Abstract
Previous data have demonstrated that doxorubicin (DOX) released from a lysolecithin-containing thermosensitive liposome (LTSL) can shut down blood flow in a human tumor xenograft (FaDu) in mice when the treatment is combined with hyperthermia (HT), suggesting that LTSL-DOX is a potential antivascular agent. To further understand mechanisms of the treatment, we investigated effects of LTSL-DOX (5 mg/kg body weight) plus HT (42°C, 1 h) on microcirculation in another tumor (a murine mammary carcinoma, 4T07) implanted in mouse dorsal skin-fold chambers and dose responses of tumor (FaDu and 4T07) and endothelial cells to LTSL-DOX or free DOX with or without HT. We observed that LTSL-DOX-HT could significantly reduce blood flow and microvascular density in 4T07 tumors. The antivascular efficacy of LTSL-DOX-HT could be enhanced through increasing tumor microvascular permeability of liposomes by using platelet activating factor (PAF). We also observed that the dose responses of FaDu and 4T07 to DOX in vitro were similar to each other and could be enhanced by HT. Taken together, these data suggested that tumor microvascular permeability was more critical than the sensitivity of tumor cells to DOX in determining the antivascular efficacy of LTSL-DOX-HT treatment.
Introduction
Liposomes accumulate preferentially in solid tumors Citation[1–5]. The accumulation is due to two mechanisms: leaky tumor vasculature and long circulation half-life of liposomes. This phenomenon makes liposomes ideal vehicles for delivery of anticancer agents. Drugs encapsulated in liposomes can be released passively through drug diffusion and liposome degradation or specifically through various mechanisms Citation[6–9], such as changes in local temperature and pH. Despite the advantages of liposomes, clinical outcomes in cancer treatment have not yet been improved significantly compared to free drug treatment. It is partly because the size of liposomes (∼ 100 nm in diameter) is larger than or comparable to the inter-fiber distance in extracellular matrix (ECM). As a result, liposomes accumulate only in perivascular regions after extravasation from tumor microcirculation. Free drugs released from liposomes may penetrate into deeper tissues but the amount of free drugs deposited in deeper regions is likely to be inadequate, due to drug diffusion into tumor microvessels and physiological barriers to interstitial transport.
One way to circumvent the problems related to drug penetration is to target liposomal drugs to tumor microvasculature since the vascular damage will reduce nutrient supply in tumors and thus kill tumor cells in deeper tissues. As mentioned above, liposomes accumulate preferentially in perivascular regions in tumors. In addition, plasma concentration of liposomes is high before liposomes are cleared from the systemic circulation. Under these conditions, vascular endothelial cells are exposed to free drugs, released locally from liposomes, at a significantly higher concentration than tumor cells. The concentration can be further increased by increasing the rate of drug release from liposomes and reducing the rate of drug clearance through microvessels Citation[6–8].
Lysolecithin-containing thermosensitive liposome (LTSL) is a drug delivery vehicle used in previous studies. It can release >90% of encapsulated doxorubicin (DOX) within 20 sec at 42°C Citation[10], Citation[11]. As a result, the average concentration of free DOX released from LTSL in solid tumors can reach a level that is approximately 30-fold higher than the average concentration after intravenous injection of free DOX Citation[10], and at least two-fold higher than the average concentration achieved by other liposomal formulations Citation[12]. If one considers the fact that the average penetration depth in extravascular regions is <10 μm for liposomes but 100–200 μm for free drugs, it can be estimated that LTSL in combination with hyperthermia (HT) is able to improve DOX delivery to endothelial cells by several orders of magnitude, compared to intravenous injection of free DOX. Furthermore, the percentage of DNA/RNA bound DOX in solid tumors at 1 h after treatment is ∼50% if DOX is delivered by LTSL but close to zero if it is delivered by other liposomes Citation[10]. These data indicate that LTSL in combination with HT is an ideal vehicle for DOX delivery to intracellular targets in vivo. Using this approach, investigators were able to completely eradicate a human pharyngeal squamous carcinoma xenograft (FaDu) in nude mic Citation[9], Citation[13].
Part of the mechanism for eradication is the destruction of tumor vasculature because there were no functional tumor microvessels, i.e. vessels with blood flow, in five out of six tumors at 6 h and 24 h after LTSL-DOX-HT treatment, and the sixth tumor contained only a few microvessels near the periphery Citation[8]. In addition, blood flow in these vessels was significantly lower than that in the control group. These data suggest that LTSL-DOX-HT is potentially a new approach to antivascular therapy of solid tumors.
