Abstract
Purpose: This study aimed to evaluate the multidrug resistance (MDR) reversal activity of quercetin (Que) in combination with hyperthermia (HT) in human myelogenous leukemia cells K562/A.
Methods: The cytotoxicity of Que alone and the effect of Que and HT to doxorubicin (Dox) cytotoxicity were determined using MTT assay in K562 and K562/A cells. K562/A cells was heated with or without Que pretreatment, and the protein and mRNA levels of heat shock protein 70 (HSP70) and P-glycoprotein (P-gp) were determined by flow cytometry (FCM) and RT-PCR, respectively. Intracellular accumulation of Dox, cell cycle and apoptosis were monitored with FCM.
Results: Que alone inhibited cell growth in a dose-dependent manner in K562 and K562/A cells. Either Que or HT alone had a weak reversal effect on Dox resistance, however, combination HT and Que showed a much more significant reversal effect on Dox resistance (reverse fold 9.49). The elevated protein expression and mRNA level of HSP70 and P-gp in response to HT were inhibited by Que. Pretreatment with Que caused the cells to accumulate Dox 8.3-fold higher than in control cells. In addition, Que induced apoptosis and G2/M arrest in a dose-dependent manner, and the combination of Que and HT was found to have a synergistic efeect on apoptosis.
Conclusions: Que pretreatment could significantly inhance the MDR reversal activity of HT in resistant cell line, by sensitizing the cell to reversing MDR activity of HT.
Introduction
In recent years, significant advancements in the treatment of solid tumors and hematological malignancies were achieved. However, multidrug resistance (MDR) represents a major obstacle to successful chemotherapy of metastatic diseases Citation[1]. Although there are several different mechanisms associated with the development of MDR, one of the best understood is that of overexpression of P-glycoprotein (P-gp). This 170-kDa plasma membrane protein belongs to a larger family of ATP-binding cassette proteins. It functions as an ATP-dependent drug efflux pump, which rapidly extrudes a variety of hydrophobic anti-cancer drugs from the target cancer cells and hence reduces intracellular drug accumulation Citation[2]. In addition, P-gp may serve as an anti-apoptotic molecule and participate in the protection against environmental stress such as heat Citation[3]. P-gp is coded by the mdr1 gene and the administration of P-gp inhibitor was found to suppress activation of the mdr1 gene expression. Accordingly, a variety of agents including verapamil (Ver), cyclosporin A(CysA) and other agents have been reported in overcoming MDR Citation[4]. However, Ver and CysA are also used as an anti-arrhythmic drug and an immunosuppressant, respectively. Therefore, possible side effects are expected when these drugs are used as MDR-reversing agents. In an attempt to search for novel agents that are less toxic, we investigated the effect of hyperthermia and in combination with quercetin (Que) in reversing MDR, in which Que was used to sensitize cells to hyperthermia.
Hyperthermia (HT) is applied as an adjuvant therapy together with various established cancer treatments such as chemotherapy and radiotherapy Citation[5]. Heat shock response is a highly conserved molecular stress response that is involved in the induction of stress proteins (heat shock proteins or HSPs). HSPs are designed to protect cells against elevated temperatures in a wide variety of stressful conditions Citation[6]. HSP70 expression is found to be elevated in many types of cancer and is supposed to represent ‘general survival proteins’ able to defend cells against a variety of pro-apoptotic stimuli Citation[7], Citation[8]. The elevated HSP70 expression is also found to be associated with various forms of drug resistance including MDR and thermotolerance Citation[9]. Que has been shown to be a broad spectrum inhibitor of HSP expression and has inhibited the induction of HSP70 at the level of mRNA accumulation since the1990s Citation[10]. Other systemic strategies that also could be used to reduce HSP expression include anti-sense strategies that target HSPs and drug intervention strategies using the inhibitor KNK437 Citation[11], Citation[12].
Que (3, 3′, 4′, 5, 7-pentahydroxyflavon) is a flavonoid which is present in many edible fruits and vegetables such as apples, grapes, lemons, onion, kale, and tomatoes. Que has a broad range of biological, pharmacological and medical applications Citation[13–15]. It has been reported to be an anti-cancer agent because it exerts anti-proliferative effects on different malignant cells by several mechanisms: freezing the cell cycle in G2/M phase, scavenging of free radicals, induction of apoptosis, inhibiting glycolysis and activities of several enzymes like protein kinase C (PKC), mitogen-activated protein (MAP) kinase, cell division cycle (CDC) kinase 2 Citation[16–18]. Que is now broadly used as a health food supplement and commercially available. It has been shown that Que could interact with several ATP-binding cassette transporters which are linked with anti-cancer drug resistance, so Que may play roles in modulating drug resistance in this way Citation[19]. Furthermore, Que is found to inhibit the expression of P-gp and mdr1 gene products by inhibiting heat shock factor DNA-binding activity Citation[2], Citation[9]. Therefore, Que has been used as an HT sentizer in previous studies Citation[20–22].
