Abstract
Purpose: Viral vectors used for cancer gene therapy are usually delivered by direct intratumoral administration. We studied the role of hyperthermia (HT) in vitro and in vivo in an attempt to achieve higher transfection rates (especially, larger volume of spread).
Materials and methods: Replication-deficient adenoviruses containing either the human sodium-iodide symporter (Ad5-CMV-hNIS) or green fluorescent protein (Ad5-CMV-eGFP) as reporter genes were used. For in vitro studies, human lung cancer A549 cells were transfected with the virus and assayed for hNIS expression by radioactive pertechnetate uptake or green fluorescence activity using a gamma-counter or fluoroscopy respectively in the presence and absence of HT. For in vivo studies, A549 tumors were established intramuscularly in CD1 athymic mice. The adenoviral constructs (1010 viral particles/tumor) were injected intratumorally when the tumors reached 10–11 mm in diameter. Different timing sequences of HT were examined and viral spread was assessed using technetium-autoradiography or GFP-fluorescence microscopy.
Results: In the in vitro studies, A549 cells infected with the adenoviral construct did not show any difference in gene expression level in the presence or absence of HT. In vivo, the effect of HT on the volume of gene expression in A549 tumors was highly variable with some groups of mice showing better spread in the presence of HT and others showing reduced spread with HT.
Conclusion: Improvements in intratumoral adenoviral spread in response to hyperthermia were not consistently observed in a mouse tumor model using two quantitative endpoints of gene expression.
Introduction
Cancer gene therapy is an experimental approach which aims at delivering an anti-cancer gene of interest to all or most of the cells comprising a tumor. Both viral and non-viral vectors have been used for in vivo gene delivery to cancer cells. One of the most common methods used to deliver the vectors to the tumor is by direct intratumoral injection. Retroviral and adenoviral vectors have shown promise in pre-clinical and animal studies. Despite some success, a limiting factor of current gene therapy research and development in the clinic is the poor efficiency of gene delivery to tumor cells. For example, using the most widely adopted vector, replication-defective adenoviruses, transfer efficiencies generally don’t exceed about 10% Citation1,Citation2. Multiple approaches to overcome suboptimal transfer efficiencies have been proposed including using replication-competent viruses, improving the bystander effect with suicide gene therapy, and improving the distribution of replication deficient viral vectors in tumors after direct intratumoral injection. With regard to the latter, the physical spread of virus in tissue is limited to 5–8 mm perpendicular to the injection track Citation3. The sparing of one tumor cell with clonogenic potential is enough to cause a recurrence and failure of treatment. There is an urgent need to better understand the conditions affecting, and ultimately improve, viral spread in vivo.
Reports in literature suggested that hyperthermia may cause changes in tumor microenvironment leading to improved distribution of viral vectors Citation4–6. Our pilot experiment to demonstrate this effect was successful and showed increased viral dissemination in the heated tumor as compared to the non-heated tumor. We decided to test this concept in greater detail and conducted in vitro and in vivo experiments using the A549 human lung cancer cell line. The results of these experiments are presented.
Materials and methods
Design of vector
The construction of the replication-defective pFL*-hNIS-pcDNA3 (a generous gift from Dr Sissy Jhiang, Ohio State University) has been described elsewhere Citation7. Both the E1 and E3 regions of this adenovirus have been deleted. The hNIS reporter gene located was inserted into the empty E1 region and is under control of a human cytomegalovirus (CMV) promoter.
The replication-defective Ad5-bCD/TK-EGFP adenovirus contains a therapeutic bacterial cytosine deaminase (CD) and thymidine kinase (TK) fusion gene in the E1 region, and the green fluorescent protein (EGFP) in the E3 region. Both genes are under control of separate human cytomegalovirus (CMV) promoters. To generate Ad5-CD/TK-EGFP, Swa I and Pac I sites were introduced by PCR Polymerase to the flanking regions of the EGFP open reading frame (ORF) which was cloned into the right-end shuttle vector, pBGH-8k. pBGH-8k is a modified version of pBGH-10 (Microbix) that contains PacI and SwaI sites in the E3 region allowing for easy directional cloning. Ad5 CD/TK-EGFP adenovirus was generated following CaPO4 transfection of pCA14-CD/TK, which contains the CD/TK fusion gene in the E1 region, and pBGH-8k into human embryonic kidney (HEK) 293 cells. Following two plaque purification steps, the Ad5-CD/TK-EGFP virus was propagated in HEK 293 cells and its identity verified by restriction enzyme digestion and DNA sequencing.