To further understand mechanism of the treatment, we examined the antivascular effects of LTSL-DOX-HT in a murine mammary carcinoma (4T07) implanted in Balb/c mice. The choice of this tumor model was based on the observation in a preliminary study in which 4T07 was the least responsive tumor to LTSL-DOX plus HT treatment among five tumor models investigated (data not shown). We hypothesized that the lack of response was partly due to the difference in free DOX concentrations between these two models. To test the hypothesis, we quantified microvascular permeability of liposome in these tumors and investigated if an increase in microvascular permeability by using platelet activating factor (PAF) would enhance the antivascular effects of LTSL-DOX-HT treatment.
Materials and methods
Liposome preparation
The LTSL was prepared, using DPPC, MPPC, and DSPE-PEG-2000 in a molar ratio of 90 : 10 : 4 Citation[13]. Doxorubicin (Sigma Chemical Co., St Louis, MO) was loaded into the liposome at a final concentration of 2 mg/ml, using a pH gradient method Citation[14]. Rhodamine labeled non-thermosensitive liposome (NTSL) was prepared using Egg PC, Cholesterol, DSPE-PEG2000, and Rhodamine-DHPE in a molar ratio of 9 : 4.5 : 0.7 : 0.1. Both LTSL and NTSL were ∼100 nm in diameter.
Dorsal skinfold window chamber and tumor models
Female athymic nude mice and Balb/c mice with an average body weight of 22 g were purchased from National Cancer Institute. Animals were housed in the Duke University animal facilities before experiments. During the chamber implantation, animals were anesthetized through an intraperitoneal (i.p.) injection of a cocktail of 80 mg ketamine and 10 mg xylazine per kg body weight. Titanium window chambers were surgically implanted on the dorsal skin flap of mice Citation[15]. After surgery, animals were allowed to recover for 48 h before tumor cells were implanted into the chambers. All procedures were under aseptic conditions. Before experiments, animals were kept ad libitum in a warm room with food and water, and a 12-h light/dark cycle.
A human pharyngeal squamous carcinoma cell line (FaDu) and a mouse mammary carcinoma cell lines (4T07) were used to prepare tumor models in mouse dorsal skinfold chambers. They were implanted in nude and Balb/c mice, respectively, by placing 10 μl of tumor cell suspension (2x105) at the center of the chambers. On days 5 to 7, angiogenesis in tumors could be observed, which triggered exponential growth of tumors. The size of tumors reached ∼ 3 mm in diameter at 10 to 12 days after tumor cell implantation, when tumors were used in our experiments.
Tumor microcirculation experiments
Red blood cells (RBCs) were labeled with 1, 1′-dioctadecyl-3, 3, 3′, 3′ tetramethylindocarbo-cyanine perchlorate (DiI, Molecular Probes, Eugene, OR), using a technique described in a previous study Citation[8]. The DiI-RBCs were used within 2 days after the labeling. The animal was anesthetized using the same method as that described above and placed on a temperature controlled microscope stage to maintain the body temperature throughout the experiment. The dorsal skinfold chamber was fixed onto a hyperthermia stage designed specially for this kind of experiments Citation[8], Citation[16–18]. DiI-RBC suspension (0.05 ml) was injected intravenously through the tail vein at 5 min before experiments. During experiments, 4T07 tumors were treated with one of the two protocols. (a) LTSL-DOX-HT: hyperthermia plus intravenous (i.v.) injection of LTSL-DOX (DOX dose: 5 mg/kg body weight; n = 7) and (b) LTSL-HT: hyperthermia plus i.v. injection of empty LTSL (with the same lipid dose as in the first group; n = 5). (Microcirculation in FaDu tumors treated with the same protocols has been investigated in our previous study Citation[8]. Thus, it was not repeated in this study.) In the protocols involving HT, tumors were first heated for ∼2 min before liposome injection in order for the tumor and surrounding normal tissues to reach a thermal equilibrium with the heating device maintained at 42°C. At 0.5 h before and 0, 6, and 24 h after each treatment, microcirculation in 4T07 tumors was examined under an intravital fluorescence microscope with either trans- or epi-illumination. For epi-illumination, a filter set for rhodamine was used. The microcirculation in five different areas of a tumor was recorded on to videotapes using a 20x objective and a SIT camera (C2400, Hamamatsu Photonics, Hamamatsu, Japan) connected to an S-VHS video recorder (BV-1000, Mitsubishi Electronics, Tokushima, Japan). The recorded videos were analysed offline for determining RBC velocity and microvascular density Citation[8].
