Abstract
Purpose: This study evaluated psychological stress reactions during hyperthermia (HT) treatments and compared them to systemic stress reactions.
Materials and methods: 27 patients with malignant disease were treated with superficial or regional hyperthermia. Cortisol and the catecholamines adrenaline and noradrenaline in venous blood were used as markers of psychological stress. The anxiety proneness of the patients was evaluated with a trait-anxiety inventory. Blood pressure and heart rate were markers of systemic stress. Patients were first grouped by superficial or regional treatment and then into two subgroups: anxious and non-anxious patients.
Results: The time course of the cortisol concentration of the superficial group showed a slight but significant decrease and that of the regional group an increase. The cortisol concentration of the regional group was sometimes slightly but significantly higher than in the superficial group, and in the group of anxious patients higher than in the group of non-anxious patients. The changes in time courses and differences in groups were not as pronounced for the catecholamine concentrations. Heart rate and blood pressure showed a significant increase only in the regional group, and there was no significant difference between the regional and the superficial groups. None of the variables showed a significant difference between the initial and the subsequent treatments; all lay well within the normal physiological range.
Conclusions: These standard hyperthermia treatments are not excessively stressful, either systemically or psychologically. The different behaviours of the variables confirm that it makes sense to consider systemic stress as well as psychological reactions.
Introduction
Hyperthermia treatment (HT) entails the well-known problem of stress-related symptoms that can adversely affect the treatment or even cause its curtailment. The existing literature on hyperthermia describes stress-related symptoms as increases in transpiration, heart rate and blood pressure Citation[1–5]. All these variables depend on physiological effects mainly caused by the heating (e.g. thermoregulation, response of the cardiovascular system), and they are primarily indicators of systemic stress. As these variables consider the psychological component of stress only non-specifically, it would be of interest to analyse indicators of psychological stress as well.
Psychological stress generally is considered to stimulate cortisol and catecholamine secretion Citation[6], Citation[7]. These stress hormones tend to be released in situations with high ego involvement, low predictability, low controllability, and novelty Citation[8]. One such extreme situation for patients is the period immediately before major surgery, when the secretion of these psychoendocrine hormones rises steeply Citation[9].
We investigated a specific hormone test to determine psychological stress with regard to its clinical utility in hyperthermia and describe its first clinical application. We used a special psychological test to assess the anxiety proneness (‘trait anxiety’) of each patient.
We were interested in the time course of the concentrations of each of these hormones as indicators for psychological stress induced by hyperthermia treatment. A further question was the relation of these time courses to variables for systemic stress, specifically any differences between superficial and regional, and initial and subsequent treatments. A final consideration was any difference between patients with more or less evidence of anxiety proneness.
We assessed the time courses of the cortisol and catecholamine concentrations in venous blood of patients as indicators of psychological stress related to hyperthermia treatments, presenting the observed effects on these variables, depending on the factor's type, number of hyperthermia treatments and anxiety proneness, and comparing these factors to the variables for systemic stress. We looked into ways in which psychological stress might be reduced during hyperthermia treatments.
Patients and methods
Patient population
The study protocol was approved by the Ethics Committee of the Medical University of Graz, Austria. From March 2003 to March 2004, 27 patients were invited to participate in this stress study after application of the inclusion and exclusion criteria. Adult patients were enrolled if a superficial or regional hyperthermia treatment was planned for tumour therapy. Inclusion criteria were age >18 years, venous catheter already in place for therapeutic purposes, and written informed consent. Exclusion criteria were a Karnofski performance score <70%, ischaemic heart disease or other major systemic diseases, known pregnancy and the need to take any steroid drugs shortly before treatment (steroids are known to significantly influence cortisol levels Citation[6], Citation[10], Citation[11]). The abort criterion was scheduled to come into effect when the patient withdrew consent. All invited patients gave written informed consent and the abort criterion was never applied. Ten patients (4 patients with breast carcinoma, 6 with lymph node metastases) were treated with superficial hyperthermia, and 17 patients with various deep-seated lesions () were treated with regional hyperthermia. Patients were aged 31–77 years (mean 56.4 ± SD 11.7). There were 27 patients, 18 female and 9 male.