In vitro experiments
Ten thousand A549 cells were plated in one well of a 4-well chamber slide in DMEM + 10% fetal bovine serum at 5% CO2 and 37°C. When the cells were 70%–80% confluent they were infected with the adenoviral vector at a multiplicity of infection of 100 viral particles per cell. For infection, the cell culture medium was withdrawn from the chamber and the adenoviral vector suspended in 100 µL normal saline was layered over the cells for 30 min at room temperature, after which fresh nutrient medium was added back to the chambers.
Heating above 37°C was carried out using a temperature-controlled water bath. At the desired time after infection and heating, AdhNIS activity was assayed using pertechnetate (). The cell culture media was withdrawn and 5µCi of
in 100 µL normal saline was added to the cells for 20 min. This was followed by phosphate buffered saline (PBS) washes three times. The
activity on each slide was then measured using a 1480 Wallac Wizard 3″ Automatic Gamma Counter (Perkin Elmer, Waltham, MA) for 1 min.
In vivo experiments
To assess the in vivo distribution, A549 tumors were used grown in CD1 athymic (nu/nu) mice (20–22 g) (Charles River Labs, Wilmington, MA) by injecting A549 cells (2 × 106 cells in 100 µL normal saline) intramuscularly into their right hind legs. Two to three weeks later, at a tumor diameter of 10–11 mm, 1010 viral particles of AdhNIS suspended in 20 µL normal saline were injected intratumorally using a 26-gauge needle.
Tumor hyperthermia was carried out using a circulating water bath with the tumor-bearing leg suspended in the water. Heating was done at 41°C for 60 min. Twenty-four hours later 0.2 mCi of in 200 µL of normal saline was injected intraperitoneally followed by euthanasia 2 h later by cervical dislocation. All animal studies were approved by the Institutional Animal Care and Use Committee.
The tumor was harvested and frozen in OCT compound at −30°C to −40°C. Sections 100 µm thick were obtained over the next 2 h and the dried sections on microscope coverslips were pasted on a cardboard sheet which in turn was placed in contact with an X-ray film for 4 half-lives of (approximately 24 h) to generate a series of autoradiographs. The autoradiographs were digitized using a VXR-8 film digitizer (Vidar Systems) and isodose diagrams depicting regions of equal optical density were constructed for regions of gene expression using MATLAB (MathWorks). When quantifying gene expression in digitized autoradiographs, pixel values over the background mean plus 2 standard deviations were considered evidence of gene expression (). The liver was also harvested, weighed and the
activity in per gram in each liver was measured using the Automatic Gamma Counter (Perkin Elmer, Waltham, MA) for 1 min.
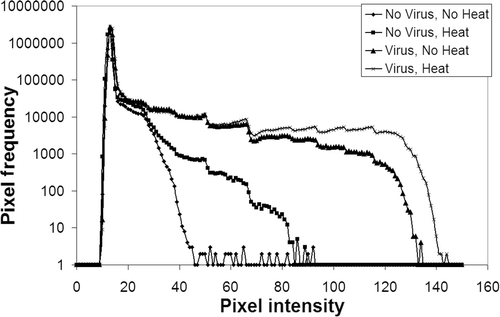
Figure 1. This figure is an example showing the results of one batch of four mice, one in each treatment group, where an increased viral dissemination was noted in the heated tumor as compared to the non-heated tumor. Pixel frequency distribution was obtained from digitized auto-radiographs of the sectioned tumors. Background was determined based on the ‘No virus, no heat’ curve. Values above this background were considered evidence of gene expression or increased uptake in the tumor section. The number of pixels demonstrating activity above the background were quantified and expressed as a percentage of the total tumor area. Similar plots were obtained for all the batches studied to compare spread of virus.
Virus distribution in the tumor was also assessed from the distribution of using enhanced green fluorescent protein (eGFP) fluorescence. A549 tumors with a diameter of 10–11mm were injected with Ad-CMV-eGFP (1010 viral proteins in 20 µL normal saline) 6 h following hyperthermia at 42°C for 30 min. The mice were euthanized 48 h after hyperthermia and the tumors and liver harvested and placed in formaldehyde. Sections 100 µm thick were obtained. These sections were then imaged at 10x magnification using an Olympus laboratory microscope model BX40. Captured images were exported to Scion Image (NIH), converted to grayscale and the total number of pixels expressing fluorescence in the image was obtained from the software. Number of positive pixels were compared between treated and control groups.