To evaluate effects of LTSL-DOX-HT plus PAF on tumor microvascular density, PAF solution (1 mg/ml, Sigma, St Louis, MO) was diluted to 300 nM with 0.1% BSA in saline before experiments. Animals were first anesthetized as described above. LTSL-DOX (DOX dose: 5 mg/kg body weight; n = 5) was injected through the tail vein. Then, 50 μl of PAF solution (300 nM) was applied topically on 4T07 tumors in dorsal skinfold chambers. One hour later, the tumors were heated for 1 h with the heating device maintained at 42°C. At the end of heating, tumor microcirculation was recorded on to videotapes using a 20x objective and the SIT camera, and the videos were analysed offline for determining the microvascular density as described above.
Quantification of tumor microvascular permeability
Animals were anesthetized and placed on a temperature controlled microscope stage as described above. A dorsal skinfold chamber was fixed on a specially designed hyperthermia stage. FaDu and 4T07 tumors in the window chambers were either heated at 42°C for 1 h (n = 7) or exposed to room temperature (n = 7) for 1 h. To evaluate the effects of PAF on tumor microvascular permeability, 50 μl of PAF solution (300 nM) was applied topically on 4T07 tumors in dorsal skinfold chambers, either immediately after the 1-hr HT or after the tumors were exposed to room temperature for 1 h. One hour later, a region in the tumor with steady blood flow and few obvious underlying vessels was selected for permeability measurement.
Microvascular permeability (P) was measured, using a method published in the literature Citation[6], Citation[17], Citation[19]. In brief, the image of tumor microvessels in the selected region was first acquired under a trans-illumination, with a 20x objective, for measurement of length and diameter of each microvessel. The data were used to calculate the vascular surface area-to-volume ratio, S/Vves,(1) where dn and Ln represent the diameter and the length of nth vessel, respectively. In addition, a background image of the tumor tissue in the same region was recorded for 10 sec under epi-illumination immediately before the injection of rhodamine labeled NTSL (0.1 ml/mouse) through the tail vein. After the NTSL injection, fluorescence images of the region were recorded continuously during the first minute and intermittently, i.e. 10 sec every 2 min, for an additional 30 min, under the intravital fluorescence microscope by using the SIT camera connected to the S-VHS video recorder. The average fluorescence intensity (Im) in the region was measured offline by using an image analysis software. Based on these data, the microvascular permeability was calculated by
(2) where HTm is the average hematocrits in tumor microvessels, which was assumed to be equal to 0. Citation[6], Citation[19]. K is the time constant of plasma clearance and was observed to be 1.78 × 104 sec for rhodamine-labeled NTSL in athymic nude mice and 1.89 × 104 sec in Balb/c mice, based on plasma clearance data. dIm/dt is the fluorescence intensity gradient. Im0 is defined as (I0 − Ib), where I0 is the value of fluorescence intensity immediately after the filling of all vessels with rhodamine-labeled NTSL. Ib is the value of background intensity.
Cytotoxicity of DOX and LTSL-DOX
Human umbilical vein endothelial cells (HUVECs) (Clonetics, MD) were cultured in EBM®-2 medium with EGM®-2MV SingleQuots (Cambrex, MD). Tumor cells (FaDu and 4T07) and HUVEC were harvested at about 90% confluency and placed in 96-well plates (1.0 × 104/10 μl/well). After allowing cells to attach for 24 h, they were incubated with culture medium containing DOX or LTSL-DOX with different DOX concentrations or equivalent DOX concentrations between 0.001 μM and 100 μM at 37°C in an incubator (NT) or 42°C in a water bath (HT) for 1 h. Subsequently, cells were incubated with fresh medium at 37°C for 24 h. The cytotoxicity was measured by the CytoTox 96 kit (Promega, Madison, WI). The percentage of viable cells was calculated, relative to untreated cells. All assays were performed in triplicate. IC50 values were calculated from the dose-response curves by curve fitting the data.
