Abstract
Purpose: This study investigated the efficacy of repeated thermotherapy for breast cancer utilising a novel sintered MgFe2O4 needle and alternating current (AC) magnetic field in xenograft animal models mimicking human breast cancer.
Materials and methods: A sintered MgFe2O4 needle and an apparatus to apply an AC magnetic field were prepared for this study. Animals bearing BT-474 tumours (mean (±standard deviation) volume, 471 ± 153 mm3) were divided into four groups. A sintered MgFe2O4 needle (length, 5 mm) was placed in the centre of each tumour. An AC magnetic field (amplitude, 4 kA/m; 2 kW; 540 kHz) was applied for 10 min once, twice or three times for the first, second and third groups, respectively, and was not applied for the control group. Temperature during treatment and tumour volume 8 weeks after first treatment were assessed.
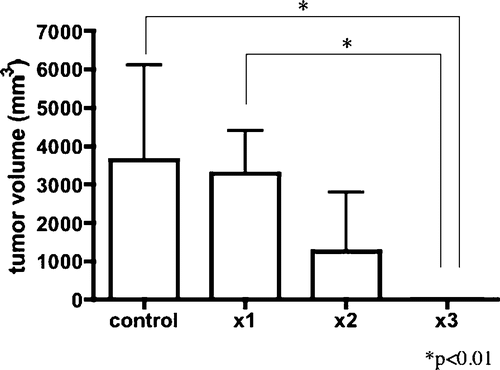
Results: Maximum tumour temperature tended to increase in repeated-application groups: group 1, 59.2 ± 4°C; group 2, 58.9 ± 3.3°C and 61.2 ± 8.9°C for the first and second applications; and group 3, 60.4 ± 4.6°C, 62.1 ± 7.8°C and 71.1 ± 6.1°C for the first, second and third applications. Tumour volumes in control, groups 1, 2 and 3 at 8 weeks after treatment were 3633 ± 2478 mm3, 3240 ± 1031 mm3, 1252 ± 1289 mm3 and 0 mm3, respectively. Tumours were significantly smaller in group 3 than in the control and group 1 at 8 weeks.
Conclusions: The efficacy of repeated inductive heating utilising a sintered MgFe2O4 needle was demonstrated. Thermotherapy using the present method may offer an effective non-surgical treatment for human breast cancer.
Introduction
In the treatment of breast cancer, improvements in chemotherapy, radiotherapy and other treatments have enabled increasingly less invasive surgical treatment. In recent years, use of radiofrequency ablation (RFA) for local control of breast cancer has been proposed Citation[1–7]. Side effects of this thermotherapy are limited to local complications such as minor burns and slight bruising Citation[1], Citation[2]. Attractive results have been achieved in these studies and the feasibility of thermotherapy against breast cancer has been identified.
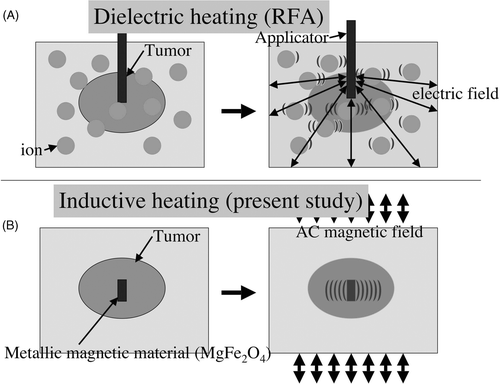
We have previously reported another method of thermotherapy using inductive heating Citation[8–10]. We investigated inductive heating with various kinds of metals and identified MgFe2O4 as offering the most effective heating. This material was then formed into a needle shape for easy and long-term placement within the target. We have also developed a new apparatus for use with the sintered MgFe2O4 needle in this ablation therapy. Conventional heating methods such as RFA involve dielectric heating, utilising thermal heating from friction produced by a high-frequency alternating current (AC) delivered into tissue, causing excitation and motion of intracellular ions. The tissue surrounding the electrode, rather than the electrode itself, is the primary source of heat Citation[11] (). Inductive heating utilises the heat produced by the hysteresis loss effect within the magnetic material itself when an alternating magnetic field is applied externally. Rather than the tissue surrounding the magnetic material, the material itself forms the source of heat (). This represents the essential difference between dielectric and inductive heating.