This study is the first description to show the combination effect of HT and Que in reversing MDR in resistant cell line. We aimed to examine treatment protocols of combining Que and HT on reversing MDR in K562/A cells.
Materials and methods
Chemicals and treatment
Que (Sigma Chemical Co., USA) was dissolved in 100% dimethyl sulphoxide (DMSO) and stored at –20°C. The stock concentration was 100 mM, and the final concentration of DMSO in medium did not exceed 0.05%. At this level, DMSO did not influence cell viability and the expression of studied proteins as indicated in preliminary experiments. Cells were pre-incubated with Que for 1 h at 37°C before the heat treatment. Doxorubicin hydrochloride (Dox, Shanghai Pharma, China) was dissolved in deionized water to 5 mg/ml, which was diluted to final concentration prior to use.
Hyperthermia
Hyperthermia was carried out by immersion of culture flasks with fully tightened screw-tops or culture plates with sealed bags in a temperature-regulated water bath (Beijing Changfeng Apparatus Co., China). The temperature was maintained within the error limits of ±0.5°C by a thermometer. The cells were heated at 41°C, 42°C or 43°C for 1 h and were immediately returned to 37°C. Cell count was performed under light microscope at 24 h using 0.4% trypan blue solution (Sigma) and a hemocytometer. The temperature of 42°C for 1 h was selected for our study, because more than 90% of cells remained viable at this temperature, and it was also consistent with whole body hyperthermia conventional temperature.
Cell culture
Human myelogenous leukemia K562 cells were obtained from Dr YF Zhu (Oncology Research Institute of PLA 307 Hospital). Dox-selected and P-gp-positive multidrug resistant human leukemia K562/A cells were purchased from Tianjin Institutes for Hematology, Chinese Academy of Medical Sciences. Both the cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS, PAA laboratories, Austria), and kept in logarithmic growth phase at 37°C in a 95% humidified atmosphere with 5% CO2.
Cytotoxicity assay
In vitro anti-proferative effect of Que and the ability of Que and HT to potentiate Dox cytotoxicity were evaluated in K562 and K562/A cells by MTT assay Citation[23]. For the determination of the anti-proliferative effect of Que in K562 and K562/A cells, various concentrations of Que diluted in 100 μl medium were added into 96-well plates (Costar). Then cells were seeded at 5 × 104/well in 100 μl medium and incubated for 24 h. To determine the reversal effect of Que and HT, graded concentrations of Dox with or without HT or Que were added to the cells. The cells were exposed to drugs for 72 h at 37°C, after which 20 µl of MTT (Sigma, 5 mg/ml) was added to each well and incubated for 4 h. The formazan formed was dissolved in 100 µl 10% SDS. The absorbance was measured at a wavelength of 570 nm using a microplate reader. The results were expressed as IC50 values, which were determined by statistical software with a method of linear regression. The reversal activity of Que and HT on drug resistance was expressed as the reversal fold (RF) calculated using the following equation: RF = IC50 of cytotoxic drug alone/IC50 of cytotoxic drug in the presence of a modulator.
P-gp and HSP70 expression analysis by flow cytometry
K562/A cells were heated with or without Que pretreatment, and incubated at 37°C for 24 h. Then 1 × 106 cells were harvested, washed with ice-cold PBS, and fixed in 1% paraformalin for at least 15 min. After fixation, cells were stained separately with mouse anti-human monoclonal antibody specific for HSP70 (reacts with both the constitutive and inducible forms of HSP70, Chemicon International, Inc.) and P-gp (clone JSB-1, Chemicon International, Inc.), detected with FITC-conjugated goat anti-mouse antibody (Sino-American Biotechnology, Co.). HSP70 and P-gp positive percentage were examined utilizing flow cytometry (FCM, FACSCaliber, Becton Dickinson).
Examination of mdr1, HSP70 mRNA by RT-PCR
Total RNA was isolated from K562/A cells using TRIzol reagent (BioDev-Tech, China). After cells were harvested, TRIzol reagent (1 ml/5–10 × 106 cells) was added to lyse the cells. The mixture was incubated for 5 min at 15–30°C. Chloroform (0.2ml/ml TRIzol) was added and mixed completely with the lysate by vortexing for 15 s. Subsequently, the mixture was incubated at 15–30°C for 2–3 min, and centrifuged at 10 000 rpm at 4°C for 15 min. The water phase supernatant was transferred to a fresh tube, and incubated with isopropyl alcohol (0.5 ml/ml TRIzol) for 20 min at 15–30°C. The mixture was centrifuged at 10 000 rpm for 10 min at 4°C. RNA pellet was washed with 75% ethanol (1 ml/ml TRIzol) by centrifugation at 5000 rpm for 3 min at 4°C. After drying at room temperature, the RNA pellet was dissolved in RNase-free water and incubated at 55°C for 10 min.
