Abstract
Of the many heat shock proteins (HSPs), hsp70 appears to correlate best with heat resistance, either permanent or transient. We have investigated various approaches to quantify the concentration of hsp70, and examined the relationship between hsp70 and cells' thermal sensitivity during the development and decay of thermotolerance in model systems. Here, experiments were performed to determine the possibility of using the rate of synthesis of hsp70 after a second test heat shock to predict the kinetics of thermotolerance. Specifically, we studied the relationship between the retained thermotolerance in a murine tumor cell line SQ-1 and a human tumor cell line, HCT-8, after fractionated heat doses and the cells' ability to re-initiate synthesis of hsp70 in response to an additional test heat dose in vitro. Monolayers of cells were exposed to a first heat treatment (e.g., 41°C, 4 h) and then incubated at 37°C for 0–72 h. At various times after the first heat treatment, cells were either challenged with a 45°C, 45 min heat shock to assess the residual thermotolerance by colony formation, or labelled with [35S]methionine before or after an additional test heat dose (e.g. 43.5°C, 15 min). We found that the cells' ability to re-initiate hsp70 synthesis in response to the test heat shock inversely correlated with retained thermotolerance. Our data suggest the level of hsp70 in thermotolerant cells regulates the rate of synthesis of additional hsp70 in response to the subsequent heat challenge. Furthermore, the results showed that the rate of re-induction of hsp70 synthesis after a test shock can be used as a rapid measure of retained thermotolerance. This study suggests an approach for quantifying the level of retained thermotolerance during a course of fractionated hyperthermia.
Introduction
One of the most intriguing aspects of thermal biology is the response of heated mammalian cells to subsequent exposures at elevated temperatures. An initial non-lethal heat treatment can induce in cells a temporary state of heat resistance, termed thermotolerance Citation1–3. The induction, development, and subsequent decay of thermotolerance have been studied extensively, both in vitro and in vivo Citation4–21. Even though many different endpoints were used the results from the in vivo and in vitro studies were qualitatively similar.
The molecular mechanism of thermotolerance is not well understood. It has been suggested that HSPs may play a role in protecting cells from thermal stress Citation22,Citation23. HSPs are a family of proteins, with molecular weights between 15 kD and 110 kD, the synthesis of which is either induced or enhanced by heat shock or other environmental stresses Citation24–27. Many investigators performed experiments to determine the relationship between thermotolerance and HSP synthesis in vitro. By and large, most studies showed a good temporal relationship between the development of thermotolerance and enhanced synthesis of HSPs Citation28–33; although there are also reports that did not demonstrate a correlation Citation32,Citation33. We have examined the quantitative relationship between cell survivals after a 45°C, 45 min heat treatment and the levels of HSPs in transient thermotolerant CHO cells and their stable heat resistant variants Citation34. Specifically, the putative protection offered by ‘induced’ HSPs in transiently thermotolerant cells at various stages of thermotolerance was compared to that conferred by the ‘constitutive’ HSPs in the heat-resistant variants. When cellular survival level was plotted against the total levels of individual HSPs (both constitutive and induced), our results showed a good correlation between the levels of hsp70 and the cells' thermal sensitivity. With the decay of tolerance the concentration of hsp70 decreased, and the cells became more sensitive to thermal stress. These data indicated that the level of hsp70 was a good predictor of cells' thermal response in vitro.
There is considerable evidence in animal systems showing that thermotolerance can be induced in tumours Citation9,Citation10,Citation12–15,Citation19. If valid extrapolation can be made to human tumours the induction of thermotolerance may influence the treatment outcome of fractionated hyperthermia. Recently we have examined the effects of hyperthermia on the induction of thermotolerance and the profiles of protein synthesis in murine tumour models Citation35,Citation36. Our data showed that tumours can synthesize HSPs during the development of thermotolerance. The kinetics of the induction of HSP synthesis and thermotolerance appeared to be similar to that found in tissue culture cell lines. Furthermore, it was the levels of hsp70, but not the rate of hsp70 synthesis, that correlated well with cell survival. These in vivo results confirmed the conclusion of the in vitro studies, that the levels of the hsp70 can be used as an indicator of the degree of retained thermotolerance. As such, it may be of clinical relevance to cancer therapy. To effectively exploit this information, a rapid assay to monitor hsp70 would be highly desirable, and even essential. We have therefore investigated various approaches to quantify this protein, and examined the relationship between hsp70 and the cells’ thermal sensitivity during the development and decay of thermotolerance in model systems.