Statistics
To determine differences between the HT and no HT groups in the in vivo experiments, a two-sided p-value for significance assuming unequal variances was obtained using Microsoft Excel®.
Results
Hyperthermia does not cause increased transgene expression in vitro
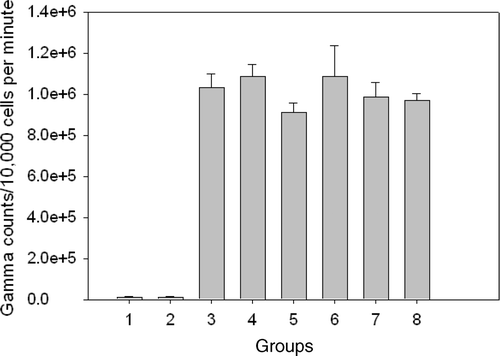
A549 cells transfected with the AdhNIS were heated immediately or 24 h after infection. No increase in transgene expression was noted as compared to non-heated cells. To ascertain that HT at 41°C for 60 min was not inactivating the virus, we heated the virus separately prior to infection and assessed the transgene expression using assay in combination with other time sequences for heating the transfected cells–viral infection followed by HT 1 h and 24 h later, HT followed 1 h later by infection and HT followed 6 h later by infection. The results of these experiments did not suggest any virus inactivation due to HT or any increased gene activity with various sequences of HT and viral infection ().
Figure 2. In vitro experiments were done to assess whether addition of heat at 41°C for 60 min would increase gene expression in transfected A549 cells. Various sequences of heating and viral infection were performed. There was no increased uptake of noted in any group as compared to the ‘Virus, no heat’ group. Group 1: No virus, no heat; group 2: No virus, heat; group 3: Virus, no heat; group 4: Virus → heat 1 h later; group 5: Heated virus, no cell heat; group 6: Virus → heat 24 h later; group 7: Heat → viral infection 1 h later; group 8: Heat → viral infection 6 h later.
Hyperthermia does not result in increased viral vector dissemination in vivo
Viral vector distribution in the tumor after direct intratumoral injection of the construct was determined using two different objective assays in three different sets of experiments.
In the first set of experiments 1010 viral particles of AdhNIS were injected into the tumors, followed 10 min later by HT at 41°C for 60 min. Tumor-bearing mice received four different types of treatment–No virus, no HT; No virus, HT; Virus, no HT (control) and Virus, HT. Using autoradiography the virus spread in the tumor tissue was compared for the four different treatment groups. The experiment was repeated four times and in one out of four cases increased spread was seen for heated tumors and in the other three cases the increased spread was seen for non-heated tumors ().
Table I. In vivo hyperthermia experiment where 1010 viral particles of AdhNIS were injected into the tumors followed 10 min later by HT at 41°C for 60 min. Area occupied by transgene expression in the tumor sections was compared. The numbers in the cells are the area occupied by the positive transgene expression as a percentage of the total area of the tumor section. In batch 4 increased area of expression was seen in the heated group and in batches 1, 2 and 3 increased area was noted in the non-heated tumors.
In the next set of experiments using autoradiography, the sequence was reversed, with HT at 41°C for 60 min delivered 15 min prior to viral injection. Again, tumor-bearing mice received the same four treatments as described above. In six repeat experiments a 1.14 to 1.88 fold increase was noted in the heated group in four instances and in the remaining two instances the heated group showed a decrease (0.36 and 0.73) as compared to control (virus, no HT) group (). A pooled analysis of the area occupied by transgene expression as a percentage of total tumor area in the two sets of experiments revealed no significant difference between the HT and no HT groups (p = 0.95).
Table II. In vivo hyperthermia experiment where 1010 viral particles of AdhNIS were injected into the tumors 15 min following HT at 41°C for 60 min. Area occupied by transgene expression in the tumor sections was compared. The numbers in the cells are the area occupied by the positive transgene expression as a percentage of the total area of the tumor section. In batches 1, 3, 4 and 5 increased area of expression was seen in the heated group and in batches 2 and 6 increased area was noted in the non-heated tumors.