The primers for HSP70, mdr1 and GAPDH genes were all synthesized by Shenggong Bioengineering, PR China. The primer sequence for the genes of interest are as following: mdr1, forward primer, 5′-CCCATCATTGCAATAGCAGG-3′ and reverse primer, 5′-GTTCAAACTTCTGCTCCTGA-3′ (167bp) Citation[24]; HSP70, forward primer, 5′-CCATGGTGCTGACCAAGATGAAG-3′, and reverse primer, 5′-TCGTCGATCGTCAGGATGGACAC -3′(284bp) Citation[25]; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, internal standard) forward primer, 5′-TCACCACCATGGAGAAGGCT-3′ and reverse primer, 5′-AAGGCCATGCCAGAGAGCTT-3′ (348 bp) [GenBank accession no. M17701]. RT-PCR kit was from BioDec-Tech, PR China. The reverse transcription mixture in a 20 µl reaction included 1 µl dNTPs, 1 µl MMLV reverse transcriptase, 4 µl 5 × MMLV buffer, 1 µl Olig(dT) primer, 0.4 µl RNasin, 2 µl template RNA and 10.6 µl sterilized deionized water. Reverse transcription was performed by incubating the mixture at 37°C for 60 min. Polymerase chain reaction (PCR) mixture was prepared in a final volume of 50 µl containing 1 µl cDNA, 1 µl dNTPs, 0.4 µl Taq DNA polymerase, 5 µl 10 × PCR reaction buffer, 1 µl each of 5′- and 3′-primers. Pre-denaturation was performed at 94°C for 10 min. Thirty cycles of denaturation at 94°C for 30 s, annealing step at 55°C for 30 s for HSP70, 60°C for 30 s for mdr1, and an extension step at 72°C for 1 min was performed, followed by a final elongation step at 72°C for 10 min. Following amplification, PCR products were electrophoresed on a 2% agarose gel at 150 volts. The gel was stained with 0.5 µg/ml ethidium bromide and photographed.
Intracellular Dox accumulation
K562/A and K562 cells at a density of 20 × 104/ml (5 ml) were exposed to 10 μg/ml Dox for 3 h in triplicate, with or without Que pretreatment and HT. The intracellular Dox fluorescence was monitored with FCM. Cells were harvested, washed and resuspended in PBS. Excitation was performed by an argon ion laser operating at 488 nm and the emitted fluorescence was collected through a 585 nm pass filter. A minimum of 10 000 cells were analyzed from each sample. Details of laser-flow-cytometric procedure for determination of cellular Dox fluorescence was published earlier Citation[26]. Data analysis was performed using Cell Quest software.
Apoptosis and cell cycle analyses
K562/A cells were seeded at a density of 40 × 104/ml (5 ml) in culture flasks, and treated with Que at various concentrations for 24 h or heated at 41°–43°C for 1 h followed by 24 h incubation at 37°C. The apoptosis synergistic effect of the combination of Que and HT was examined by treating the cells for 24 h. Cells were harvested and washed with PBS, fixed in 70% ice-cold ethanol for at least 30 min. The cell pellets were resuspended in a 1 ml solution containing 100 µg/ml of RNase (Sigma) and 50 µg/ml of propidium iodide (PI, Sigma), and incubated for 30 min at 37°C. Cell cycle distributions were examined with FCM and the data were analyzed by Modifit program.
Apoptosis assessments by annexin V–FITC and PI staining
Phosphatidylserine (PS) translocation from the inner to the outer leaflet of the plasma membrane is one of the earliest apoptotic features. The PS exposure in K562/A cells was detected with an annexin V–FITC/PI apoptosis detection kit (Biosea, China). After being exposed to Que, HT or combination for 24 h, cells were harvested and washed with ice-cold PBS. The supernatant was discarded and the cell pellets were resuspended in ice-cold binding buffer to 5 × 105–1 × 106 cells/ml. 10 μl of annexin V–FITC solution and 5 μl of dissolved PI were added to 200 μl of the cell suspensions. The samples were mixed gently and incubated on ice for 15 min in the dark. Then 300 μl of ice-cold binding buffer was added and mixed gently before the cell preparations were examined by FCM.
Data analysis
All data were presented as mean ± standard deviation (SD) and different groups were compared using Student's t-test. IC50 was analyzed with the linear regression (SPSS, version 12).