Statistics
Data are reported as mean ± standard error of the mean (SEM) in all figures. A Mann-Whitney U-test was used to determine the difference between different groups. The difference was considered to be statistically significant if the p-value was smaller than 0.05.
Results
RBC velocity in tumors
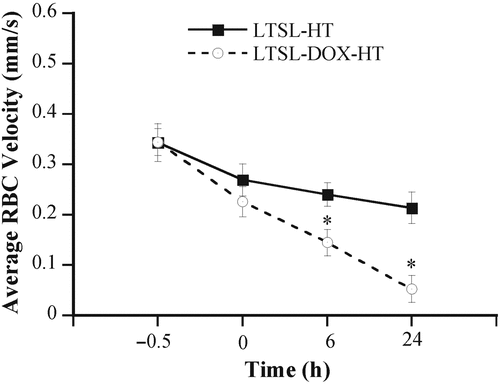
Tumors in dorsal skinfold chambers were treated with one of the two protocols as described in the Materials and methods section. They are (a) LTSL-DOX-HT: hyperthermia plus i.v. injection of LTSL-DOX and (b) LTSL-HT: hyperthermia plus i.v. injection of empty LTSL. Before any treatment, there was no significant difference in RBC velocities (mm/sec) between the two groups (0.344 ± 0.027 versus 0.343 ± 0.038) (see ). After LTSL-DOX-HT treatment, the RBC velocity (mm/sec) was decreased to 0.227 ± 0.03, 0.145 ± 0.026, and 0.05 ± 0.027 at 0, 6, and 24 h, respectively; and the amounts of decrease at these time points were statistically significant. In the LTSL-HT group, the RBC velocity was also decreased after treatment, but the amount of decrease was much less than that in the LTSL-DOX-HT group and was statistically significant only at 24 h.
Figure 1. Average RBC velocities in 4T07 tumors treated with LTSL-DOX-HT (n = 7) and LTSL-HT (n = 5). The velocity was measured at 0.5 h before, at 0, 6 and 24 h after the treatment. Error bars, SEM; *, p < 0.05, when comparing data between LTSL-DOX-HT and LTSL-HT groups. In addition, the velocities at 0, 6, and 24 h were significantly less than that before the treatment in the LTSL-DOX-HT group (p < 0.05), whereas the decrease in RBC velocity after the treatment was statistically significant (i.e. p < 0.05) only at 24 h in the LTSL-HT group.
Tumor microvascular permeability
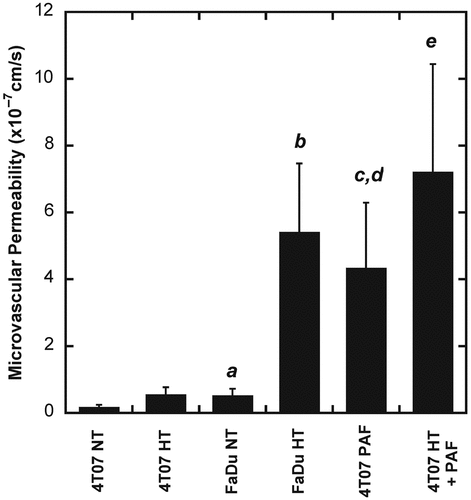
The microvascular permeability of liposomes was quantified in FaDu and 4T07 tumors under different experimental conditions. The results are shown in . HT treatment increased the permeability in both FaDu and 4T07 tumors. However, the amount of increase in 4T07 tumors was insignificant. In FaDu tumors, the permeability increase was approximately 9-fold and was statistically significant (p < 0.05). When comparing the microvascular permeabilities between 4T07 and FaDu tumors, the former was significantly lower than the latter in both HT and non-HT groups. To increase the permeability in 4T07 tumors, a solution of PAF was applied topically to the tumor in the dorsal skin fold chamber before permeability measurement. We observed that PAF alone could significantly increase the microvascular permeability to the level that was statistically the same as that in HT treated FaDu tumors (see ). When PAF was combined with HT, the permeability could be further increased although the amount of increase was statistically insignificant.
