotoxicity of NK cells. PBMC from donor patients were recovered before, and at 3 h and 1 week after hyperthermia. Mixtures of effector-target cells were incubated at 37°C for 4 h followed by 15 min incubation with PI. Cytotoxicity was determined by flow cytometry as described above. Percentage means of specific lysis are displayed (n = 8). P was assessed by paired t-test.
Discussion
In this article, we have shown that at 5 h after heat shock treatment, the cytotoxicity of human NK cells was suppressed but at 24 h after heat shock, the NK cytolytic activity of heat shock-treated cells was similar to that of its respective control. Moreover, we observed a similar temporary suppression of NK cell activity in tumor patients receiving hyperthermia. The inhibition of human NK cell-mediated cytotoxicity by hyperthermia has also been reported by other researchers Citation[7], Citation[18–21], however, these studies did not clearly establish the mechanism underlying the regulation of NK cell cytotoxicity by heat. In our previous work, we showed that cytotoxicity of NK cells from whole-body heat-shocked mice was suppressed via down-regulation of perforin expression Citation[9]. However, mouse NK biology differs in certain aspects to human NK biology, notably, the lack of murine homolog of CD56 subsets Citation[22]. Hence, we have undertaken the investigation of the effect of heat shock on human NK cells. In the present article, we show that the suppression of human NK cytotoxicity by heat shock involved the perforin protein expression in CD56dim NK cell subset (), a novel finding that could not be noted in mouse model Citation[9]. But although we have shown that heat shock predominantly affected the expression of perforin in CD56dim NK cell subset, the actual mechanism of how heat shock preferentially regulates perforin in CD56dim NK cells remains to be elucidated. Further studies into the selective regulation by heat on subsets of human NK cells may provide insight into the distinct functional properties of these human NK subsets in heat-regulated immune response.
We observed that the cytolytic activity was fully recovered in heat shock-treated cells after 24 h but the level of perforin protein at this time point in treated cells was slightly lower than that of nonheat shock control (). However, the difference in perforin expression between control and heat shock-treated cells was not significant and the level of perforin in the treated cells at 24 h might be enough for the NK cells to be fully functional. Nonetheless, we cannot totally rule out the possibility of a perforin-independent-pathway Citation[23].
The initial suppression of NK cytotoxicity by heat shock was not attributed to the reduction of cell count caused by cell death or to an altered expression of the major NK cell receptors and adhesion molecules (). Surface density of natural cytotoxicity receptors (NCRs) on NK cells has been reported to correlate with the magnitude of NK cytotoxicity against target cells Citation[24]. However, the heat regulation of NK cytotoxicity might not involve these surface receptors since there were no alterations by heat on these molecules. As shown by Yang et al., even though NK cells were completely inactivated for target cell lysis at a high-thermal condition of 44°C, they maintained their recognition and binding functions Citation[20].
Finally, the purpose of this study was to accumulate knowledge about the effects of heat treatment on NK cells. We anticipate that further investigations will yield a deeper understanding on the response of the immune system to hyperthermia, which could further improve current hyperthermia modalities.
Acknowledgments
This work was supported by grants from the Ministry of Education, Science, Sports and Culture (MEXT) of Japan, the Global COE Program (Cell Fate Regulation Research and Education Unit), MEXT, Japan and the Sasakawa Scientific Research Grant from the Japan Science Society.
References
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002; 3: 487–497
- Baronzio G, Gramaglia A, Fiorentini G. Hyperthermia and immunity. A brief overview. In vivo (Athens, Greece) 2006; 20: 689–695
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001; 22: 633–640
- Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol 1986; 136: 4480–4486
- Azocar J, Yunis EJ, Essex M. Sensitivity of human natural killer cells to hyperthermia. Lancet 1982; 1: 16–17
- Onsrud M. Effect of hyperthermia on human natural killer cells. Recent Results Cancer Res. 1988; 109: 50–56
- Fuggetta MP, Alvino E, Tricarico M, D'Atri S, Pepponi R, Prete SP, Bonmassar E. In vitro effect of hyperthermia on natural cell-mediated cytotoxicity. Anticancer Res. 2000; 20: 1667–1672
- Yang H, Lauzon W, Lemaire I. Effects of hyperthermia on natural killer cells: Inhibition of lytic function and microtubule organization. Int. J. Hyperthermia 1992; 8: 87–97
- Koga T, Harada H, Shi TS, Okada S, Suico MA, Shuto T, Kai H. Hyperthermia suppresses the cytotoxicity of NK cells via down-regulation of perforin/granzyme B expression. Biochem Biophys Res Commun 2005; 337: 1319–1323
- Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 2006; 176: 1517–1524
- Harada H, Suzu S, Ito T, Okada S. Selective expansion and engraftment of human CD16+ NK cells in NOD/SCID mice. Eur J Immunol 2005; 35: 3599–3609
- Robins HI, Dennis WH, Neville AJ, Shecterle LM, Martin PA, Grossman J, Davis TE, Neville SR, Gillis WK, Rusy BF. A nontoxic system for 41.8°C whole-body hyperthermia: Results of a Phase I study using a radiant heat device. Cancer Res 1985; 45: 3937–3944
- Sawaji Y, Sato T, Takeuchi A, Hirata M, Ito A. Anti-angiogenic action of hyperthermia by suppressing gene expression and production of tumour-derived vascular endothelial growth factor in vivo and in vitro. Br J Cancer 2002; 86: 1597–1603
- Lanier LL, Chang C, Azuma M, Ruitenberg JJ, Hemperly JJ, Phillips JH. Molecular and functional analysis of human natural killer cell-associated neural cell adhesion molecule (N-CAM/CD56). J Immunol 1991; 146: 4421–4426
- Shacklett BL, Cox CA, Quigley MF, Kreis C, Stollman NH, Jacobson MA, Andersson J, Sandberg JK, Nixon DF. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: Implications for immune control of HIV-1 infection. J Immunol 2004; 173: 641–648
- Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti MA, Hayakawa Y. Activation of NK cell cytotoxicity. Mol Immunol 2005; 42: 501–510
- Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer 2002; 2: 850–861
- Yoshioka A, Miyachi Y, Imamura S. Immunological effects of in vitro hyperthermia. J Clin Lab Immunol 1989; 29: 95–97
- Kalland T, Dahlquist I. Effects of in vitro hyperthermia on human natural killer cells. Cancer Res 1983; 43: 1842–1846
- Yang HX, Mitchel RE. Hyperthermic inactivation, recovery and induced thermotolerance of human natural killer cell lytic function. Int J Hyperthermia 1991; 7: 35–49
- Shen RN, Lu L, Young P, Shidnia H, Hornback NB, Broxmeyer HE. Influence of elevated temperature on natural killer cell activity, lymphokine-activated killer cell activity and lectin-dependent cytotoxicity of human umbilical cord blood and adult blood cells. Int J Radiat Oncol Biol Phys 1994; 29: 821–826
- Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunological Rev 2006; 214: 47–55
- Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nature Rev 2002; 2: 735–747
- Sivori S, Parolini S, Marcenaro E, Castriconi R, Pende D, Millo R, Moretta A. Involvement of natural cytotoxicity receptors in human natural killer cell-mediated lysis of neuroblastoma and glioblastoma cell lines. J Neuroimmunol 2000; 107: 220–225