In this study, experiments were designed to study the ability of thermotolerant cells to re-initiate synthesis of hsp70 after a second test heat dose in vitro. Specifically, we examined the possibility of using the cells' ability to re-initiate hsp70 synthesis as an indicator of retained thermotolerance during fractionated hyperthermia. Our working hypotheses are: (1) when tolerance is at its maximum, the cells’ ability to re-initiate synthesis of hsp70 in response to a second test heat shock is less than that of control non-tolerant cells; and (2) when tolerance gradually decays, the cells' ability to re-initiate synthesis of hsp70 in response to a second test heat shock will return to that of control non-tolerant cells. A very good correlation was obtained between the magnitude of the re-induction rate of hsp70 synthesis in thermotolerant cells following a test heat shock and the decay of thermotolerance. Our data suggest that the level of hsp70 in these thermotolerant tumour cells regulates the rate of synthesis of additional hsp70 in response to the subsequent heat challenge. In addition, the results show that the rate of re-induction of hsp70 after a test heat shock can be used as a rapid measure of the retained thermotolerance of cultured cells during fractionated heat treatments. Our study suggests an approach for quantitating the level of retained thermotolerance during a course of fractionated hyperthermia.
Materials and methods
Cells and culture conditions
Murine cells, designated SQ-1, were derived from squamous cell carcinoma of oesophageal origin Citation37. Cultures were grown in Eagle's minimal essential medium supplemented with 20 per cent heat-inactivated calf serum and antibiotics. The cultures were kept in a humidified incubator with a mixture of 95 per cent air and 5 per cent CO2 and routinely checked for mycoplasma contamination. Exponentially growing cell cultures were prepared by plating approximately 1 × 104 cells/cm2 in 60 mm Petri dishes on day 0. All experiments were performed on day 2 or day 3, when the cell density had reached 0.5–1 × 105 cells/cm2. After each treatment, cells were trypsinized, counted with a Coulter counter and plated after appropriate dilutions had been made. After 10 days incubation at 37°C, colonies were stained and counted. Whenever possible, dishes containing 50–150 colonies were used for calculation of survival. Plating efficiencies were between 70 and 90 per cent.
The human HCT-8 cells were originally derived from a human colorectal adenocarcinoma Citation38. The cultures were maintained in RPMI 1640 medium supplemented with insulin (0.2 IU/ml), 10 per cent fetal calf serum and antibiotics. Under our culture conditions the doubling time was 20 h. For tissue culture experiments, 1–5 × 105 cells were plated on 60 mm Petri dishes on day 0. Experiments were always performed on day 3 when the cultures were in exponential growth. Post-treatment procedures were similar to these used for SQ-1 cells. The plating efficiencies were 75–85 per cent.
Heating
Heating of monolayers of cells was carried out in hot water baths in specially designed incubators Citation34. The pH of the medium overlying the cells was maintained at 7.2–7.4 by a regulated gas flow of air and CO2, and monitored immediately before, during and after heating. The temperature of the water bath was controlled to within ±0.1°C. Fresh medium was placed over the cells immediately prior to and after heating.
Labelling and gel electrophoresis
Monolayers of cells were labelled with [35S]methionine (specific activity 1200°Ci/mmol; Amersham) at concentrations of 20–40 µCi/ml for one-dimensional gels or 200–400 µCi/ml for two-dimensional gels. After labelling at 37°C (2 h for onedimensional gels and 4 h for two-dimensional gels), cells were washed twice with ice-cold phosphate-buffered saline and lysed in SDS-sample buffer for one-dimensional analysis Citation30, Citation34, or in isoelectric focusing-sample buffer for two-dimensional analysis Citation39. One- and two-dimensional gel electrophoresis were performed as previously described Citation40, Citation39. Following electrophoresis, gels were stained with Coomassie blue G-250 in 3.5 per cent perchloric acid, de-stained in 7 per cent acetic acid, dried, and autoradiographed on Kodak SB-5 X-ray film. Care was taken to remain within the linear exposure range of the film.
Autoradiograms of one-dimensional gels were quantified with an LKB model 927 laser densitometer. The enhanced synthesis of HSPs were identified by comparing proteins synthesized in heat-shocked cells with proteins synthesized in control non-tolerant cells. Since the rate of synthesis of actin remains constant during the development and decay of thermotolerance Citation34, Citation36, we chose to express the rate of HSP synthesis by simply taking the ratio of the peak height of the specific HSP peak to that of actin, e.g. hsp70/actin. Although, on two-dimensional gel, we can separate the Mr 68,000–70,000 proteins into at least two distinct spots; separation on one-dimensional gel for individual quantification of Mr 68,000–70,000 protein was difficult. We therefore analysed this region as a composite peak and referred to it as hsp70.
Experimental protocols
Experiments were designed to determine whether the relative rate of hsp70 synthesis in thermotolerant cells after a second heat treatment can predict the level of retained thermotolerance during fractionated hyperthermia. shows a schematic representation of our experimental protocols. Briefly, we first determined the level of retained thermotolerance in cells, at various times after a first heat dose, by assaying their survival after a second heat treatment. In parallel, we measured the rates of synthesis of hsp70 in such thermotolerant cells, before and after a second heat dose. The ratio of the synthesis rates obtained after and before the test heat shock gave a measure of the ability of thermotolerant cells to re-initiate the synthesis of additional hsp70 in response to the test heat dose. The relative hsp70 synthesis rate can then be compared to retained thermotolerance to determine if a correlation exists. As a comparison, we obtained the same relative rates for non-tolerant cells, i.e. cells which did not receive a priming heat dose.