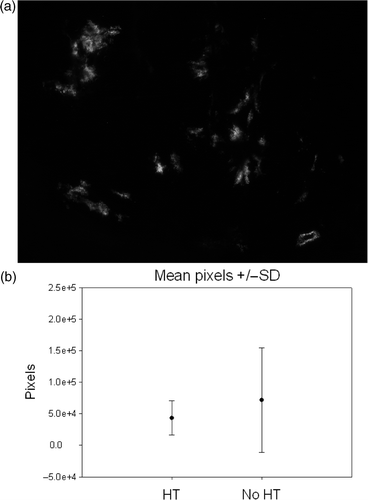
The last set of experiments was done using the GFP assay. The tumor-bearing leg was heated in a circulating water bath at 42°C for 30 min followed 6 h later by intratumoral injection of 1010 viral particles of Ad-CMV-eGFP. The mice were euthanized 48 h after hyperthermia and 100 µm thick tumor sections imaged for green fluorescence. A total of 12 mice were treated (6 mice–virus, no HT; 6 mice–Virus, HT). shows the fluorescence distribution box-plots for the two groups. Again, no significant difference was noted between the total numbers of pixels between the HT and no HT groups (p = 0.17).
Figure 3. In vivo experiments were performed using an eGFP containing adenoviral vector. Intratumoral injection of the vector was performed 6 h after HT at 42°C for 30 min. (a) Example of green fluorescence as seen in one of the tumor sections. Total number of pixels in approximately 25–30 such images, 100 µm thick after every 200 µm, was quantified for each tumor (173 images for HT group and 162 for no HT group). (b) Box-plots of mean ± standard deviation showed no difference in the amount of fluorescence in the two groups (p = 0.17).
To ascertain that delivery of heat to the tumors did not lead to increased leakage out of the tumor after the intratumoral injections, the livers from the mice were also harvested. After injection of , the radio-activity per gram of liver was determined using the automatic gamma counter. There was no difference in activity noted in the livers between the four groups of mice. There was a high degree of variability noted in the first few batches of mice, hence we did not rigorously pursue this method. In addition, livers were also dissected in the eGFP in vivo experiment. As outlined in the Materials and methods section, sections of the liver were examined for any fluorescence activity suggesting any virus spread to the liver. None of the livers examined in the HT and no HT groups of mice revealed any increased eGFP activity. This suggested that there was no systemic dissemination of the hepatotropic adenoviruses to the liver.
Discussion
Tumor hyperthermia to temperatures of 41°C to 42°C for 30 to 60 min has been shown to result in changes in tumor microenvironment including changes in pH, blood flow and oxygen concentration Citation8,Citation9. Mild hyperthermia has the potential to oxygenate both diffusion-limited chronically hypoxic and perfusion-limited acutely hypoxic cells Citation10. There may also be as yet unknown effects of hyperthermia on the expression and affinity of the coxsackie and adenovirus receptor (CAR). Hence we decided to use hyperthermia in an attempt to increase viral dissemination in the tumor. We postulated that increased tumor blood flow, possibly fibrous tissue septae breakdown and other undefined factors such as the effect of temperature on enzyme efficiencies, may alter the physical architecture of the tumors allowing better virus dissemination. We conducted in vitro and in vivo studies to determine whether mild hyperthermia would improve the distribution or expression of replication-deficient adenovirus after direct intratumoral injection. As there is very little or no data available in literature to suggest what temperature should be used for what time and how the sequencing of intratumoral adenoviral injections should be carried out, we used various temperatures (that would be feasible in a clinical situation) and sequences of injections to determine if any approach would prove beneficial. If any of the sequence/temperature/time combinations had shown any consistent results we had planned to use that for further study. However, based on our results, the spread of intratumorally injected viral particles was not consistently improved with hyperthermia. The possibility remains that other routes of injection may be more amenable to increased hyperthermic spread of virus.
Adenoviruses are primarily hepatotropic and tend to be sequestered in the liver in the event of a systemic dissemination. Hence, in addition to the tumors, we also harvested the livers in the mice. The radioactivity in the livers, measured per gram, was not significantly different in the four groups of mice. Also in the GFP assay, no increase in fluorescence was noted in the livers. This suggested that there was no increased systemic dissemination after intratumoral injection of the virus in response to heating.