Results
Evaluation of the cytotoxic effect of Que
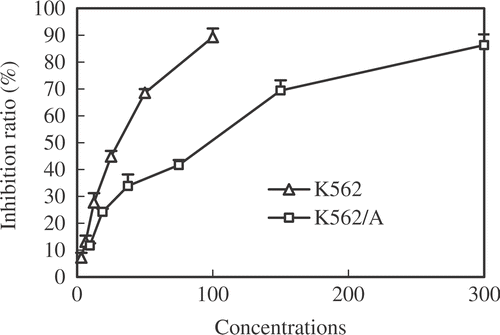
The cytotoxicity of Que alone in K562 and K562/A cells was determined using MTT assay. As shown in , Que inhibited cell growth in a dose-dependent manner at 24 h. IC50 values of Que in K562 and K562/A cells at 24 h were 47.00 ± 1.25 µM and 121.91 ± 3.56 µM respectively. Que at a final concentration of 100 µM, which is lower than IC50, was used in the experiments on the basis of our preliminary experiments and observations of other authors Citation[17], Citation[27].
Effect of Que and HT on Dox cytotoxicity
The effect of Que and HT in reversing the resistance of K562/A cells to Dox is shown in . Que (100 µM) or HT (42°C, 1 h) only reversed the cellular resistance to Dox weakly, while a combination of HT and Que significantly reversed the cellular resistance to Dox, and RF was up to 9.49, which has never been observed in K562 cells.
Table I. Effect of Que and HT to Dox cytotoxicity in K562 and K562/A cells.
FCM analysis of HSP70 and P-gp expression
P-gp and HSP70 expression in resistant K562/A cells were evaluated using FCM analysis. As shown in , the fluorescence intensity of P-gp and HSP70 increased when cells were heat shocked at 42°C for 1h. The pretreatment of Que inhibited the elevated expression P-gp and HSP70 upon HT ( & ).
Figure 2. The expression of HSP70 and P-gp in K562/A cells. K562/A cells were heated with or without Que pretreatment and expression of HSP70 and P-gp was evaluated by FCM after 24 h recovery period at 37°C. A P-gp expression; B HSP70 expression; a-d: different groups (a, control; b, HT; c, Que; d, Que + HT, the filled area represents the normal naked cells).
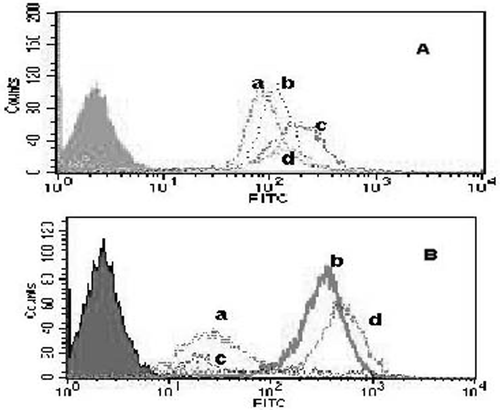
Table II. Percentage of K562/A cells with positive P-gp and HSP70 in different groups (gate%).
Effects of Que on mdr1 and HSP70 gene expression
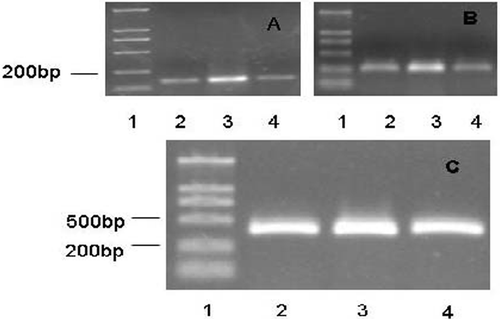
To determine the effects of Que on mdr1 and HSP70 mRNA expression in K562/A cells, cells were incubated for 24 h after HT with or without the pretreatment of Que. As shown in , K562/A cells expressed both mdr1 and HSP70 gene. HT up-regulated the expression of mdr1 and HSP70 mRNA, whereas Que inhibited the elevated mdr1 and HSP70 gene expression induced by HT.
Effect on intracellular accumulation of Dox
MDR tumor cells accumulate and retain less anti-cancer drug than their drug-sensitive cells. Accumulation of Dox in K562/A cells was much lower than in K562 cells. When cells were heated, Dox content increased more in K562 cells than in K562/A cells. Pretreatment with Que increased the accumulation of Dox to a level of 8.3-fold higher than the level in untreated control cells. On the other hand, there was only a weak increase in K562 cells ( & ).
Figure 4. Effect of HT and Que on intracellular Dox accumulation in K562 and K562/A cells. Cells were incubated with medium containing 10 μg/ml Dox for 3 h with or without Que pretreatment when cells were heated. (A) Sensitive K562 cells; (B) resistant K562/A cells; a-d: different groups (a, Dox; b, HT + Dox; c, Que + Dox; d, Que + HT + Dox; the filled area represents normal naked cells).