Figure 1. (A) Dielectric heating. Rather than the applicator, tissues around the applicator produce heat by friction of ions agitated by a high-frequency electric current. (B) Inductive heating. The magnetic material in the target itself produces heat by the hysteresis loss effect.
With inductive heating, once the magnetic material is placed in the target, the target has only to move into an alternating magnetic field to receive treatment. This means the patient can receive treatments repeatedly simply by moving into an alternating magnetic field, with no need for further puncture or contact as required in conventional thermotherapies. This characteristic of inductive heating may provide patients with less invasive tumour control.
The objective of this study was to evaluate the efficacy of repeated inductive heating using a sintered MgFe2O4 needle in xenograft animal models mimicking human breast cancer.
Methods
Animals
Female immunodeficient mice (4–6 weeks old) were obtained from Charles River Japan (Yokohama). All animals were treated in accordance with the guidelines for animal research of Ehime University School of Medicine. Mice were anaesthetised using intraperitoneal administration of 50 µg/g pentobarbital (Nembutal; Dainippon Sumitomo Pharma, Osaka).
Human breast cancer cells from the BT-474 line (JCRB Cell Bank, Osaka) were used. A suspension of 1 × 106 BT-474 cells in 0.1 mL of phosphate-buffered saline was injected subcutaneously into the flank of a mouse using a 27-G needle. After 12 weeks, formation of a subcutaneous tumour 2 cm in diameter was confirmed. The tumour was resected and cut into 2-mm blocks. Blocks were inoculated into the right flank of 30 mice through small skin incisions. The study was started after 6–8 weeks, once the tumour had exceeded 10 mm in diameter (mean diameter, 11.8 ± 1.6 mm). Mean weight of mice at this stage was 23.8 ± 2 g.
MgFe2O4 needle and electromagnetic heating device
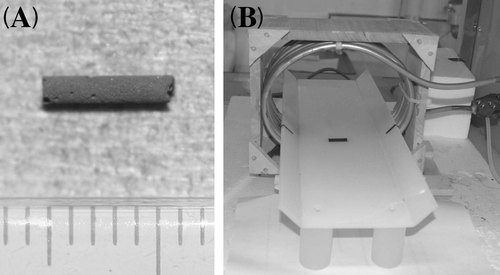
MgFe2O4 particles (mean particle size, approximately 1 µm) were purchased from Wako Pure Chemicals (Osaka). Particles were forced into a thin quartz tube before sintering at 1100°C for 27 h. The completed MgFe2O4 needle is 1 mm in diameter and contains 2.21 mg of MgFe2O4/mm (). The entire manufacturing process was performed by the Department of Materials Science in the Faculty of Engineering at Ehime University.
Figure 2. (A) The MgFe2O4 needle (diameter, 1 mm; length, 5 mm; containing 2.21 mg of MgFe2O4/mm). (B) The device for applying alternating current (AC) magnetic fields comprises loops of copper pipe connected to a high-frequency power supply through an adjuster. Loops are cooled from within by flowing water in a cooling unit, so that the coil can consistently produce reproducible magnetic fields.
The device for applying an AC magnetic field comprised loops of copper pipe connected to a T162-5712B high-frequency power supply (Thamway, Shizuoka, Japan) through an adjuster. Loops were cooled from within by flowing water in a CLU33 cooling unit (Iwaki, Tokyo), so that the coil could produce consistently reproducible magnetic fields ().
Elevation of temperature at the centre of the tumour was monitored using an AMOTH FL 2000 sensor FS100-M fluorescence optic fibre thermometer (Anritsu Meter, Tokyo).