Table I. Patients’ anthropometric data, indication for hyperthermia and application type, trait-anxiety score.
Hyperthermia system and treatment
Superficial hyperthermia treatments were applied with our BSD500/BSD2000-V system (BSD Medical Corporation, Salt Lake City, Utah) using the MA-100 and MA-120 applicators at 915 MHz and SA-115 at 130 MHz. The RF power ranged from 80–140 W. Regional hyperthermia was delivered by our BSD2000-V System using the Sigma-60 applicator at 95 MHz with RF power in from 450–600 W.
Hyperthermia was scheduled twice a week (minimum two days between treatments). The treatments aimed to reach a tumour temperature of 43°C within a maximum of 60 min. Temperatures in normal tissue were not to exceed 42.5°C. As the patients did not receive analgesics or sedation, they could report any pain.
Measurement of endocrine hormone concentrations
The standard markers of psychological stress used were the concentrations of cortisol and the catecholamine adrenaline and noradrenaline in venous blood Citation[6], Citation[7], Citation[10], Citation[11]. The concentrations of these hormones were determined three times per hyperthermia treatment (): first, within 5 minutes of the beginning (0 min) of the warm-up phase; second, within 5 minutes of the beginning of the therapeutic time (20 min); and third, within 5 minutes of the end of the treatment (60 min). We always drew 8 mL of venous blood at these time points (3.5 mL for cortisol and 4.5 mL for catecholamine concentrations).
Figure 1. Measurement cycles of psychoendocrine hormone concentrations in venous blood, heart rate and blood pressure.
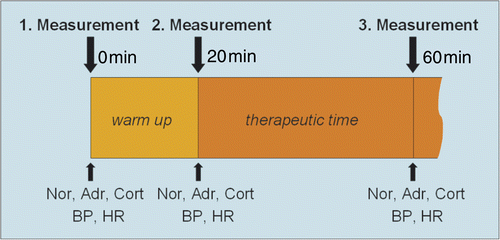
The samples were always put on ice immediately and processed within a few minutes in our laboratory.
Analysis of noradrenaline, adrenaline and cortisol
All blood samples were immediately centrifuged at 4°C for 10 min. Decanted plasma was stored at −70°C until analysis for total plasma noradrenaline and adrenaline concentrations. Plasma levels of catecholamines were measured with high pressure liquid chromatography (HPLC) and electrochemical detection on an Agilent HPLC Chemstation chromatographic system (Agilent Technologies Corporation, Santa Clara, CA) using a Chromsystem® test kit and a Chromsystem® reverse phase analytical column for HPLC analysis of catecholamines (Chromsystems® Instruments and Chemicals, Munich) fitted with an ESA Coulochem II electrochemical detector (ESA Laboratories, Chelmsford, MA) Citation[12]. The sensitivity for catecholamine measurement was 0.05 ng/mL. The technician who analysed the samples was unaware of any patient data.
Serum cortisol levels were determined with a fluorescence polarization immunoassay kit (TDx/TDx Cortisol, Abbott Laboratories, Abbott Park, IL) according to the manufacturer's instructions.
Trait anxiety inventory
To evaluate the anxiety proneness of each patient we used a standardised questionnaire, the German version of the Spielberger Trait Anxiety Inventory form X2 (STAI X2). This is one of the two sections of the Spielberger State-Trait Anxiety Inventory (the other measures state anxiety–‘how one feels at a particular moment’), which has been validated for various situations and populations in several studies Citation[13]. Trait anxiety–‘how one usually feels’–has been defined as anxiety proneness, that is, the tendency to respond to situations perceived as threatening with elevations in the intensity of state anxiety. The trait anxiety score is based on 20 items which a person rates from 1 to 4 in terms of frequency categories (almost never, sometimes, often, and almost always). The analysis of each questionnaire produces a raw score for trait anxiety. The conversion of the raw value with normative tables that consider age (three age groups: 15–29, 30–59, >60 years) and sex delivers the percentile value, the T-value as measure for the intergroup comparable trait anxiety and the stanine value as measure for the individual comparable trait anxiety and the individual percentile (). The scores are positively related to the attribute trait anxiety (i.e. high stanine scores represent high scores on the attribute trait anxiety). The questionnaire requires 3–5 min for completion. Every patient was asked to fill in the form at the time he or she gave informed consent to participate in the study.