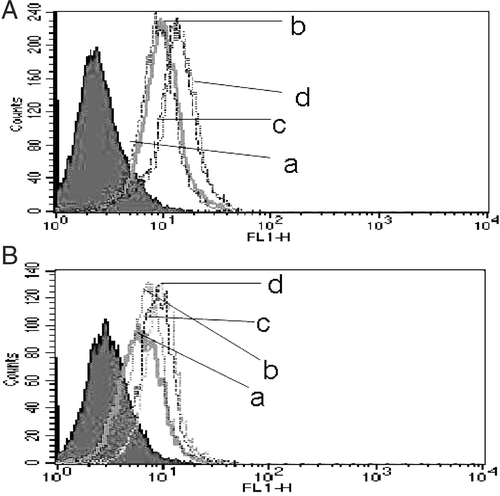
Table III. Intracellular Dox content of K562 and K562/A cells (gate%).
Que and HT induced cell cycle arrest and apoptosis synergistic effect
To confirm apoptosis and determine if growth inhibition involves cell cycle changes, we examined cell cycle phase distribution by FCM. K562/A cells were treated with 0, 100, 120 and 140 µM of Que for 24 h. shows a significant increase of a G2/M population after the treatment. Percentage of cells with a DNA content <2N (Sub-G1, apoptotic cells) was increased in dose-dependent manner. The cell cycle phase distribution of K562/A cells after HT with 41°, 42° and 43°C for 24 h is also shown in . G2/M and G1 phase cell cycle arrest was observed in heated cells. In Que + HT group, the percentage of apoptotic cells was increased to 11.4%. The number of cells in the G1 and S phase of cell cycle was decreased and remained at G2/M arrest in this group.
Table IV. Effects of Que and HT on cell cycle in K562/A cells.
Effects of Que and HT on apoptosis and necrosis induction
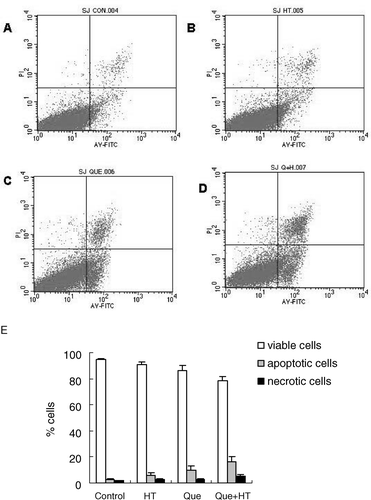
As dead cells are reactive to PI, we examined the externalized phosphatidylserine residues, present in apoptosis, by annexin V–FITC. Flow cytometric analysis with annexin V/PI staining showed that when cells were exposed to Que in combination with HT for 24 h, the proportion of AV+/PI+ (necrotic cells) was 5.21%, while it was only 1.67% in the control cells. In addition, when cells were exposed to Que in combination with HT for 24 h, the number of AV+/PI− (apoptotic cells) was 16.1%, and in the untreated cells, it was only 2.91%. The number of AV+/PI− (apoptotic cells) in the cells treated with Que and HT was 6 times higher than in control cells, suggesting that the combination of Que and HT could increase the number of cells committing to undergoing apoptosis. On the other hand, compared with 94.91% of control cells were viable, only 78.3% of combination treated cells were AV−/PI− (viable cells) (). These results suggested that both necrosis and apoptosis contributed to Que or HT-induced death of K562/A cells and apoptosis was the main cause.
Discussion
Despite recent advancement in treatment and supportive care in leukemia, the long term survival rate remains low, which may mainly due to chemo-resistance. In this study, the effect of Que, a potentially non-toxic agent, was tested along with HT in multidrug resistant cell line. First, we demonstrated Que's reversal effect on MDR, when it is combined with HT. Next, we showed that Que could regulate the expression of HSP70 and P-gp at the level of transcription and increase the intracellular accumulation of Dox. Therefore, Que may act as a potential HT sensitizer in reversing MDR in K562/A cells.
Previous studies indicated that Que acts as an inhibitor of HSP expression in several tumor cell lines Citation[10], Citation[28], Citation[29]. It was suggested that inhibition of HSP expression has an impact on increased tumor sensitivity and apoptosis induction. However, the mechanism of inhibition of HSP70 expression by Que is not clearly understood. Some studies suggested that Que may inhibit HSP expression at the level of transcription by preventing the heat shock factor 1 and 2 (HSF-1and HSF-2) from binding to the conserved DNA sequence known as the heat shock element (HSE) in the promoter region of HSP genes Citation[9], Citation[29–31]. While other studies suggested that Que may function at early events before HSP synthesis by blocking additional modifications necessary for activation of HSF, and inhibiting its interactions with other DNA-binding proteins in the promoter region Citation[32], Citation[33].