Figure 2. Tumor microvascular permeability in FaDu and 4T07 tumors treated with (HT) or without hyperthermia (NT) (n = 7 in each group), PAF alone or PAF plus HT (n = 5 in each group). Error bars, SEM; (a) p < 0.05, FaDu NT versus 4T07 NT; (b) p < 0.01, FaDu HT versus FaDu NT and 4T07 HT; (c) p < 0.01, 4T07 PAF versus 4T07 NT; (d) p < 0.05, 4T07 PAF versus FaDu NT; (e) p < 0.01, 4T07 HT+PAF versus 4T07 NT, 4T07 HT or FaDu NT.
Microvascular density in tumors
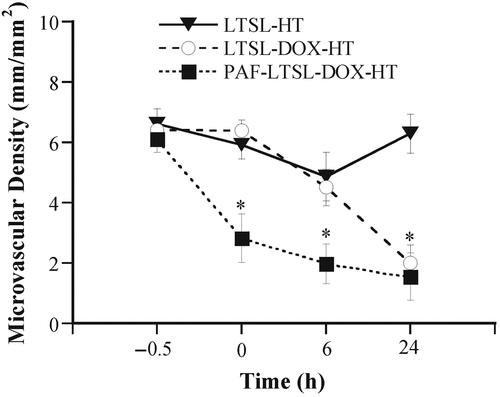
The treatment of 4T07 tumors with LTSL-DOX plus HT could significantly decrease the microvascular density. The qualitative results are shown in . Quantitatively, the average microvascular density in the LTSL-DOX-HT group was 6.40 ± 0.38 mm/mm2 before the treatment, and reduced to 2.00 ± 0.60 mm/mm2 at 24 h after the treatment (see ). Empty LTSL plus HT resulted in only a transient reduction in the microvascular density since the reduction became insignificant at 24 h after the treatment. To enhance the efficacy of LTSL-DOX-HT treatment on microvessels in 4T07 tumors, we increased the microvascular permeability by using PAF prior to LTSL-DOX-HT treatment. The results of treatment are also shown in . The microvascular density was decreased from 6.11 ± 0.45 at 0.5 h before treatment, to 2.83 ± 0.81, 1.98 ± 0.66, 1.55 ± 0.78 at 0, 6 and 24 h, respectively, after the tumors were treated with LTSL-DOX-HT plus PAF.
Figure 3. Typical images of tumor surfaces in (A) control and (B) LTSL-DOX-HT treated groups. In each group, the images show the network of tumor vasculature at (a) 0.5 h before treatment, and at (b) 0 h, (c) 6 h, (d) 24 h, and (e) 72 h after treatment.
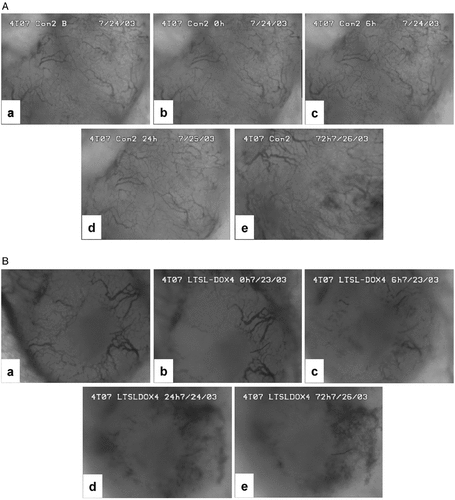
Figure 4. Microvascular density in 4T07 tumors treated with LTSL-DOX-HT (n = 7), LTSL-HT (n = 5), and PAF-LTSL-DOX-HT (n = 5). Error bars, SEM; *p < 0.05, when comparing data between PAF-LTSL-DOX-HT and LTSL-HT or LTSL-DOX-HT groups at 0 and 6 h. In addition, the difference in data at 24 h between LTSL-HT and LTSL-DOX-HT or PAF-LTSL-DOX-HT groups was statistically significant (p < 0.05).