Figure 1. A schematic representation of our experimental protocols. Monolayers of cells were exposed to a first heat treatment and then incubated at 37°C for 0–72 h. At various times after the first heat treatment, (a) cells were heated at 45°C for 45 min and the survival levels determined; (b) cells were labelled with [35S]methionine at 37°C for 1 h and cellular proteins analysed by gel electrophoresis; (c) cells were given a second heat dose (43.5°C for 15 min), labelled with [35S]methionine at 37°C for 1 h, and then the cellular proteins were analysed.
![Figure 1. A schematic representation of our experimental protocols. Monolayers of cells were exposed to a first heat treatment and then incubated at 37°C for 0–72 h. At various times after the first heat treatment, (a) cells were heated at 45°C for 45 min and the survival levels determined; (b) cells were labelled with [35S]methionine at 37°C for 1 h and cellular proteins analysed by gel electrophoresis; (c) cells were given a second heat dose (43.5°C for 15 min), labelled with [35S]methionine at 37°C for 1 h, and then the cellular proteins were analysed.](/cms/asset/49cdff8b-6250-471f-b4a9-4d6344c288af/ihyt_a_392666_f0001_b.gif)
For experiments, monolayers of cells were exposed to a first heat treatment (e.g. 41°C for 4 h) and then incubated at 37°C for 0–72 h. At various times after the first heat treatment these cells were divided into three groups and treated in the following manner. (a) The first group of cells was heated at 45°C for 45 min, and the kinetics of development and decay of thermotolerance were determined by survival assay. (b) The second group of cells was labelled with [35S]methionine at 37°C for 1 h. Cellular proteins were then analysed by gel electrophoresis. This procedure measures the rate of hsp70 synthesis of thermotolerant cells at various times after the first heat shock. This rate can be expressed as a percentage of that of control non-tolerant cells. (c) The third group of cells was given a second test heat dose (43.5°C for 15 min), and then labelled with [35S]methionine at 37°C for 1 h. Similarly, cellular proteins were analysed. The rate of hsp70 synthesis, relative to that of control cells, was also calculated. The ratio of the synthesis rates obtained in (c) and (b) was calculated, and designated as (hsp70)TT,Δ. This ratio provided a measure of the ability of cells to re-initiate the synthesis of hsp70 in response to a test heat dose, at various stages of thermotolerance. We also determined, for control non-tolerant cells, the ratio of hsp70 synthesis rates before and after the same test heat dose. Based on our working hypothesis, the value of (hsp70)TT,Δ should approach that of control non-tolerant cells, as thermotolerance decays.
Results
The rate of hsp70 synthesis in response to a second heat shock inversely correlates with the decay of thermotolerance in murine SQ-1 cells
shows an autoradiograph of [35S]methionine labelled proteins obtained in one set of experiments. For these experiments, monolayers of squamous cell carcinoma SQ-1 cells were exposed to 41°C for 4 h in vitro, and then returned to 37°C incubation for various periods from 0 to 72 h. At each time point, cells were either (a) labelled, or (b) subjected to another heat dose of 43.5°C for 15 min, and then labelled. In , lanes are designated by the time period (in number of hours) for which the cells were placed at 37°C; lanes representing (b) are marked with an additional subscript Δ. The lanes marked C and CΔ are for control cells; cells corresponding to C did not receive any heat dose, whereas cells corresponding to CΔ were treated only with the test dose of 15 min at 43.5°C. From data such as these we measured the rate of hsp70 synthesis, the results of which are presented below.
Figure 2. An autoradiograph of [35S]methionine labelled proteins obtained in one set of experiments. SQ-1 cells were exposed to 41°C for 4 h, incubated at 37°C for various time periods, and (a) labelled, (b) or heated at 43.5° for 15 min, and then labelled. Lanes are designated by the time period (in number of hours) for which the cells were placed at 37°C; lanes representing (b) are marked with an additional subscript Δ. The lanes marked C and CΔ are for control cells, i.e. cells which did not receive a priming heat dose.
![Figure 2. An autoradiograph of [35S]methionine labelled proteins obtained in one set of experiments. SQ-1 cells were exposed to 41°C for 4 h, incubated at 37°C for various time periods, and (a) labelled, (b) or heated at 43.5° for 15 min, and then labelled. Lanes are designated by the time period (in number of hours) for which the cells were placed at 37°C; lanes representing (b) are marked with an additional subscript Δ. The lanes marked C and CΔ are for control cells, i.e. cells which did not receive a priming heat dose.](/cms/asset/5c404dca-ec0a-489e-b2a1-830ae6c1126b/ihyt_a_392666_f0002_b.gif)
(left panel) shows the kinetics of hsp70 synthesis after the 41°C, 4 h priming heat dose, i.e. during the development and decay of thermotolerance, as derived from measurements such as those in . The curve labelled ‘SINGLE HEATING’ (open circles) represents hsp70 synthesis rates at various times after the priming dose. The rate of hsp70 synthesis was enhanced immediately after the heat treatment, and then returned to the control value by 16–24 h. The solid circles (with the curve labelled ‘DOUBLE HEATING)’ are hsp70 synthesis rates following the second heat dose (15 min at 43.5°C). These latter data measured the response of cells to a second heat treatment, in terms of its ability to elevate hsp70 synthesis, during the decay of thermotolerance which was induced by the first priming heat shock. Initially, the rate of hsp70 synthesis decreased with time, to a minimum at 16 h, and then increased, reaching a plateau between 48 and 72 h.