Our study demonstrated that HSP70 expression is up-regulated by heat and down-regulated by Que. RT-PCR results showed that pretreatment with Que inhibited the elevated expression of HSP70 mRNA induced by HT. However, the intensity shift result in does not support this statement. That is because the overall counts might be lower but the intensity is higher in a sub-population of cells. So, we decided to examine the effect of Que on the HT-induced apoptosis. We found that Que induced apoptosis in a dose-dependent manner and this induction of apoptosis was promoted by HT. In addition, Que inhibited the induction of HSP70 expression and enhanced the cytotoxic effect of HT thereby. On the other hand, HSP70 released from tumor cells stimulates a potent anti-tumor immune response Citation[12]. Therefore, strategies that suppress the expression of HSPs may also reduce the immune response directed against the tumors. However, it was found that Que partially inhibited stress response, and reduced the expression of Hsp27 or Hsp70 but not Hsp90 or Hsp110 Citation[12].
The reports of the roles of HT on reversing MDR and the expression of P-gp appeared to be controversial. Pre-clinical data suggested that HT is a good candidate to overcome various modes of drug resistance, and this has been demonstrated in particular for the platinum derivate cisplatin (DDP) Citation[34]. Overcoming DDP resistance by HT is exemplary, since the MDR phenotype is not involved in DDP resistance Citation[6]. Although HT has been shown to induce resistance to heat and drugs (thermotolerance and MDR) under certain conditions Citation[35–37], some clinical trials showed that some refractory diseases were successfully treated with the addition of HT to a given chemotherapeutic schedule Citation[38], Citation[39]. It has been speculated that HT may induce multidrug resistance gene (mdr1) expression by HSF-DNA binding in the promoter region of genes, which could result in the disadvantageous MDR phenotype Citation[20]. Some observations supported this Citation[40], Citation[41], but some experiments suggested that HT may reduce P-gp expression Citation[42], Citation[43]. In this study we found that the expressions of P-gp and mdr1 mRNA were induced by HT and inhibited by Que, indicating that drug resistance could be induced by HT on principle, and Que could antagonize this effect.
Effect of modulator on Dox cytotoxicity and intracellular Dox accumulation are two important assays to evaluate synergistic reversal effect of MDR by Que and HT. Our results showed that HT in combination with Dox exerted more evident cytotoxicity effect and drug accumulation in sensitive cells compared to that in resistant cells. This may suggest that the elevated expression of P-gp and HSP70 induced by HT in resistant cells may protect the cells from heat and drugs. Our study also showed that the combination of HT and Que had a more significant reversal effect on Dox resistance (RF 9.49), than HT or Que alone. In addition, pretreatment with Que accumulated Dox 8.3-fold higher than that in untreated control cells. Therefore, HT in combination with Que pretreatment could be more effective treatment to reverse the MDR activity of Dox in K562/A cells.
Que treatment significantly increased the percentage of G2/M phase of cell cycle. In general, highest heat sensitivity can be observed during the mitotic phase Citation[6]. Microscopic examinations of HT treated M-phase cells showed of mitotic apparatus damage which led to inefficient mitosis and consecutive polyploidy Citation[44]. Therefore, Que treatment could promote cellular sensitivity to HT by arresting the cell cycle. From this point of view, it increased the synergistic effect of HT and Que, especially on apoptosis induction.
Data presented in demonstrated that Que and HT can affect membrane annexin V staining in K562/A cells, but that is not the best indicator of apoptosis. It is known that caspase-3 activation is one of the crucial pathways resulting in apoptosis. Proteolytic cleavage of poly (ADP-ribose) polymerase PARP by caspases is a hallmark of apoptosis. So, further study is needed to study the levels of caspase-3 and PARP cleavage in these backgrounds.
In this study, we used Que at high concentrations which exceed the level that can be achieved in vivo. Based on epidemiological and human absorption data, it is possible that the concentration of Que in colon is 40–80 µM Citation[17]. Elevation of Que plasma levels to levels approaching 100 µM requires intravenous injection in vivo, since low bioavailability of Que and the high first pass effect makes elevation of Que levels to these high concentrations via oral supplementation unlikely Citation[45]. Human pharmacokinetic studies have demonstrated serum concentrations of Que to range from 1 to 400 µM after a non-toxic intravenous dose of Que with a half-life of 1–2 h Citation[46]. Therefore, repeated intravenous doses or infusion seems to be the preferred way to administer Que in in vivo studies. On the other hand, the results obtained in the present study have also demonstrated that Que was likely to be effective synergistic reversing MDR reagent at concentrations lower than 100 µM.
Although Que alone has been shown to play a role in reversing MDR in some resistant cell lines Citation[19], Citation[44] and recognized as HT sensitizer Citation[21], Citation[22], this study is the first description of using Que as a sensitizer to facilitate HT to reverse MDR. In conclusion, HT in combination with Que pretreatment showed significant MDR reversal activity in K562/A cells. Its activity may be related to the inhibition of HSP70 and P-gp overexpression and the increase in intracellular accumulation of Dox. Therefore, the treatment with Que in combination with HT had a synergistic reverse effect on MDR.