Table II. Mean values of patients’ anthropometric data and trait-anxiety scores. The patients are grouped according to type of hyperthermia application, sex and applicator used.
Measurement of variables for systemic stress
Blood pressure (maximum systolic, minimum diastolic) and heart rate were measured with a vital sign monitor (Dinamap 1846 SX®, Critikon, Tampa, FL) every 5 min and recorded in the case report form.
Study design and analysis
This was a pilot study, open, monocentric, dual-arm. We grouped patients by superficial or regional treatment. These two groups were divided into two subgroups each: anxious and non-anxious patients. We defined ‘anxious’ as a trait anxiety stanine value greater than 6, and ‘non-anxious’ as a stanine value equal to or less than 6. Treatments were classified as initial or subsequent.
Circadian rhythms in plasma cortisol: the literature reports an antemeridian physiologic decrease in cortisol concentration Citation[10], Citation[11], Citation[14], Citation[15]. As virtually all patients were treated in the morning, we took this into consideration with an additional correction of the cortisol data with a physiologic decrease of 15% of the initial value for the cortisol concentration per hour. This value should approximate the mean value of the antemeridian decrease in cortisol concentration observed in larger studies Citation[15] and should correct the influence of the circadian rhythms on plasma cortisol.
All recorded data were analysed with repeated measures ANOVA (p < 0.05 was considered significant) to find changes in the variables time course, type of treatment and treatment order (initial or subsequent treatments), and between the groups and subgroups.
Results
Hormone concentrations
Raw data on cortisol concentration (): among 94 analysed standard treatments (27 patients, 1–8 treatments, 81 treatments includable) in the combined group regional and superficial treatment we observed a slight but significant decrease of the cortisol concentration in venous blood during the warm-up phase (from beginning of warm up to beginning of therapeutic time) and a slight but significant decrease from the beginning to the end of treatment as well. The cortisol concentration of the superficial group also showed a slight but significant decrease from the beginning of warm up to the end of treatment. In contrast, the cortisol concentration of the regional group showed an increase (but not significant; p = 0.06) during the second phase of treatment (beginning of therapeutic time to end of treatment). During the second phase of treatment, the cortisol concentration of the regional group was slightly but significantly higher than in the superficial group. During all phases of treatment the cortisol concentration in the regional group was higher (but not significantly) than that in the superficial group. The cortisol concentration was higher as well (but not significantly) in the group of anxious patients than in the group of non-anxious patients. The cortisol concentration did not show significant differences between the initial and the subsequent treatments.
Figure 2. Time course of the raw data on cortisol concentration in venous blood during superficial and regional standard HT treatments (mean, 95% confidence interval): average of non-anxious group and anxious group (A), non-anxious group (B), anxious group (C). Significant changes see .
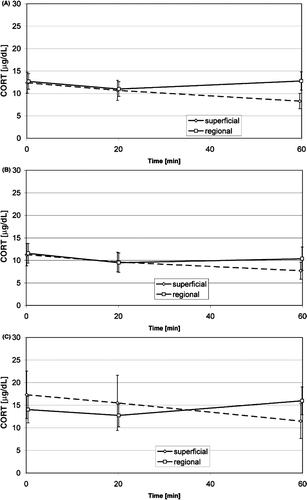
Cortisol data with correction of the influence of the circadian rhythms: the corrected data showed nearly the same differences in time course and differences between groups as the raw data for cortisol concentration (same significances in time course, except the decrease in time course in the combined group was not significant), but they showed two additional significant findings. On the one hand, the slight increase in cortisol concentration in the regional group was significant during the second phase of treatment. On the other hand, the cortisol concentration in the group of anxious patients was slightly but significantly higher than in the group of non-anxious patients.
