Abstract
Aim: Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemoperfusion (HIPEC) is the treatment regime most likely to achieve prolonged survival in patients with peritoneal carcinomatosis from gastroenteric cancer. To date, few publications have focused on the treatment of patients with gastric cancer alone. Several controversies remain unsolved, including the safety and effectiveness of the CRS–HIPEC combination regime, particularly in cases where HIPEC is used as adjuvant treatment after CRS. Therefore, in the current study, we aimed to evaluate the safety and effectiveness of CRS combined with HIPEC in patients with gastric cancer.
Method: Data from 231 patients with a median age of 55.1 years treated with the CRS–HIPEC combination regime between January 2009 and December 2014 were retrospectively reviewed. All patients underwent the combination therapy (mean of 2.4 cycles per patient, range, 1 to 4 cycles).
Results: Median overall survival was 37.0 months, with 1-, 2- and 3-year survival rates recorded as 83.4%, 68.5%, and 38.7%, respectively. The serum levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 199 (CA199) were significantly decreased after combination therapy in the completeness of cytoreduction (CCR)-0 and CCR-1 groups, while no significant changes observed in marker levels were observed in the CC ≥2 group. The post-operative morbidity and mortality rates were 6.9% and 0.9%, respectively. Multivariate analysis revealed low TNM tumour stage, ascites condition and CCR score as independent predictors for better survival.
Conclusion: In view of the acceptable morbidity and mortality rates we propose that CRS combined with HIPEC presents an effective and safe treatment modality for patients with gastric cancer, especially in cases where optimal cytoreduction is achieved before the HIPEC procedure.
Introduction
Gastric cancer is the fifth most common cancer type and the third leading cause of cancer-related mortality worldwide [Citation1]. For decades, surgical resection combined with systemic chemotherapy has remained the most widely accepted treatment approach [Citation2]. However, despite remarkable advances in medical technology and anti-cancer drug development, outcomes of patients with advanced gastric cancer are still poor. The overall 5-year survival rate of patients with resectable gastric cancer is unsatisfactory owing to their high risk of predominantly lymphatic spread, haematogenous metastasis, and in particular, peritoneal recurrence [Citation3].
Peritoneal carcinomatosis is the most common type of recurrence and cause of death after surgery in patients with gastric cancer. The disease, encountered in 5–20% of gastric cancer patients, is partly attributable to tumour spillage during surgery, and nearly half of the patients with potentially curable advanced gastric cancer die from peritoneal recurrence [Citation4–6]. Once gastric peritoneal carcinomatosis is manifest, intravenous chemotherapy and radiotherapy as adjuvant treatments provide no significant survival advantage. With growing understanding of the mechanisms underlying peritoneal tumour spread, multimodal approaches combining aggressive cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemoperfusion (HIPEC) have been proposed as promising treatment options for advanced gastric cancer. HIPEC, which has the advantage of an ability to eradicate micronodules and micrometastases that cannot be managed by conventional surgery, is particularly effective for the treatment of peritoneal carcinomatosis [Citation7,Citation8]. CRS combined with HIPEC is reported to improve locoregional control of the disease and ultimately enhance survival [Citation9,Citation10].
The CRS-HIPEC combination regime is most likely to achieve prolonged survival in patients with disease due to appendiceal, colorectal, and mesothelioma primaries. However, few reports have focused on the utility of this regime in gastric cancer alone. Several controversies remain unsolved, including the safety and effectiveness of the CRS-HIPEC combination, especially in cases where HIPEC is used as adjuvant treatment after CRS [Citation11]. An earlier meta-analysis of randomised controlled trials by Yan et al. [Citation12] showed significantly increased incidence of intra-abdominal abscess and neutropenia in the HIPEC group. The most commonly encountered post-operative complications included leucopenia, anaemia, thrombopenia, and heart, liver or renal toxicity, among other side effects, with morbidity rates ranging from 14–41% [Citation13–17]. Earlier randomised controlled trials have demonstrated significant long-term survival benefits and decreased peritoneal recurrence in patients with advanced gastric cancer without visible carcinomatosis subjected to the CRS-HIPEC regime [Citation18]. However, less encouraging results have been reported by other authors. A number of prospective trials have demonstrated limited improvement in the long-term survival of patients with gastric peritoneal carcinomatosis following combination therapy [Citation19,Citation20].