Toxicity of DOX and LTSL-DOX to tumor cells and HUVEC
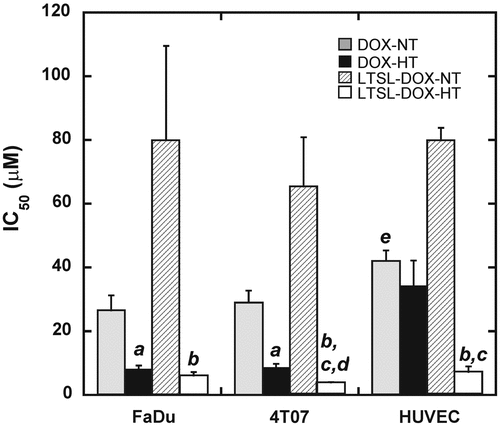
Cytotoxicities of DOX and LTSL-DOX with or without HT were characterized by IC50 (μM), defined as the DOX concentration at which the amount of viable cells was equal to 50% of that in the control group. It was determined by curve fitting the dose response data measured at 24 h after treatment. The results are shown in . For all three cell lines, IC50 was the lowest after LTSL-DOX-HT treatment and the highest after LTSL-DOX treatment without HT. The data also showed that during HT, LTSL-DOX was more toxic than free DOX for 4T07 cells and HUVECs but similar to free DOX for FaDu cells, suggesting that the LTSL or components released from it (e.g. MPPC Citation[20]) were toxic to cells at high temperature and that the death of LTSL-DOX-HT treated cells was caused by a combined effect, involving free DOX released from LTSL, LTSL itself, and thermal injury. Furthermore, it was observed that HT could enhance the cytotoxicity of free DOX in FaDu and 4T07 cells but had insignificant effects on the IC50 of free DOX in HUVEC (see ). Finally, IC50s for 4T07 cells in different groups were similar to those for FaDu cells in corresponding groups, suggesting that there were insignificant differences between 4T07 and FaDu cells in terms of dose responses to DOX treatment.
Figure 5. Cytotoxicity of DOX and LTSL-DOX to tumor cells and HUVEC. FaDu and 4T07 cells as well as HUVEC were treated with DOX or LTSL-DOX at a range of exponentially increasing DOX concentrations or equivalent DOX concentrations between 0.001 μM and 100 μM at 37°C (NT) or 42°C (HT) for 1 h. Then, the cells were incubated in fresh medium for 24 h, followed by the cytotoxicity assay. The percentage of viable cells was calculated relative to untreated cells. All assays were performed in triplicate. IC50 values were calculated from the dose–response curves by curve fitting the data. Error bars, SEM; (a) p < 0.01, DOX-HT versus DOX-NT; (b) p < 0.05, LTSL-DOX-HT versus LTSL-DOX-NT; (c), p < 0.05, LTSL-DOX-HT versus DOX-HT; (d) p < 0.05, LTSL-DOX-HT from 4T07 group versus LTSL-DOX-HT from FaDu group; (e) p < 0.05, DOX-NT versus LTSL-DOX-NT.
Discussion
Antivascular effects of LTSL-DOX-HT were evaluated in 4T07 tumors, in terms of the RBC velocity and the microvascular density. To enhance the antivascular effects, microvascular permeability of liposomes was increased in 4T07 tumors via local application of PAF solution prior to LTSL-DOX-HT treatment. Finally, the dose responses of tumor cells and HUVECs to free DOX and LTSL-DOX with or without HT were quantified in vitro to determine IC50s of DOX for these cells. Our data demonstrated that LTSL-DOX with HT could significantly reduce blood flow in 4T07 tumors, but the amount of reduction was not as much as that in FaDu tumors observed in our previous study Citation[8]. In addition, we observed in the in vitro experiment that HT could increase the toxicity of both free DOX and LTSL-DOX to 4T07 and FaDu cells, the toxicity of LTSL-DOX-HT was similar to or higher than that of DOX-HT, and the responses of FaDu and 4T07 cells to DOX treatment were similar in vitro.
One of the major findings in this study was that microvascular permeability of liposomes was lower in 4T07 than in FaDu, and HT could increase the permeability in both tumors. However, the amount of increase in FaDu tumors was approximately 12-fold higher than that in 4T07 tumors, which would result in a relatively lower concentration of LTSL-DOX in perivascular regions of 4T07 tumors. Based on this observation, we hypothesized that the antivascular effects of LTSL-DOX-HT in 4T07 tumors could be increased significantly if the permeability of liposome was enhanced. The hypothesis was supported by the data in a follow-up experiment, in which local application of PAF solution prior to LTSL-DOX-HT treatment led to an increase in the permeability by a factor of 7 (see ) and a significant reduction in the microvascular density, compared to 4T07 tumors in the LTSL-DOX-HT group at 0 and 6 h after treatment (see ). These data suggested that local concentration of LTSL-DOX was one of the key factors in determining the extent of antivascular effects of LTSL-DOX-HT.