Inductive heating
A total of 30 tumour-bearing mice were divided into four groups. MgFe2O4 needles were placed in the centre of the tumour. All animals were anaesthetised and placed in the centre of the loops for 15 min 3 times at 24-h intervals. An AC magnetic field of amplitude 4 kA/m (2 kW, 540 kHz) was applied for 10 min once for group 1 (n = 7), twice for group 2 (n = 8) and 3 times for group 3 (n = 7), and was not applied for the control group (n = 8). One animal that died from anaesthesia in group 2 was excluded from the study.
Tumour volume
Tumour volume before and 8 weeks after first treatment was calculated as an elliptocyte according to the following formula:where a and b represent the long and short diameters, respectively, and c is the diameter of the vertical axis.
Microscopic observations
Animals were sacrificed 8 weeks after the first treatment. Tumours or residual tissue were fixed in 10% formalin and embedded in paraffin for 24 h. Paraffin-embedded sections were cut at a thickness of 4 µm, dewaxed, dehydrated and stained using haematoxylin and eosin (HE). Sections were then examined for microscopic evidence of residual viable cancer cells.
Statistical analysis
All values are expressed as mean ± standard deviation. Comparison of maximum tumour temperatures in groups 2 and 3 were made using a two-sided Wilcoxon's signed-rank test and Kruskal-Wallis test with Dunn's multiple comparison test. Tumour sizes at 8 weeks were compared using the Kruskal-Wallis test with Dunn's multiple comparison test. Differences of P < 0.05 were considered statistically significant in all tests.
Results
Elevation of temperature
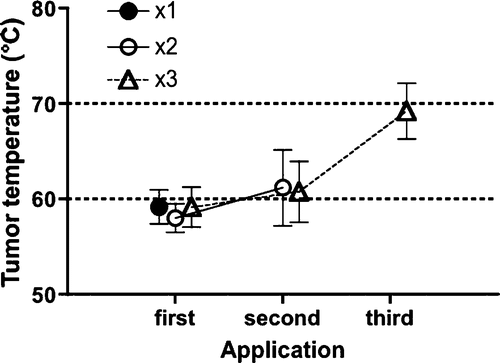
Baseline tumour temperature was approximately 32°C before treatment. After starting AC application, temperature plateaued within a few minutes and fell immediately to baseline level after the end of application. Maximum temperature in group 1 was 59.2° ± 4.0°C. In group 2, maximum temperatures were 58.9° ± 3.3°C and 61.2° ± 8.9°C for the first and second applications. In group 3, maximum temperatures were 60.4° ± 4.6°C, 62.1° ± 7.8°C and 71°.1 ± 6.1°C for the first, second and third applications. No significant differences were seen between first and second applications in group 2 or first, second and third applications in group 3 ().
Figure 3. Maximum temperature was 59.2° ± 4°C in group 1, 58.9° ± 3.3°C and 61.2° ± 8.9°C for the first and second applications in group 2, and 60.4° ± 4.6°C, 62.1° ± 7.8°C and 71.1° ± 6.1°C for the first, second and third applications in group 3. Although no significant differences were seen between groups 2 and 3, maximum temperature tended to increase with increased application time.
Tumour volume
One animal in the control group died from tumour 5 weeks after first application. Tumour volumes in control, and groups 1, 2 and 3 were 438 ± 121 mm3, 461 ± 158 mm3, 515 ± 198 mm3 and 474 ± 86.0 mm3 before application, and 3633 ± 2478 mm3, 3240 ± 1031 mm3, 1252 ± 1289 mm3 and 0 mm3 at 8 weeks. Tumour volume was significantly smaller in group 3 compared with the control and group 1 (P < 0.01 each) ().