Table III. Changes in time course of the measured variables during standard HT treatments and differences between groups.
Adrenaline () and noradrenaline (): the adrenaline concentrations in the anxious patient group indicated an increase in the first and a decrease in the second phase of treatment, and the concentration in the regional treatments seems to be higher than in the superficial treatments of the anxious patient group, but neither is significant. The noradrenaline concentration in the superficial treatment group showed a slight but significant decrease during warm up, and a slight but significant increase from the beginning of warm up to the end of treatment in the regional treatment group. The noradrenaline concentration seems to be higher in the anxious patient group than in the non-anxious group, but this is not significant. Both concentrations of these hormones in venous blood did not show significant differences between groups, either between regional treatments and superficial treatments, or between anxious and non-anxious patients, or between initial and subsequent treatments.
Figure 3. Time course of the adrenaline concentration in venous blood during superficial and regional standard HT treatments (mean, 95% confidence interval): average of non-anxious group and anxious group (A), non-anxious group (B), anxious group (C). No significant changes.
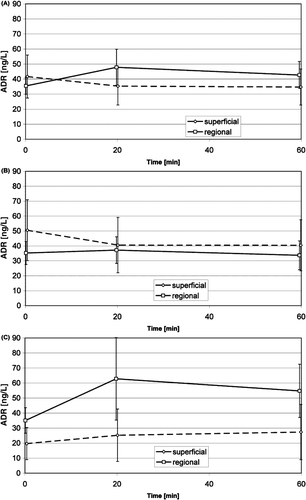
Figure 4. Time course of the noradrenaline concentration in venous blood during superficial and regional standard HT treatments (mean, 95% confidence interval): average of non-anxious group and anxious group (A), non-anxious group (B), anxious group (C). Significant changes see .
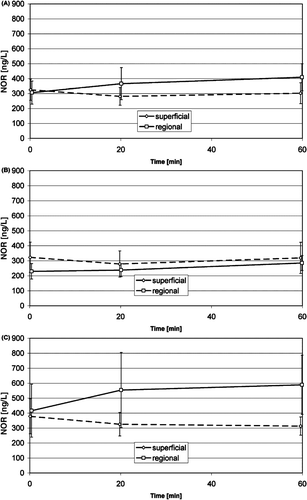
None of all the measured hormone concentration in venous blood showed a significant difference between the initial and the subsequent treatments. All values for the hormone concentrations lay well within the physiological normal range.
Variables for trait anxiety
The response rate to our questionnaire was 81% (22 of 27). The mean values as well the standard deviations of T-values and stanine values of trait anxiety are shown in , classified by local and regional treatment and by gender.
Heart rate and blood pressure
The heart rate () showed a slight increase from the first to the second phase of treatment. This increase was significant both in the combined group (superficial and regional treatments) and in the regional group, but not in the superficial group alone. The heart rate seemed to be higher in the regional group than in the local group, especially among the anxious patients, but without significance. The mean systolic pressure () increased from the first to the second phase of treatment, but both the mean systolic and diastolic arterial blood pressures () did not change significantly and did not show differences between regional and superficial treatments. Neither the heart rate nor the blood pressure showed a significant difference between the initial and the subsequent treatments. The values for heart rate and blood pressure also lay well within the normal physiological range.
Figure 5. Time course of the heart rate during superficial and regional standard HT treatments (mean, 95% confidence interval): average of non-anxious group and anxious group (A), non-anxious group (B), anxious group (C). Significant changes see .