Acknowledgements
This work was supported by Morestep Science & Technology Development Co., Ltd, Jilin Province of China. We are grateful to Ms W. Zhou and Ms Y.X. Li for their assistance in FCM analysis. We thank Ms Y.C. Tai and M.J. Jin from Singapore for word processing and Temin Duan for his expert technique support.
References
- Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: The multidrug resistance-associated proteins. J Natl Cancer Inst 2000; 92: 1295–1302
- Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: Role of P-glycoproein, MRP1, MRP2 and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 2005; 204: 216–237
- Pirity M, Hever-Szabo A, Venetianer A. Overexpression of P-glycoprotein in heat- and/or drug-resistant hepatoma variants. Cytotechnology 1996; 19: 207–214
- Ji BS, He L, Liu GQ. Reversal of p-glycoprotein-mediated multidrug resistance by CJX1, an amlodipine derivative, in doxorubicin-resistant human myelogenous leukemia (K562/DOX) cells. Life Sci 2005; 77: 2221–2232
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497
- Hildebrandt B, Wust P, Ahlers O, Diening A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002; 43: 33–56
- Wu C. Heat shock transcription factors: Structure and regulation. Ann Rev Cell Dev Biol 1995; 11: 441–469
- Agashe VR, Hartl FU. Roles of molecular chaperones in cytoplasmatic protein folding. Sem Cell Dev Biol 2000; 11: 15–25
- Kim SH, Yeo GS, Lim YS, Kang CD, Kim CM, Chung BS. Suppression of multidrug resistance via inhibition of heat shock factor by quercetin in MDR cells. Exp Mol Med 1998; 30: 87–92
- Hosokawa N, Hirayoshi K, Nakai A, Hosokawa Y, Marui N, Yoshida M, Sakai T, Nishino H, Aoike A, Kawai K. Flavonoids inhibit the expression of heat shock proteins. Cell Struct Funct 1990; 15: 393–401
- Matsuda K, Nakagawa SY, Nakano T, Asaumi J, Jagetia GC, Kawasaki S. Effects of KNK437 on heat-induced methylation of histone H3 in human oral squamous cell carcinoma cells. Int J Hyperthermia 2006; 22: 729–735
- Coss RA. Inhibiting induction of heat shock proteins as a strategy to enhance cancer therapy. Int J Hyperthermia 2005; 21: 695–701
- Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol Therapeut 2001; 90: 157–177
- Perez-Vizcaino F, Duarte J, Andriantsitohaina R. Endothelial function, & cardiovascular disease: Effects of quercetin and wine polyphenols. Free Rad Res 2006; 40: 1054–1065
- Le Marchand L. Cancer preventive effects of flavonoids–A review. Biomed Pharmacother 2002; 56: 296–301
- Murota K, Terao J. Antioxidative flavonoid quercetin: Implication of its intestinal absorption and metabolism. Arch Biochem Biophys 2003; 417: 12–17
- Dihal AA, Woutersen RA, van Ommen B, Rietjens IM, Stierum RH. Modulatory effects of quercetin on proliferation and differentiation of the human colorectal cell line Caco-2. Cancer Lett 2006; 238: 248–259
- Jakubowicz-Gil J, Paduch R, Piersiak T, Głowniak K, Gawron A, Kandefer-Szerszeń M. The effect of quercetin on pro-apoptotic activity of cisplatin in HeLa cells. Biochem Pharmacol 2005; 69: 1343–1350
- Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J 2005; 272: 4725–4740
- Piantelli M, Tatone D, Castrilli G, Savini F, Maggiano N, Larocca LM, Ranelletti FO, Natali PG. Quercetin and tamoxifen sensitize human melanoma cells to hyperthermia. Melanoma Res 2001; 11: 469–476
- Asea A, Ara G, Teicher BA, Stevenson MA, Calderwood SK. Effects of the flavonoid drug quercetin on the response of human prostate tumours to hyperthermia in vitro and in vivo. Int J Hyperthermia 2001; 17: 347–356
- Debes A, Oerding M, Willers R, Göbel U, Wessalowski R. Sensitization of human Ewing's tumor cells to chemotherapy and heat treatment by the bioflavonoid quercetin. Anticancer Res 2003; 23: 3359–3366
- Jin J, Wang FP, Wei H, Liu G. Reversal of multidrug resistance of cancer through inhibition of P-glycoprotein by 5-bromotetrandrine. Cancer Chemother Pharmacol 2005; 55: 179–188
- Wang DH, Wei HL, Zhao HS, Hao CY, Min ZH, Liu JM. Arsenic trioxide overcomes apoptosis inhibition in K562/ADM cells by regulating vital components in apoptotic pathway. Pharmacol Res 2005; 52: 376–385