We have previously reported that the tumor blood flow could be shut down by LTSL-DOX-HT in FaDu tumors Citation[8]. The average RBC velocity was reduced from 0.428 mm/s to 0.003 mm/s and the microvascular density was reduced from 3.93 mm/mm2 to 0.86 mm/mm2 at 24 h after the treatment. In addition, we observed blood flow stasis and severe hemorrhage immediately after treatment, and no microvessels with blood flow in five out of six tumors at 6 h and 24 h after the treatment. To investigate whether the treatment had the same effects on tumor blood flow in other tumors, we treated 4T07 tumors with LTSL-DOX plus HT. As described above, LTSL-DOX-HT treatment indeed reduced blood flow in 4T07 tumors, but the amount of decrease was less than that observed in FaDu tumors. And we did not observe a complete shutdown of blood flow in any 4T07 tumors. Therefore, the question was why did the antivascular effects differ in these tumors? The answer to the question may depend on two factors.
One is that FaDu cells are more sensitive to DOX than 4T07 cells. However, the data shown in suggested that it was unlikely to be true at least in vitro. The second factor was that concentration of free DOX was higher in FaDu tumors than in 4T07 tumors because of the difference in microvascular permeabilities between these tumors (see ). Our data showed that during HT, the difference in the permeability between 4T07 and FaDu tumors was approximately 10-fold, suggesting that the difference in LSTL-DOX concentrations between these tumors was also 10-fold. Mechanisms for the difference are still unknown. They are related to both intrinsic structures in microvessel wall and tumor responses to HT since the permeability difference was only 3-fold in unheated tumors. Gaber et al. Citation[21] have reported that liposome extravasations was increased 47-fold when the tumor was heated at 42°C for 1 h. However, there are also reports in the literature, showing that HT has minimal effects on the permeability Citation[22], Citation[23].
The increased vascular permeability may last several hours, which allows long circulating liposomes to deliver a large amount of drugs to tumors Citation[21], Citation[24]. More importantly, it can result in a very high concentration of DOX in perivascular regions. This is because liposome distribution in tumor tissues is heterogeneous and tissue penetration of free DOX is limited due to its binding to DNA/RNA in cells, lipids in membranes, and proteins in cells and interstitial space. Therefore, DOX concentration in perivascular regions is much higher than that in other regions. In a previous study, the DOX concentration averaged over the entire volume of a FaDu tumor heated at 42°C could reach ∼25 ng/mg tissue (or ∼50 μM in tissues) at 1 h after i.v. administration of LTSL-DO Citation[9], Citation[10], indicating that the local concentration of DOX in perivascular regions must be much higher than 50 μM. The high concentration of free DOX would cause damage of tumor endothelial cells and thus further increase in the microvascular permeability. This positive feedback loop provides a mechanism for significant enhancement of DOX delivery to endothelial cells.
Previous studies have shown that changes in microvascular permeability may influence RBC velocity in tumors Citation[25], Citation[26], i.e. these parameters are coupled together. An increase in microvascular permeability will improve fluid exchange across microvessel wall. As a result, it will reduce the pressure gradient, i.e. the driving force for blood flow, within tumor microvessels Citation[26], and increase the viscosity of blood due to fluid leak out into the interstitial space. Both changes will decrease tumor blood flow and thus increase the residence time of LTSL-DOX and the local concentration of free DOX.
In addition to HT, we have explored other methods to increase the microvascular permeability of liposome in tumors. The most effective method identified in our study was to treat tumors with PAF, which is a biologically active phospholipid. It is considered to be a major mediator of acute lung injury by increasing pulmonary microvascular permeability, which causes pulmonary edem Citation[27], Citation[28]. We observed that PAF could greatly increase the permeability in 4T07 tumors and thus the antivascular effects of LTSL-DOX-HT (see ).
The cytotoxicity assay showed that the IC50 of DOX for LTSL-DOX-HT treated HUVECs was 7.3 μM, which was lower than DOX concentration in perivascular regions discussed above. In other words, DOX concentration in perivascular regions is higher than the level required to damage half of the endothelial cells. Mechanisms of this damage are related to free radical mediated toxicity and DNA cross-linking Citation[29]. We had also noticed that the IC50 for LTSL-DOX-HT treated HUVECs (i.e. 7. 3 μM) was significantly lower than that for DOX-HT treated cells (34.4 μM), suggesting that LTSL-DOX was more toxic to endothelial cells than free DOX under HT.