Discussion
The method we used in this study involves inductive heating. The heating principle in this method differs from the prevailing method of dielectric heating employed in RFA and microwave coagulation therapy (MCT). In dielectric heating, heat is produced by friction from the excitation of ions or water molecules surrounding the needle-shaped applicator positioned within the target. In comparison, the magnetic material itself achieves heating effects when entering a magnetic field in inductive heating. Hysteresis loss is thought to be greatly involved in the heating effect, although the precise mechanisms remain unclear. Using this method, repeated episodes of thermotherapy can be provided without subsequent contact with the body.
The first trial of inductive heating using iron oxide and an AC magnetic field was reported by Gilchrist in 1957 Citation[13]. Subsequent experimental studies have reported using inductive heating as a method of local control for cancer. Almost all of these studies used FeFe2O4 as the metallic magnetic material, reaching temperatures of 42°–48°C by applying stronger AC magnetic fields with longer application times than those used in the present study Citation[14–25]. Our previous study determined the heating ability (W/g) of various ferrite powders by placing 1 g of each powder into 10 mL of water under an alternating magnetic field Citation[10]. This alternating magnetic field (370 kHz, 4.0 kA/m) was produced using a copper pipe 6 mm in diameter connected to a power supply through an impedance tuner. Heating abilities of MFe2O4 where M = Mg, Mn, Fe, Co, Ni, Cu and Sr were 1.44, 0.31, 0.16, 0.20, 0.41 and 0.45 W/g, respectively. That study identified MgFe2O4 as the most effective material for inductive heating, raising therapeutic temperature and reducing the time required in the AC magnetic field. Using this material with high potential for temperature elevation, inductive heating with a weaker AC magnetic field and shorter duration of application can be achieved. Another advantage of using MgFe2O4 is that this material has a lower possibility of further oxidisation than FeFe2O4. Ion oxidation and long-term insertion in the body may cause adverse events such as surrounding tissue injury, elution of materials into the circulation and finally reduced heating efficacy. For this reason, MgFe2O4 may provide equal or greater efficacy of heating after long-term placement, representing a potential advantage with repeated inductive heating.
The present study showed the efficacy of repeated treatment, with three applications at 24-h intervals appearing sufficient to achieve complete local control. Some studies have shown the efficacy of multiple thermal cycles compared to a single continuous session in vivo Citation[20],Citation[25]. The interval in those studies was set at 24 h, but the ideal interval remains unclear. We applied the alternative magnetic field with a 24-h interval on the basis of convenience for outpatients in clinical use. A preliminary study comparing double thermal sessions with 24-, 72- and 120-h intervals (data not shown) suggested the 24-h interval as the most effective for BT-474 tumour models. When we consider clinical use in outpatients, triple sessions with intervals of 6- or 12-h may prove impractical for use on an outpatient basis.
Maximum temperature during the treatment tended to rise with increasing treatment time. Friedman et al. suggested that the decrease in tumour blood flow makes following treatment more effective in combination therapy using radiotherapy and RFA Citation[26]. The tendency for temperature increases seen in our study may be attributable to the same mechanisms. This may indicate that when a single session is insufficient, another session may complete total ablation of the tumour. Although conventional thermotherapies (RFA or MCT) require additional puncture for each session, our method has no such requirement once the needle has been placed.
Complete removal of the needle may be required in clinical use. Ohno et al. Citation[20] formed a stick-type FeFe2O4 by mixing with carboxymethylcellulose for inductive heating. After thermotherapy, they showed diffused distribution of FeFe2O4 particles throughout the tissue. This diffusing characteristic of the stick may be advantageous in achieving diffused heat effects, but removing the material may be impossible. Our MgFe2O4 needle does not dissolve within the target, was easily removed and did not move until removal was performed. Although our HE sections showed no marked presence of residual particles in surrounding tissues, coating the needle in another material may facilitate complete removal of the needle.