Figure 3. Rate of HSP synthesis of thermotolerant cells before and after exposure to a test heat shock. Left panel: kinetics of hsp70 synthesis. The open circles represent rates of hsp70 synthesis at various times after the priming dose of 4 h at 41°C. The solid circles are rates of hsp70 synthesis following the second heat dose (15 min at 43.5°C). These latter data measured the response of cells to a second heat treatment, in terms of their ability to re-initiate the synthesis of additional hsp70 during the decay of thermotolerance induced by the first priming heat shock. Right panel: Similar measurements performed for hsp87. For both panels, rates were calculated by taking the ratio of the peak height of hsp70 or hsp87 peak to that of actin.
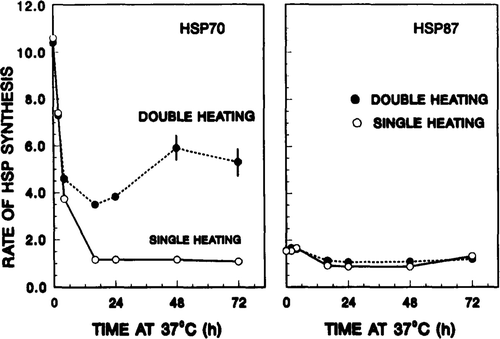
Similar measurements were performed on hsp87 for comparison, with the data shown in (right panel). In contrast to that observed for hsp70, neither the first nor the second treatment had any significant effect on hsp87 synthesis.
The data presented in , then, permitted the extraction of relative rates of HSP synthesis, i.e., the quantity (hsp70)TT,Δ. As defined previously, these are the ratios of HSP synthesis rates for thermotolerant cells after and before the second heat dose, respectively (see , and section on Experimental protocols). To reiterate, it is an estimate of the capacity of thermotolerant cells to re-initiate the synthesis of HSP in response to the second heat shock at various stages of thermotolerance. The values of (hsp70)TT,Δ are shown in (right panel). For hsp70, the relative synthesis rate increased with 37°C incubation time after the priming dose. In contrast, no time-dependent effect was observed for hsp87. Now, the stage is set for correlating (hsp70)TT,Δ with the level of thermotolerance.
Figure 4. Rate of hsp70 synthesis after the second heat shock inversely correlates with the decay of thermotolerance. Left panel: SQ-1 cell survival resulting from a second heat treatment (45 min at 45°C) at various times after the 41°C, 4 h priming dose. The 41°C, 4 h treatment reduced survival to 60 per cent. The solid circle represents the survival of control cells, i.e. cells which did not receive a first heat dose. Right panel: the quantity (hsp70)TT,Δ as extracted from the data of . As defined in the text, these are the ratios of hsp70 synthesis rates for thermotolerant cells after given a test heat shock to that for cells before given the test heat dose. This ratio is an estimate of the ability of thermotolerant cells to re-initiate the synthesis of hsp70 at various stages of thermotolerance. For hsp70 the relative synthesis rate increased with 37°C incubation time after the priming dose. In contrast, no time-dependent effect was observed for hsp87.
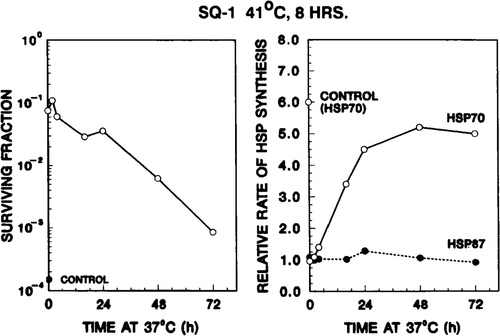
As indicated in reference to , the level of thermotolerance was assessed by measuring cell survival after a second heat treatment (45 min at 45°C) at various times after the priming dose. Cell survival thus obtained is displayed in (left panel). The solid circle represents the survival of control cells, i.e. cells which did not receive a first dose. In the thermotolerance induced by 4 h at 41°C, and its subsequent decay, are clearly observed.
The comparision of the two panels of tests the central hypothesis of this study: cells' ability to re-initiate hsp70 synthesis is related to the level of thermotolerance. A cursory review of shows that, as thermotolerance decays (i.e. as survival level decreases), the relative rate of hsp70 synthesis increases. A more careful examination follows.
Immediately after the first heat dose, when the cells are extremely thermotolerant, (hsp70)TT,Δ is unity, indicating that the hsp70 synthesis rate remains constant prior to and after the second test dose. Stated another way, cells at a high level of thermotolerance cannot further elevate their hsp70TT,Δ synthesis rate in response to the second heat challenge. As tolerance gradually decays, the cells’ ability to re-initiate hsp70 synthesis in response to a test heat dose increased, as indicated by the higher (hsp70)TT,Δ values with incubation at 37°C. In fact, (hsp70)TT,Δ eventually reached a value close to that of the non-heated controls.