Acknowledgement
This research was supported by grants from the National Institutes of Health (CA87630 and CA42745).
References
- Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, et al. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA 1991; 88: 11460–11464
- Ranson M, Howell A, Cheeseman S, Margison J. Liposomal drug delivery. Cancer Treat Rev 1996; 22: 365–379
- Tardi PG, Boman NL, Cullis PR. Liposomal doxorubicin. J Drug Target 1996; 4: 129–140
- Gregoriadis G. Liposome technology. CRC press, Boca Raton 1993
- Lasic DD, Papahadjopoulos D. Liposomes revisited. Science 1995; 267: 1275–1276
- Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res 1994; 54: 3352–3356
- Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev 2001; 46: 149–168
- Chen Q, Tong S, Dewhirst MW, Yuan F. Targeting tumor microvessels using doxorubicin encapsulated in a novel thermosensitive liposome. Mol Cancer Ther 2004; 3: 1311–1317
- Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev 2001; 53: 285–305
- Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res 2000; 60: 6950–6957
- Anyarambhatla GR, Needham D. Enhancement of the phase transition permeability of DPPC liposomes by incorporation of MPPC: A new temperature-sensitive liposome for use with mild hyperthermia. J Liposome Res 1999; 9: 491–506
- Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, Winer E, et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 2002; 94: 25–36
- Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res 2000; 60: 1197–1201
- Mayer LD, Bally MB, Cullis PR. Uptake of adriamycin into large unilamellar vesicles in response to a pH gradient. Biochim Biophys Acta 1986; 857: 123–126
- Huang Q, Shan S, Braun RD, Lanzen J, Anyrhambatla G, Kong G, Borelli M, Corry P, Dewhirst MW, Li CY, et al. Noninvasive visualization of tumors in rodent dorsal skin window chambers. Nat Biotechnol 1999; 17: 1033–1035
- Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res 2000; 60: 4440–4445
- Yuan F, Leunig M, Berk DA, Jain RK. Microvascular permeability of albumin, vascular surface area, and vascular volume measured in human adenocarcinoma LS174T using dorsal chamber in SCID mice. Microvasc Res 1993; 45: 269–289
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001; 61: 3027–3032
- Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA 1996; 93: 14765–14770
- Sandstrom MC, Ickenstein LM, Mayer LD, Edwards K. Effects of lipid segregation and lysolipid dissociation on drug release from thermosensitive liposomes. J Control Release 2005; 107: 131–142
- Gaber MH, Wu NZ, Hong K, Huang SK, Dewhirst MW, Papahadjopoulos D. Thermosensitive liposomes: Extravasation and release of contents in tumor microvascular networks. Int J Radiat Oncol Biol Phys 1996; 36: 1177–1187
- Song CW, Kang MS, Rhee JG, Levitt SH. Effect of hyperthermia on vascular function in normal and neoplastic tissues. Ann N Y Acad Sci 1980; 335: 35–47
- Gerlowski LE, Jain RK. Effect of hyperthermia on microvascular permeability to macromolecules in normal and tumor tissues. Int J Microcirc Clin Exp 1985; 4: 363–372
- Huang SK, Stauffer PR, Hong K, Guo JW, Phillips TL, Huang A, Papahadjopoulas D, et al. Liposomes and hyperthermia in mice: Increased tumor uptake and therapeutic efficacy of doxorubicin in sterically stabilized liposomes. Cancer Res 1994; 54: 2186–2191
- Yuan F. Transvascular drug delivery in solid tumors. Semin Radiat Oncol 1998; 8: 164–175
- Netti PA, Roberge S, Boucher Y, Baxter LT, Jain RK. Effect of transvascular fluid exchange on pressure-flow relationship in tumors: A proposed mechanism for tumor blood flow heterogeneity. Microvasc Res 1996; 52: 27–46
- Lewis JC, O'Flaherty JT, McCall CE, Wykle RL, Bond MG. Platelet-activating factor effects on pulmonary ultrastructure in rabbits. Exp Mol Pathol 1983; 38: 100–108
- Clavijo LC, Carter MB, Matheson PJ, Wilson MA, Wead WB, Garrison RN. PAF increases vascular permeability without increasing pulmonary arterial pressure in the rat. J Appl Physiol 2001; 90: 261–268
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 1999; 57: 727–741