As reported, we observed variability in virus spread in response to hyperthermia using two different objective user-independent assays; however, on careful analysis, there was no consistent improvement observed with heating. We initially used autoradiography, as the human sodium iodide symporter (hNIS) gene is able to take up
very efficiently and the cumulative number of pixels in the tumor volumes could be easily quantified. However, we felt that this may not be the most efficient way to determine virus spread in the tumor. Many variables could potentially affect results in
uptake studies. The
was administered intraperitoneally and reached the tumor cells through circulation and tumor vasculature. Hence tumor characteristics including volume, vascularity and interstitial pressure could affect the delivery of substrate to the transfected cells. Technetium has a half-life of approximately 6 h and this gives a small window of time within which tumor has to be harvested, sectioned and autoradiographs obtained. The film sensitivity (Kodak MR vs. MS), exposure duration, processor protocol and digitizer settings are other variables that could affect results. Therefore we decided to use the GFP assay. This offered the advantages of no dependence on substrate (
) delivery to tumor cells and uptake, slower fading of fluorescence as compared to
decay, no need to set ‘cut-off’ values on autoradiographs, the samples and slides could be stored and revisited after weeks or months, and a smaller number of mice were needed with only 2 groups to compare.
These findings are contrary to what has been reported by others. Chang et al. Citation5 had demonstrated that hyperthermia significantly increases transgene expression in tumors when heated at 40°C to 41.5°C for 30 min. They established MC-38 subcutaneous tumors in C57Bl6 mice. Vaccinia virus expressing the luciferase gene was introduced by tail vein injection. The tumors were harvested 6 h later and viral DNA was measured using quantitative PCR. More than a 100-fold increase in vaccinia marker gene activity was noted in the hyperthermically treated tumors. In this study, spatial distribution of virus was not studied. They used real-time PCR to show increased gene expression in treated tumors. However, there was no evidence to demonstrate increased viral spread in the tumor. We had not noted any increased gene expression in our in vitro experiments. Hence, we did not test this in the in vivo situation. Our primary end-point was increased viral distribution in the tumor volume.
The combination of lidocaine and heat on transfection efficiency of luciferase gene in PC-3 cells using ultrasound mediated transfection showed that hyperthermia at 42°C enhanced luciferase activity more than 8-fold Citation11. More in vitro evidence came from experiments done in bovine kidney cells. These cells had been heated at 42°C for 30 min and allowed to recover at 37°C for 6 h. A two-fold increase in the number of plaques per culture was noted in the heat-treated cells compared to controls Citation4.
Li et al. Citation6 used micro-PET imaging to study the effect of heat in increasing virus spread. They injected a replication deficient adenoviral construct expressing eGFP and thymidine kinase in one mouse with a subcutaneous tumor and subjected this mouse to hyperthermia and compared the virus spread to another mouse where the tumor had not been heated. They reported increased virus spread in response to heat. We had also noted an increased volume of viral dissemination in some experiments but this was not consistently reproducible.
Of the studies quoted above, the only report of successfully increasing virus mediated transgene expression in vivo was in the study reported by Chang et al. Citation5. Similar to our results, no increased gene expression due to hyperthermia was noted in the in vitro setting. The authors explained the in vivo increase in gene expression as being due to an increased leakiness of the tumor vasculature thus allowing more extravasation of the vaccinia viral particles into the tumor. This phenomenon has been previously described by Kong et al. Citation12. We did not use tail vein injections as in their study. Adenoviruses are highly hepatotropic and a systemic injection of the adenoviral vector would result in a large amount of the vector being sequestered in the liver Citation13. This would cause undesirable hepatic toxicity with minimal virus reaching the intended tumor. Hence, we used the direct intratumoral approach. The lack of a beneficial effect of heat in our study was probably because the effect on tumor vasculature was inconsequential. The other reports quoted above used non-viral methods Citation11 or viral vectors Citation4 in in vitro settings. These reports did not examine actual viral dissemination in tumor/tissue specimens.
The current results are of significance because of the widespread use of replication-deficient adenoviruses in cancer gene therapy studies. The Journal of Gene Medicine Database reports that in the year 2007 the total number of clinical trials using gene therapy was 1309, of which 871 (66.5%) were for cancer. The majority of these trials are phase I (61.2%) while a very small percentage makes up phase II/III or phase III trials. Adenoviruses are being used as gene delivery vectors in approximately 25% of trials.