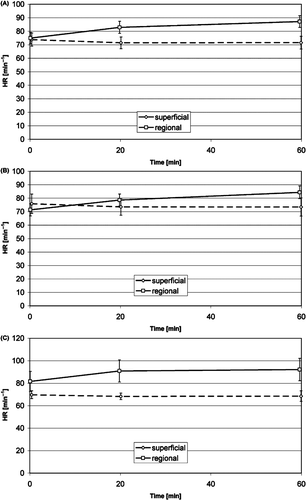
Figure 6. Time course of the systolic arterial blood pressure during superficial and regional standard HT treatments (mean, 95% confidence interval): average of non-anxious group and anxious group (A), non-anxious group (B), anxious group (C). No significant changes.
Figure 7. Time course of the diastolic arterial blood pressure during superficial and regional standard HT treatments (mean, 95% confidence interval): average of non-anxious group and anxious group (A), non-anxious group (B), anxious group (C). No significant changes.
summarizes all significant changes, differences between groups and differences between the initial and the subsequent treatments, of the measured variables.
Discussion
An interesting characteristic of the results of the present study is that neither the analysed indicators for systemic stress (heart rate, blood pressure) nor the analysed indicators for psychological stress (cortisol and catecholamine concentrations in venous blood) exceeded the normal physiological range. In all, this allows the assumption that the standard hyperthermia treatments we studied were not excessively stressful for the patients, either systemically or psychologically.
To say anything conclusive about the feasibility of measuring psychological stress induced by hyperthermia, we must consider the plausibility of the time courses of the analysed variables and the comparison of the different groups (superficial, regional, anxious and non-anxious patients).
In the time courses, we could observe a slight but significant increase in the heart rate during the treatments, similar to the results of other studies that investigated this variable during hyperthermia treatments. A significant increase in the heart rate is reported in Citation[2] and a significant increase in heart rate as well in blood pressure in Citation[5]. The studies Citation[1] and Citation[4] report an increase in blood pressure, but do not say whether the changes were significant. Another study Citation[3] reports a trend for blood pressure to increase slightly in normotensive patients and a trend for blood pressure to decrease slightly in hypertensive patients during heating sessions. It is in slight contrast to the results of Citation[5] that the time course of our patients’ blood pressure did not show such a significant increase during the treatments, but the behaviours of heart rate and blood pressure showed that the ‘systemic stress level’ of our patients during standard hyperthermia treatments generally was not very high.
The slight changes in time course of the cortisol and catecholamine concentrations in venous blood suggest the same for psychological stress, i.e. that our patients also had a low ‘psychological stress level’. The low concentration of cortisol and catecholamine additionally could indicate that the anticipation of the treatment might cause more psychological stress reactions than the treatment itself. However, for a clear statement regarding the latter relation, it would be necessary to measure the variables before the patient is prepared for hyperthermia treatment. This should be considered in further studies.
Because neither heart rate nor blood pleasure showed significant differences between the superficial and the regional treatment groups, nothing can be said about the stress level of patients during hyperthermia treatment as indicated by these two variables. It does, however, seem to make sense to use the cortisol concentration in venous blood to quantify psychological stress reactions during standard hyperthermia treatments: the raw data of the cortisol concentration in the regional group were slightly but significantly higher than in the superficial group during the second phase of treatment. This is plausible because superficial treatments should indeed be less stressful–both, systemically and psychologically–than regional treatments.
Further support for using primarily the cortisol concentration as a psychological stress marker for hyperthermia treatments is offered by the fact that the plasma cortisol values in the group of anxious patients corrected for circadian rhythms were slightly but significantly higher than those in the group of non-anxious patients during the second phase of treatment. It also seems plausible that anxious patents will respond to hyperthermia treatments with a higher stress level than non-anxious patients.
A limitation of this study could be that it is difficult to say how representative the sample of the enrolled patients is of all cancer patients treated with hyperthermia, because we do not know the magnitude of the influence of the inclusion and exclusion criteria, e.g. on the anxiety proneness of the studied patient population.