In contrast, similar analysis showed that (hsp87)TT,Δ remained unchanged whatever the heat treatment or the sequence. Likewise, the rate of synthesis of actin was unaffected by the second test heat dose, and independent of the level of thermotolerance (data not shown).
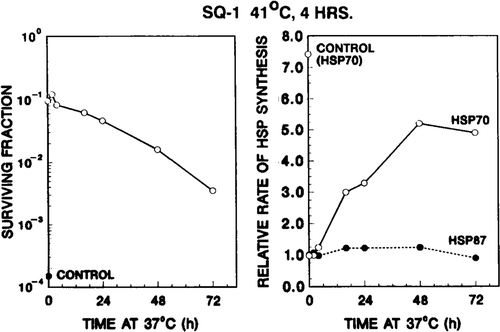
In a second set of experiments the same experimental protocols () were followed, except the SQ-1 cells were exposed to a first heat dose of 41°C for 8 h. This treatment makes cells maximally thermotolerant, as previously shown in CHO cells Citation11, and confirmed in this study for SQ-1 cells (data not shown). The hsp70 protein synthesis profiles, as analysed by one-dimensional gel electrophoresis and densitometry, are shown in . In contrast to the first set of experiments, in which SQ-1 cells were heated for 4 h at 41°C, the synthesis rate of hsp70 after the first heat dose was not elevated relative to that of control cells; and it remained at the same value throughout the 72 h incubation at 37°C (, lanes corresponding to 0–72 h incubation). For these maximally thermotolerant cells the relative synthesis rate (hsp70)TT,Δ in response to a second test heat shock (43°C for 15 min) was near unity for at least the first 4 h after the 41°C priming dose. It increased with 37°C incubation time, approaching that of control cells by 48–72 h (, right panel). Again, the increase in (hsp70)TT,Δ with 37°C incubation time corresponded to the decrease in surviving fraction of the same cells treated with 45°C for 45 min (, left panel). Thus, for this set of experiments as well, the ability of thermotolerant cells to re-initiate the synthesis of hsp70 in response to the second heat exposure inversely correlated with the decay of thermotolerance.
Figure 5. An autoradiograph of [35S]methionine labelled proteins obtained in a second set of experiments. SQ-1 cells were exposed to 41°C for 8 h, incubated at 37°C for various periods, and (a) labelled, or (b) heated at 43.5° for 15 min, and then labelled. Lanes are designated by the time period (in number of hours) for which the cells were placed at 37°C; lanes representing (b) are marked with an additional subscript A. The lanes marked C and CΔ are for control cells, i.e. cells which did not receive a priming heat dose.
![Figure 5. An autoradiograph of [35S]methionine labelled proteins obtained in a second set of experiments. SQ-1 cells were exposed to 41°C for 8 h, incubated at 37°C for various periods, and (a) labelled, or (b) heated at 43.5° for 15 min, and then labelled. Lanes are designated by the time period (in number of hours) for which the cells were placed at 37°C; lanes representing (b) are marked with an additional subscript A. The lanes marked C and CΔ are for control cells, i.e. cells which did not receive a priming heat dose.](/cms/asset/4e016dc7-c988-469a-9376-cfba81eef5a4/ihyt_a_392666_f0005_b.gif)
Figure 6. Rate of hsp70 synthesis of thermotolerant cells after the second test heat shock inversely correlates with the decay of thermotolerance. Left panel: development and decay of thermotolerance after a priming heat dose at 41°C for 8 h. The 41°C, 8 h treatment reduced survival to 30 per cent. Right panel: ability of thermotolerant SQ-1 cells to re-initiate the synthesis of additional hsp70 in response to a second heat dose. For detailed description see .
Human tumour cells' ability to elevate hsp70 synthesis after a second heat shock correlates with the decay of thermotolerance
Similar experiments were performed using a human colorectal adenocarcinoma cell line HCT-8. The results are shown in . Examining the protein profiles of heated and control HCT-8 cells by two-dimensional gel electrophoresis, we identified at least three major polypeptides in the Mr 68,000–70,000 region, designated as hsp70-a, hsp70-b, and hsp70-c (). Several points emerged from the comparison of these patterns. First, hsp70-a and hsp70-b were synthesized by both heated and control HCT-8 cells. Second, the hsp70-c was found exclusively in the heat shocked cells. Third, after exposure of cells to a mild heat shock (45°C for 15 or 30 min), the synthesis of hsp70-b was significantly enhanced, and that of hsp70-c induced.
Figure 7. Autoradiogram of a two-dimensional gel showing the enhanced synthesis of hsp70 in heat shocked human tumour HCT-8 cells in vitro. Monolayers of HCT-8 cells were heated at 45°C for 15 min, labelled at 37°C for 4 h, and cellular proteins analysed. First dimension (IEF) was from left to right, and the second dimension was from top to bottom. Three members of hsp70 family were identified: (a) hsp70-a; (b) hsp70-b; and (c) hsp70-c. The protein synthesis profile of non-heated control was shown in the left panel. The heat shock enhanced the expression of hsp70-b and induced that of hsp70-c.