- Lu HJ, Guo CB, Jin XQ, Wang LH. Influence of heat shock on expression of HSP70 in human glioma cells. Chin J Cancer Prev Treat 2005; 12: 1606–1608
- Krishan A, Ganapathi R. Laser flow cytometric studies on the intracellular fluorescence of anthracyclines. Cancer Res 1980; 40: 3895–3900
- Kudo M, Naito Z, Yokoyama M, Asano G. Effects of quercetin and sunphenon on responses of cancer cells to heat shock damage. Exp Mol Pathol 1999; 66: 66–75
- Jakubowicz-Gil J, Pawlikowska-Pawlega B, Piersiak T, Pawelec J, Gawron A. Quercetin suppresses heat shock-induced nuclear translocation of Hsp72. Folia Histochem Cytobiol 2005; 43: 123–128
- Jakubowicz-Gil J, Rzymowska J, Gawron A. Quercetin, apoptosis, heat shock. Biochem Pharmacol 2002; 64: 1591–1595
- Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med 1999; 31: 261–271
- Hosokawa N, Hirayoshi K, Kudo H, Takechi H, Aoike A, Kawai K, Nagata K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol 1992; 12: 3490–3498
- Hansen PK, Oesterreich S, Lemieux P, Sarge KD, Fuqua SA. Quercetin inhibits heat shock protein induction but not heat shock factor DNA-binding in human breast carcinoma cells. Biochem Biophys Res Commun 1997; 239: 851–856
- Nagasaka Y, Nakamura K. Modulation of the heat-induced activation of mitogen-activated protein (MAP) kinase by quercetin. Biochem Pharmacol 1998; 56: 1151–1155
- Hettinga JV, Konings AW, Kampinga HH. Reduction of cellular cisplatin resistance by hyperthermia - A review. Int J Hyperthermia 1997; 13: 439–457
- Li GC, Mivechi NF, Weitzel G. Heat shock proteins, thermotolerance, and their relevance for clinical hyperthermia. Int J Hyperthermia 1995; 11: 459–488
- Kampinga HH. Hyperthermia, thermotolerance and topoisomerase II inhibitors. Br J Cancer 1995; 72: 333–338
- Oh HJ, Chen X, Subjeck JR. Hsp 110 protects heat-denaturated proteins and confers cellular thermoresistance. J Biol Chem 1997; 272: 31636–31640
- Douwes F, Bogovic J, Douwes O, Migeod F, Grote C. Whole-body hyperthermia in combination with platimum-containing drugs in patients with recurrent ovarian cancer. Int J Clin Oncol 2004; 9: 85–91
- Westermann AM, Grosen EA, Katschinski DM, Jäger D, Rietbroek R, Schink JC, Tiggelaar CL, Jäger E, Zum Vörde sive Vörding P, Neuman A, et al. A pilot study of whole body hyperthermia and carboplatin in platinum-resistant ovavian cancer. Eur J Cancer 2001; 37: 1111–1117
- Stein U, Rau B, Wust P, Walther W, Schlag PM. Hyperthermia for treatment of rectal cancer: Evaluation for induction of multidrug resistance gene (mdr1) expression. Int J Cancer 1999; 80: 5–12
- Tang WB, Ma PQ, Zeng WT, Zhu KL, Yang W. An experimental study on the effect of hyperthermia and chemotherapy combined with quercetin on liver hepatocar cinoma HepG2 cells. Cancer Res Clin 2006; 18: 77–80
- Walther W, Stein U, Schlag PM. Use of the human MDR1 promoter for heat-inducible expression of therapeutic genes. Int J Cancer 2002; 98: 291–296
- Komdeur R, Plaat BE, Hoekstra HJ, Molenaar WM, Hollema H, van den Berg E, Mastik MF, van der Graaf WT. Expression of P-glycoprotein, multidrug resistance-associated protein 1, and lung resistance-related protein in human soft tissue sarcomas before and after hyperthermic isolated limb perfusion with tumor necrosis factor-alpha and melphalan. Cancer 2001; 91: 1940–1948
- Coss RA, Dewey WC, Bamburg JR. Effects of hyperthermia on dividing Chinese hamster ovary cells and on microtubules in vitro. Cancer Res 1982; 42: 1059–1071
- van Zanden JJ, van der Woude H, Vaessen J, Usta M, Wortelboer HM, Cnubben NH, Rietjens IM. The effect of quercetin phase II metabolism on its MRP1 and MRP2 inhibiting potential. Biochem Pharmacol 2007; 74: 345–351
- Ferry DR, Smith A, Malkhandi J, Fyfe DW, de Takats PG, Anderson D, Baker J, Kerr DJ. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res 1996; 2: 659–668
- Limtrakul P, Khantamat O, Pintha K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J Chemother 2005; 17: 86–95