Even if the psychological stress reactions during standard hyperthermia treatment do not seem to be very high, to maximise patient tolerance, it would surely be desirable to reduce stress reactions as far as possible. There is some evidence that stress can promote tumour development by suppressing natural killer cell activity Citation[16]. The question, then, is how can stress be best reduced? As a reduction in heating would worsen treatment outcome, and technical innovations to improve patient comfort, e.g. by reducing bolus pressure, are unlikely in the near future, other means of reducing treatment stress must be sought.
Reducing the psychological component of stress could be one aim. Since we are able to determine the level of psychological stress during hyperthermia treatments, we can in turn evaluate measures for the targeted reduction of psychological stress. Such measures could be, for example, special room design, interior decoration and lighting, or music during the treatments. Studies have shown that listening to music can reduce patient stress reactions and anxieties before and during medical interventions such as venipuncture Citation[17], gastroscopy Citation[18], Citation[19], cardiac catherisation Citation[20] or surgical procedures Citation[21–23]. To minimise stress exposure, a friendly atmosphere combined with relaxing music of the patient's choice Citation[24–27] would be desirable prior to HT treatments. Further studies could be designed to optimise the environment for hyperthermia treatments.
Another interesting challenge for further study would be to analyse the stress reactions of patients undergoing non-standard HT treatments (e.g. extra-high bolus temperature, additional heater blankets, or other measures to increase the tumour temperature that deviate from standard HT treatments).
A reduction in treatment-related stress, whether systemic or psychological, could be expected to improve patient tolerance and reduce adverse treatment effects and treatment interruptions.
Conclusion
The results of the study indicate slight differences in time courses as well as between the patient and treatment groups for the hormone concentrations and cardiovascular variables during the hyperthermia treatments. This confirms our supposition that it makes sense to investigate psychological and systemic stress reactions in order to evaluate the stress exposure of patients during treatments. Cardiovascular variables such as blood pressure and heart rate are primarily representative of systemic stress reactions but not of psychological stress reactions of the patient during standard hyperthermia treatments. Our results further indicate that the psychological stress reactions during standard hyperthermia treatments generally are not very pronounced, and there are indications that the anticipation of the treatment might cause more psychological stress reactions than the treatment itself. It is also remarkable that there is no significant difference in hormone concentration between the initial treatment and the subsequent treatments; this could indicate that anticipatory stress of the standard hyperthermia treatment is rather low. This is in contrast to our empirical experience that the systemic stress reactions of patients during hyperthermia treatments can be problematic.
Acknowledgements
The authors thank Gabriele Gartner for her excellent technical assistance and Gerald Kügerl, Dr Marcus-Yves Rigler and Dr Gabriele Höss for their interest and support. The authors gratefully acknowledge Eugenia Lamont for language editing of the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Van Es CA, Wyrdeman HK, de Leeuw AA, et al. Regional hyperthermia of pelvic tumours using the Utrecht ‘Coaxial TEM’ system: a feasibility study. Int J Hyperthermia 1995; 11: 173–186
- De Leeuw AA, van VM, Van de Kamer JB, Warlam-Rodenhuis CC, Lagendijk JJ. Increasing the systemic temperature during regional hyperthermia: effect of a cooling strategy on tumour temperatures and side-effects. Int J Hyperthermia 2003; 19: 655–663