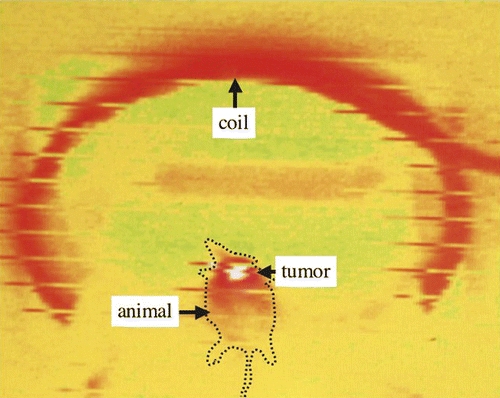
In the future, a study using larger animals and larger tumours may be required for clinical use. Using multiple needles or applying the AC magnetic field for longer or more frequently may allow control of larger tumours. In this study, almost all animals developed skin burns over the tumour (data not shown). These lesions healed within 2 weeks, but may represent an issue in human patients in terms of cosmetic results. Animals in this study were very small, about 24g in weight. This may be the reason for severe skin burns. Monitoring of skin temperature during treatment by infrared thermometer showed a selective temperature elevation (). Controlling the strength of the alternative magnetic field under a strict monitoring of skin temperature or active skin cooling may therefore help to avoid this problem.
Figure 6. Monitoring of skin temperature during treatment by infrared thermometer shows a selective temperature elevation.
This treatment appears to offer an effective, less invasive thermotherapy for clinical use against breast cancer.
Conclusion
This study showed that repeated inductive heating using a sintered MgFe2O4 needle achieved effective local control of breast cancer in xenograft animal models mimicking human breast cancer. This heating method may well become a treatment of choice for local control of human breast cancer in future.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jeffrey SS, Birdwell BR, Ikeda DM, Daniel BL, Nowels KW, Dirbas FM, Griffey SM. Radiofrequency ablation of breast cancer: First report of an emerging technology. Arch Surg 1999; 134: 1064–1068
- Izzo F, Thomas R, Delrio P, Rinaldo M, Vallone P, DeChiara A, Botti G, D’Aiuto G, Cortino P, Curley SA. Radiofrequency ablation in patients with primary breast carcinoma: A pilot study in 26 patients. Cancer 2001; 92: 2036–2044
- Elliott LR, Rice BP, Suits AJ, Ostrowe JA, Head FJ. Radiofrequency ablation of a stereotactically localized nonpalpable breast carcinoma. Am Surg 2002; 68: 1–5
- Burak Jr WB, Agnese DM, Povoski SP, Yassens TL, Bloom KJ, Wakely PE, Spigos DG. Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer 2003; 98: 1369–1376
- Fornage BD, Sneige N, Ross MI, Mirza AN, Kuerer HM, Edeiken BS, Ames FC, Newman LA, Babiera GV, Singletary SE. Small (≤2-cm) breast cancer treated with US-guided radiofrequency ablation: Feasibility study. Radiology 2004; 231: 215–224
- Klimberg VS, Kepple J, Shafirstein G, Adkins L, Henry-Tillman R, Youssef E, Brito J, Talley L, Korourian S. eRFA: Excision followed by RFA–A new technique to improve local control in breast cancer. Ann Surg Oncol 2006; 13: 1422–1433
- Noguchi M, Earashi M, Fujii H, Yokoyama K, Harada K, Tsuneyama K. Radiofrequency ablation of small breast cancer followed by surgical resection. J Surg Oncol 2006; 93: 120–128
- Bland KL, Gass J, Klimberg S. Radiofrequency, cryoablation and other modalities for breast cancer ablation. Surg Clin N Am 2007; 87: 539–550
- Maehara T, Konishi K, Kaminori T, Aono H, Naohara T, Kikkawa H, Watanabe Y, Kawachi K. Heating of ferrite powder by an AC magnetic field for local hyperthermia. Jpn J Appl Phys 2002; 12: 1620–1621
- Konishi K, Maehara T, Kamimori T, Aono H, Naohara T, Kikkawa H, Watanabe Y, Kawachi K. Heating ferrite powder with AC magnetic field for thermal coagulation therapy. J Magn Magn Mater 2004; 272–276: 2428–2429