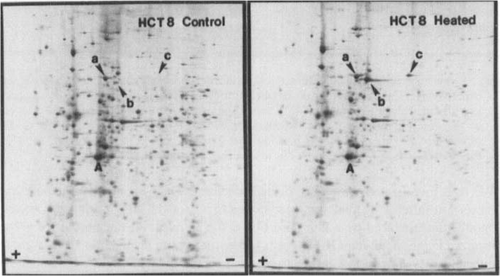
Figure 8. Autoradiograms of two-dimensional gels showing the ability of thermotolerant HCT-8 cells to re-initiate the synthesis of hsp70 after a test heat dose. HCT-8 cells were exposed to a first heat treatment at 45°C for 30 min, returned to 37°C incubation for 4–72 h, and labelled (for 4 h) either before or after a second test heat dose (45°C for 15 min). Left panels (top to bottom): control non-tolerant cells, 4, 8, 48, 72-h thermotolerant cells; all were labelled without receiving the second test heat shock. Right panels (top to bottom): control non-tolerant cells, 4, 8, 48, 72-h thermotolerant cells; all cells were labelled immediately after receiving the second test heat shock (45°C, 15 min).
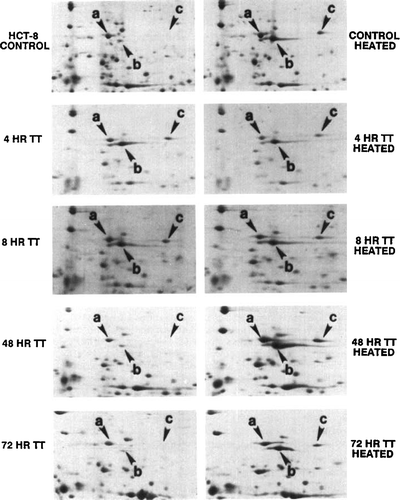
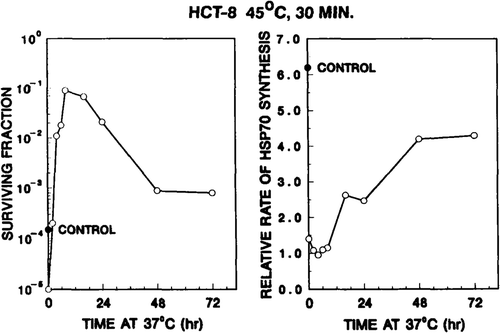
Figure 9. Ability of thermotolerant HCT-8 cells to re-initiate the synthesis of hsp70 inversely correlates with the decay of thermotolerance. Left panel: HCT-8 cell survival resulting from a second heat treatment (75 min at 45°C) at various times after the 45°C, 30 min priming dose. The 45°C, 30 min priming treatment reduced survival to ∼15 per cent. The solid circle represents the survival of control cells, i.e. cells did not receive a first heat dose. Right panel: relative rate of hsp70 synthesis, in response to a second heat shock (45°C for 15 min), were calculated from densitometer tracings of one-dimensional gels, as described in Materials and methods section.
We then investigated the ability of thermotolerant HCT-8 cells to re-initiate hsp70 synthesis rate after a second test heat dose (45°C for 15 min) during the decay of thermotolerance induced by a first dose (45°C for 30 min). Again, profiles of proteins synthesized in thermotolerant HCT-8 cells before and after a second heat shock were then examined by two-dimensional gel electrophoresis (). When cells were at their most tolerant state (, right panels, 4, 8 h points), the amounts of hsp70-b and hsp70-c synthesized after the second test heat shock were almost identical to those before the test heat shock (, left panels, 4, 8 h points). One the other hand, as thermotolerance gradually decayed, the cells' ability to enhance the synthesis of hsp70-b and to induce the synthesis of hsp70-c, in response to the second test heat shock, returned to that of control non-tolerant cells (compare the control, 48 h and 72 h data points in ). For HCT-8 cells the (hsp70)TT,Δ were also determined at various stages of tolerance. Again, our data clearly indicated that the re-initiation of hsp70 synthesis rate after the test heat dose correlates inversely with the decay of thermotolerance (). In contrast, the relative rate of hsp87 synthesis was affected less by the second test heat dose, and did not show a clear correlation with the level of thermotolerance (data not shown).