- Cho CH, Wust P, Hildebrandt B, et al. Regional hyperthermia of the abdomen in conjunction with chemotherapy for peritoneal carcinomatosis: Evaluation of two annular-phased-array applicators. Int J Hyperthermia. 2008; 24: 399–408
- Sapozink MD, Joszef G, Astrahan MA, et al. Adjuvant pelvic hyperthermia in advanced cervical carcinoma. I. Feasibility, thermometry and device comparison. Int J Hyperthermia 1990; 6: 985–996
- Matsuoka H, Furusawa M, Tomoda H, Seo Y, Sugimachi K. Efficacy of indomethacin pretreatment with regional hyperthermia for treating upper abdominal malignancies. Int J Hyperthermia 1995; 11: 169–171
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994; 19: 313–333
- Hubert W, Moller M, Nieschlag E. Stress reactions in response to the procedure of LHRH tests as measured by salivary and serum cortisol and psychological variables. Horm Res 1989; 32: 198–202
- Peters ML, Godaert GL, Ballieux RE, van VM, Willemsen JJ, Sweep FC, Heijnen CJ. Cardiovascular and endocrine responses to experimental stress: Effects of mental effort and controllability. Psychoneuroendocrinology 1998; 23: 1–17
- Bodley PO, Jones HV, Mather MD. Preoperation anxiety: A qualitative analysis. J Neurol Neurosurg Psychiatry 1974; 37: 230–239
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: An overview. Neuropsychobiology 1989; 22: 150–169
- Kirschbaum C, Hellhammer D. Methodological Aspects of Salivary Cortisol Measurement. Assessment of Hormones and Drugs in Saliva in Biobehavioral Research, C Kirschbaum, G Read, D Hellhammer. Hogrefe & Huber, Seattle 1992; 19–32
- Goldstein DS, Feuerstein G, Izzo JL, Jr, Kopin IJ, Keiser HR. Validity and reliability of liquid chromatography with electrochemical detection for measuring plasma levels of norepinephrine and epinephrine in man. Life Sci 1981; 28: 467–475
- Spielberger CD, Porsuch RL, Lushene RH. State-Trait-Anxiety Inventory. Palo Alto. Consulting Psychologists Press, California 1970
- Nomura S, Fujitaka M, Sakura N, Ueda K. Circadian rhythms in plasma cortisone and cortisol and the cortisone/cortisol ratio. Clin Chim Acta 1997; 266: 83–91
- Stone AA, Schwartz JE, Smyth J, et al. Individual differences in the diurnal cycle of salivary free cortisol: A replication of flattened cycles for some individuals. Psychoneuroendocrinology 2001; 26: 295–306
- Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer 1999; 80: 880–888
- Caprilli S, Anastasi F, Grotto RP, Abeti MS, Messeri A. Interactive music as a treatment for pain and stress in children during venipuncture: A randomized prospective study. J Dev Behav Pediatr 2007; 28: 399–403
- Escher J, Hohmann U, Anthenien L, et al. Music during gastroscopy. Schweiz Med Wochenschr 1993; 123: 1354–1358
- Kotwal MR, Rinchhen CZ, Ringe VV. Stress reduction through listening to Indian classical music during gastroscopy. Diagn Ther Endosc 1998; 4: 191–197
- Hamel WJ. The effects of music intervention on anxiety in the patient waiting for cardiac catheterization. Intensive Crit Care Nurs 2001; 17: 279–285
- Reilly M. Incorporating music into the surgical environment. Plast Surg Nurs 1999; 19: 35–38
- Nilsson U, Unosson M, Rawal N. Stress reduction and analgesia in patients exposed to calming music postoperatively: A randomized controlled trial. Eur J Anaesthesiol 2005; 22: 96–102
- Miluk-Kolasa B, Obminski Z, Stupnicki R, Golec L. Effects of music treatment on salivary cortisol in patients exposed to pre-surgical stress. Exp Clin Endocrinol 1994; 102: 118–120
- Khalfa S, Bella SD, Roy M, Peretz I, Lupien SJ. Effects of relaxing music on salivary cortisol level after psychological stress. Ann N Y Acad Sci 2003; 999: 374–376
- Labbe E, Schmidt N, Babin J, Pharr M. Coping with stress: The effectiveness of different types of music. Appl Psychophysiol Biofeedback 2007; 32: 163–168
- Chafin S, Roy M, Gerin W, Christenfeld N. Music can facilitate blood pressure recovery from stress. Br J Health Psychol 2004; 9: 393–403
- Smith JC, Joyce CA. Mozart versus new age music: Relaxation states, stress, and ABC relaxation theory. J Music Ther 2004; 41: 215–224