Adenoviruses are highly efficient vectors consistently achieving transfection rates of 90–95% in vitro. However, these rates are reduced to 0–10% in the in vivo setting Citation1,Citation2. This suggests that it is not inherently an inability of the viruses to infect the target cells, rather it is the inability to gain access to the target cells. Possible reasons contributing to this are poor mobility of viral particles in the tumor microenvironment, host immune response blocking viral dissemination, and short duration of viral oncolytic action in the tumor Citation14. Tumor architecture has high cell density with large amounts of fibrous tissue which can prevent tumor spread Citation15. Intratumoral injection of viral vectors is the most direct and safe method of delivery to tumor cells but it has been demonstrated that using this method only about five layers of tumor cells surrounding the injection site may be infected Citation16. Adenoviruses are highly immunogenic and host immune responses may also limit viral dissemination in the tumor Citation17,Citation18.
In summary, we consider this a negative experiment as no consistent and reproducible effect was noted in repeat sets of experiments for the effect of hyperthermia on both gene expression in vitro and virus spread in vivo after direct intratumoral injection. Different time-temperature sequences were explored to determine the most optimal way of delivering hyperthermia which could potentially be used in clinical situations. However, no unequivocal benefit for hyperthermia could be demonstrated. We therefore concluded that hyperthermia does not result in increased viral dissemination in the tumors after direct intratumoral injection. These results are important because of the widespread use of replication-deficient adenoviruses and the general perception that hyperthermia may enhance efficacy. There may be as yet unexplored mechanisms that could be used to potentiate viral dissemination in the tumor, however, elevated temperatures alone did not appear to reproducibly increase viral gene expression in tissue.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Puumalainen AM, Vapalahti M, Agrawal RS, Kossila M, Laukkanen J, Lehtolainen P, Viita H, Paljarvi L, Vanninen R, Yla-Herttuala S. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther 1998; 9: 1769–1774
- Siddiqui F, Ehrhart EJ, Charles B, Chubb L, Li CY, Zhang X, Larue SM, Avery PR, Dewhirst MW, Ullrich RL. Anti-angiogenic effects of interleukin-12 delivered by a novel hyperthermia induced gene construct. Int J Hyperthermia 2006; 22: 587–606
- Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, Berger MS, McDermott MW, Kunwar SM, Junck LR, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: Biological and clinical results. J Clin Oncol 2003; 21: 2508–2518
- Kilgore JL. Severity of viral infection is promoted by hyperthermic pretreatment. J Sci Med Sport 2004; 7: 259–263
- Chang E, Chalikonda S, Friedl J, Xu H, Phan GQ, Marincola FM, Alexander HR, Bartlett DL. Targeting vaccinia to solid tumors with local hyperthermia. Hum Gene Ther 2005; 16: 435–444
- Li GC, He F, Ling CC. Hyperthermia and gene therapy: Potential use of microPET imaging. Int J Hyperthermia 2006; 22: 215–221
- Cho JY, Xing S, Liu X, Buckwalter TL, Hwa L, Sferra TJ, Chiu IM, Jhiang SM. Expression and activity of human Na+/I− symporter in human glioma cells by adenovirus-mediated gene delivery. Gene Ther 2000; 7: 740–749
- Bicher HI, Hetzel FW, Sandhu TS, Frinak S, Vaupel P, O'Hara MD, O'Brien T. Effects of hyperthermia on normal and tumor microenvironment. Radiology 1980; 137: 523–530
- Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005; 21: 761–767
- Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res 2001; 155: 515–528
- Ogawa R, Kagiya G, Feril LB, Jr, Nakaya N, Nozaki T, Fuse H, Kondo T. Ultrasound mediated intravesical transfection enhanced by treatment with lidocaine or heat. J Urol 2004; 172: 1469–1473
- Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res 2000; 60: 4440–4445
- Li Q, Kay MA, Finegold M, Stratford-Perricaudet LD, Woo SL. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther 1993; 4: 403–409
- Jia W, Zhou Q. Viral vectors for cancer gene therapy: Viral dissemination and tumor targeting. Curr Gene Ther 2005; 5: 133–142
- Sauthoff H, Hu J, Maca C, Goldman M, Heitner S, Yee H, Pipiya T, Rom WN, Hay JG. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: Virus persists and spreads systemically at late time points. Hum Gene Ther 2003; 14: 425–433
- Goldstein DJ, Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: Isolation and characterization of an ICP6 lacZ insertion mutant. J Virol 1988; 62: 196–205
- Chen D, Murphy B, Sung R, Bromberg JS. Adaptive and innate immune responses to gene transfer vectors: Role of cytokines and chemokines in vector function. Gene Ther 2003; 10: 991–998
- Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther 2003; 10: 935–940