Discussion
The results of this study can be interpreted in two ways. First, our data suggest that the cellular level of hsp70 regulates the rate of synthesis of additional hsp70 in response to a second heat shock. This is based on the following reasoning. Previously, we observed that the level of hsp70 is inversely correlated with cellular thermal sensitivity during the development and decay of thermotolerance Citation34, i.e. when the intracellular concentrations of hsp70 decrease, the cells become more sensitive to thermal stress. The present experiments showed that the degree of thermotolerance retained, as assayed by cellular survival, is inversely correlated with the ability of thermotolerant cells to re-initiate the synthesis of hsp70 after a second test heat shock. When tolerance is at its maximum, the cells' ability to elevate the synthesis of hsp70 following a second test heat shock is much less than that of control non-tolerant cells. When tolerance gradually decays, the cells' ability to re-initiate synthesis of hsp70 following a second test heat shock return to that of control non-tolerant cells. Taken together, these results indicate that the levels of hsp70 is positively related to the degree of thermotolerance retained; and both quantities are inversely related to the relative rate of hsp70 synthesis after a second test heat shock. Following this line of reasoning, we hypothesize that the level of hsp70 in thermotolerant cells down-regulates the synthesis of additional hsp70 in response to the second heat challenge. It is not known whether this regulation process is operated at the transcriptional or post-transcriptional levels.
An alternative hypothesis can be offered: the rate of hsp70 synthesis is related to the amount of thermal damage induced by the treatment. If hsp70 plays a role in protecting cells from thermal stress, then thermotolerant cells, because of their higher intracellular concentration of hsp70, register less thermal damage from the test dose than do the non-tolerant cells. Since there is less thermal damage, the rate of synthesis of hsp70 is reduced. It is possible, though in our view unlikely, that thermotolerant cells are resistant to thermal stress by mechanisms totally unrelated to hsp70. However, based on the same reasoning, the less thermal damage seen by these resistant cells, the less hsp70 will be synthesized.
Two priming doses were used for SQ-1 cells, i.e. 41°C for 4 h or 8 h. The 4 h treatment elicited an increase in hsp70 synthesis rate, but 8 h heat treatment did not. Presumably the longer heating period caused elevation in the level of hsp70 to such an extent that further increase in hsp70 synthesis rate is neither necessary nor possible. This interpretation is consistent with the hypothesis that hsp70 level down-regulates its own synthesis rate in thermotolerant cells.
Secondly, the present experiments showed that the degree of retained thermotolerance, as assayed by cellular survival, was inversely correlated to the rate of hsp70 synthesis after a second test heat shock. Our experimental results thus suggest that the relative rate of hsp70 synthesis after a second test heat shock may be used as an index of the level of retained thermotolerance. We used [35S]methionine labelling and gel electrophoresis to measure the rate of hsp70 synthesis after the test heat shock. This technique is highly reproducible and yields results within 2–3 days. Using this method we determined that a test heat shock of 43.5°C for 15 min induced up to a 7-fold increase in the rate of hsp70 synthesis, depending on the degree of thermotolerance retained. In contrast, the level of hsp70 only varies at most by a factor of 2 between control and maximally thermotolerant cells Citation34. Thus, in determining the degree of thermotolerance retained, measuring the relative rate of hsp70 synthesis after a test heat shock is a more sensitive test than monitoring the relative level of hsp70. If these in vitro results are applicable to in vivo situations, the relative rate of hsp70 synthesis in response to a second heat shock may be used as a reliable assay to predict the retained thermotolerance during fractionated hyperthermia.
Acknowledgements
We thank Drs George M. Hahn and William C. Dewey for stimulating discussions, and Dr C. Clifton Ling for critically reading the manuscript. This work is supported by grant no. CA 31397 from the National Cancer Institute, USA.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Gerner EW, Schneider MJ. Induced thermal resistance in HeLa cells. Nature (London) 1975; 256: 500–502
- Henle KJ, Leeper DB. Interaction of hyperthermia and radiation in CHO cells: Recovery kinetics. Radiat Res 1976; 66: 505–518
- Henle KJ, Dethlefsen LA. Heat fractionation and thermotolerance: A review. Cancer Res 1978; 38: 1843–1851
- Law MP, Coultas PG, Field SB. Induced thermal resistance in the mouse ear. Brit J Radiol 1979; 52: 308–314
- Li GC, Hahn GM. A proposed operational model of thermotolerance based oneffects of nutrients and the initial treatment temperature. Cancer Res 1980; 40: 4501–4508