- Maehara T, Konishi K, Kamimori T, Aono H, Hirazawa H, Nomura S, Kikkawa H, Watanabe Y, Kawachi K. Selection of ferrite powder for thermal coagulation therapy with alternating magnetic field. J Mat Sci 2005; 40: 135–138
- Organ LW. Electrophysiologic principles of radiofrequency lesion making. Appl Nerophysiol 1976/77; 39: 69–76
- Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph nodes. Ann Surg 1957; 146: 596–606
- Jordan A, Scholz R, Wust P, Fähling H, Krause J, Wlodarczyk W, Sander B, Vogl T, Felix R. Effects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo. Int J Hyperthermia 1997; 13: 587–605
- Jordan A, Scholz R, Wust P, Fähling H, Krause J, Wlodarczyk W, Sander B, Vogl T, Felix R. Effects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo. Intracellular hyperthermia for cancer using magnetite cationic liposomes: An in vivo study. Jpn J Cancer Res 1998; 89: 463–469
- Minamimura T, Sato H, Kasaoka S, Saito T, Ishizawa S, Takemori S, Tazawa K, Tsukada K. Tumor regression by inductive hyperthermia combined with hepatic embolization using dextran magnetite-incorporated microspheres in rats. Int J Oncol 2000; 16: 1153–1158
- Minamimura T, Sato H, Kasaoka S, Saito T, Ishizawa S, Takemori S, Tazawa K, Tsukada K. Tumor regression by inductive hyperthermia combined with hepatic embolization using dextran magnetite-incorporated microspheres in rats. Int J Oncol 2000; 16: 1153–1158
- Hilger I, Andra W, Hergt R, Hiergeist R, Schubert H, Kaiser WA. Electromagnetic heating of breast tumors in interventional radiology: In vitro and in vivo studies in human cadavers and mice. Radiol 2001; 218: 570–575
- Moroz P, Jones SK, Gray BN. The effect of tumour size on ferromagnetic embolization hyperthermia in a rabbit liver tumour model. Int J Hyperthermia 2002; 18: 129–140
- Ohno T, Wakabayashi T, Takemura A, Yoshida J, Ito A, Shinkai M, Honda H, Kobayashi T. The effect of tumour size on ferromagnetic embolization hyperthermia in a rabbit liver tumour model. Effective solitary hyperthermia treatment of malignant glioma using stick type CMC-magnetite. In vivo study. J Neurooncol 2002; 56: 233–239
- Hamaguchi S, Tohnai I, Ito A, Mitsudo K, Shigetomi T, Ito M, Honda H, Kobayashi T, Ueda M. Selective hyperthermia using magnetoliposomes to target cervical lymph node metastasis in a rabbit tongue tumor model. Cancer Sci 2003; 94: 834–835
- Ito A, Tanaka K, Kondo K, Shinkai M, Honda H, Matsumoto K, Saida T, Kobayashi T. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci 2003; 94: 308–313
- Wada S, Tazawa K, Furuta I, Nagae H. Antitumor effect of new local hyperthermia using dextran magnetite complex in hamster tongue carcinoma. Oral Dis 2003; 9: 218–223
- Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldöfner N, Scholz R, Deger S, Wust P, Loening SA, Jordan A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int J Hyperthermia 2005; 21: 637–647
- Kawai N, Ito A, Nakahara Y, Honda H, Kobayashi T, Futakuchi M, Shirai T, Tozawa K, Kohri K. Complete regression of experimental prostate cancer in nude mice by repeated hyperthermia using magnetite cationic liposomes and a newly developed solenoid containing a ferrite core. Prostate 2005; 66: 718–727
- Friedman MA, Altemus R, Wood BJ. Radiofrequency ablation in a previously irradiated liver. J Vasc Interv Radiol 2003; 14: 1345–1348