- Hume SP, Marigold JCL. Transient, heat-induced thermal resistance in the small intestine of mouse. Radiat Res 1980; 82: 526–535
- Urano M, Rice L, Kahn J, Sedlacek RC. Studies on fractionated hyperthermia in experimental animal systems. I. The foot reaction after equal doses: Heat resistance and repopulation. Int J Radiat Oncol Biol Phys 1980; 6: 1519–1523
- Law MP. The induction of thermal resistance in the ear of the mouse by heating at temperatures ranging from 41.5 to 45.5°C. Radiat Res 1981; 85: 126–134
- Maher J, Urano M, Rice L, Suit HD. Thermal resistance in a spontaneousrnurine tumor. Brit J Radiol 1981; 54: 1086–1090
- Kamura T, Nielsen OS, Overgaard J, Andersen AH. Development of thermotolerance during fractionated hyperthermia in a solid tumor in vivo. Cancer Res 1982; 42: 1744–1748
- Li GC, Fisher GA, Hahn GM. Induction of thermotolerance and evidence for a well-defined, thermotropic cooperative process. Radiat Res 1982; 89: 361–368
- Nielsen OS, Overgaard J. Importance of preheating temperature and time for the induction of thermotolerance in a solid tumour in vivo. Brit J Cancer 1982; 46: 894–903
- Rhee JG, Song CW, Levitt SH. Changes in thermosensitivity of mouse mammary carcinoma following hyperthermia in vivo. Cancer Res 1982; 42: 4485–4489
- Urano M, Rice LC, Montoya V. Studies on fractionated hyperthermia in experimental animal systems. II. Response of murine tumors to two or more doses. Int J Radiat Oncol Biol Phys 1982; 8: 227–233
- Wheldon TE, Hingston EC, Ledda JL. Hyperthermia response and thermotolerance capacity of an experimental rat tumour with occluded blood flow. Eur J Cancer Clin On 1982; 18: 1007–1015
- Kapp DS, Lord PF. Thermal tolerance to whole body hyperthermia. Int J Radiat Oncol Biol Phys 1983; 9: 917–921
- Li GC, Meyer JL, Mak JY, Hahn GM. Heat-induced protection of mice against thermal death. Cancer Res 1983; 43: 5758–5760
- Martinez AA, Meshorer A, Meyer JL, Hahn GM, Fajardo LF. Thermal sensitivity and thermotolerance in normal porcine tissues. Cancer Res 1983; 43: 2072–2075
- Rofstad EK, Brustad T. Development of thermotolerance in human melanoma xenograft. Cancer Res 1984; 44: 525–530
- Overgaard J, Nielsen OS. Influence of thermotolerance on the effect of multi-fractionated hyperthermia in a C3H mammary carcinoma in vivo. Hyperthermia Oncology, J Overgaard. Taylor & Francis Ltd, London 1984; 1: 211–214
- Field SB. Clinical implication of thermotolerance. Hyperthermia Oncology, J Overgaard. Taylor & Francis Ltd, London 1985; 2: 235–244
- McAlister L, Finkelstein DB. Heat shock proteins and thermal resistance in yeast. Biochem Bioph Res Co 1980; 93: 819–824
- Mitchell HK, Moller G, Petersen NS, Lipps-Sarmiento L. Specific protection from phenocopy induction by heat shock. Dev Genet 1980; 1: 181–192
- Ashburner M, Bonner JJ. The induction of gene activity in Drosophila by heat shock. Cell 1979; 17: 241–254
- Craig EA. The heat shock response. CRC Cr Rev Bioche Mol 1985; 18: 239–280
- Lindquist S. The heat shock response. Ann Rev Biochem 1986; 55: 1151–1191
- Bienz M, Pelham RB. Mechanisms of heat-shpck gene activation in higher eukaryotes. Adv Genet 1987; 24: 31–72
- Landry J, Bernier D, Chretien P, Nicole LM, Tanguay RM, Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res 1982; 42: 2457–2461
- Li GC, Petersen NS, Mitchell HS. Induced thermal tolerance and heat shock protein synthesis in Chinese hamster ovary cells. Int J Radiat Oncol Biol Phys 1982; 8: 63–67
- Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proceedings of the National Academy of Sciences, USA 1982; 79: 3219–3222
- Subjeck JR, Sciandra JJ, Johnson RJ. Heat shock proteins and thermotolerance: A comparison of induction kinetics. Brit J Radiol 1982; 55: 579–584
- Landry J, Chretien P. Relationship between hyperthermia induced heat shock proteins and thermotolerance in Morris hepatoma cells. Can J Biochem Cell B 1983; 61: 428–437
- Tomasovic SP, Steck PA, Heitzman D. Heat-stress proteins and thermal resistance in rat mammary tumor cells. Radiat Res 1983; 95: 399–413
- Li GC. Elevated levels of 70,000 dalton heat shock protein in transiently thermotolerance Chinese hamster fibroblasts and in their stable heat resistant variants. Int J Radiat Oncol Biol Phys 1985a; 11: 165–177
- Li GC. Possibility of using hsp70 as a predictor of thermal response in tumor during fractionated hyperthermia. Hyperthennia in Cancer Therapy. MAG Bros. Inc., Tokyo 1985b; 46–49
- Li GC, Mak JY. Induction of heat shock protein synthesis in murine tumors during the development of thermotolerance. Cancer Res 1985; 45: 3816–3824
- Fu KK, Rayner PA, Lam KN. Modification of the effects of continuous low dose irradiation by concurrent chemotherapy infusion. Int J Radiat Oncol Biol Phys 1984; 10: 1473–1478
- Tomkins W, Watrach A, Schmale J, Schultz R, Harris J. Cultural and anti-genic properties of newly established cell strains derived from adenocarcinomas of the human colon and rectum. J Nat Cancer I 1979; 52: 1101–1110
- O’Farrell PH. High-resolution two-dimensional polyacrylamide gel electrophoresis of proteins. J Biol Chem 1975; 250: 4007–4021
- Laemmli UK. Cleavage of structure proteins during the assembly of the head ofbacteriophage T4. Nature (London) 1970; 227: 680–685