The current study provides a review of our experience with treatment of gastric cancer using the CRS-HIPEC combination, with emphasis on the safety and effectiveness of the regime. The results indicated the median overall survival was 37.0 months. The post-operative morbidity and mortality rates were 6.9% and 0.9%, respectively. The most commonly encountered complication after treatment was hypoproteinaemia (3.9%). HIPEC-specific toxicity ranged from 0.9–3.0%, and was associated with gastrointestinal, hepatic, haematological, and metabolic manifestations. Our findings collectively support the utility of CRS combined with HIPEC as an effective and safe treatment modality for patients with gastric cancer.
Patients and methods
Patients
Clinico-pathological data of 231 patients exposed to CRS combined with HIPEC at the Cancer centre of Guangzhou Medical University (Guangzhou, China) between January 2009 and December 2014 were retrospectively reviewed. All cases were confirmed as gastrointestinal malignancy using CT or magnetic resonance imaging (MRI), and histological diagnosis. TNM stage was assessed according to the seventh edition TNM staging system proposed by the American Joint Committee on Cancer using endoscopy with biopsy, chest and abdominal CT, laparoscopy, and endoscopic ultrasound with fine needle aspiration where necessary. Ethical approval for this study was obtained from the Ethics Committee of the Cancer Center of Guangzhou Medical University.
Data collection
The following data were collected for all patients: gender, age, primary diagnosis, total hospital stay (days), preoperative and post-operative chemotherapy, surgical procedures performed, CCR score, primary tumour location, estimated blood loss, and complications associated with CRS-HIPEC combination therapy. The grades of complications resulting from the procedure were documented in accordance with Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [Citation21]. Clinical efficacy in terms of ascites control was divided into three grades according to the World Health Organization criteria, with some modifications: 1) complete remission (CR): complete disappearance of ascites for at least 4 weeks, 2) partial remission (PR): ascites reduced by 50% and sustained over 4 weeks, and 3) no response (NC): failure to meet either of the above criteria. Only CR and PR were considered effective control. The extent of CRS was determined according to the Sugarbaker criteria for CCR classification [Citation9]. A score of CCR-0 indicates no residual peritoneal disease after CRS, CCR-1, <2.5 mm residual disease, CCR-2, residual tumour between 2.5 mm and 2.5 cm, and CCR-3, >2.5 cm residual tumour or the presence of a sheet of unresectable tumour nodules. Patients were evaluated retrospectively from the date of treatment with CRS combined with HIPEC to a specific end-point consisting of either the date of last follow-up or death.
CRS combined with HIPEC for treatment of patients with gastric cancer
All procedures of the combination regime were performed by a team of surgical oncologists, anaesthesiologists and operating room staff. Eligibility criteria for the combined treatment determined during multidisciplinary meetings were as follows: 1) age 18 to 75 years, no concomitant severe heart, respiratory, liver, haematological or kidney diseases, functional status (Eastern Cooperative Oncology Group) ≤2, 2) prophylactic HIPEC for gastric cancer: patients with serosal invasion (T3 or T4), lymph node metastasis (N1) or positive peritoneal cytology [Citation10], 3) inclusion criteria for patients with peritoneal carcinomatosis according to the report of Losa et al. [Citation22]. All patients underwent CRS combined with HIPEC. The CRS in cases with no visible disease after gastrectomy were radical resection with respect to the surgical cytoreduction of the primary tumour, and regional lymph nodes. In these cases, they underwent a complete cytoreduction (CCR-0 or R0 resection). Surgical treatment options included gastrectomy, peritonectomy or lymphadenectomy. In terms of curative procedures, maximal CRS was performed according to the peritonectomy technique developed by Sugarbaker, including resection of the primary tumour with acceptable margins, any involved adjacent structures, lymphadenectomy, omentectomy and peritoneotomy where peritoneal surfaces were involved with tumour [Citation9]. In terms of palliative procedures, only large tumour masses or those causing pain were removed. Upon opening (via midline incision in all cases), the diagnosis of peritoneal carcinomatosis was confirmed by frozen section pathological examination. The extent of peritoneal disease was assessed. When peritoneal disease was considered resectable, resection of afflicted peritoneal areas was performed. The CCR score was used to classify the extent of residual disease. Finally, peritoneal lavage for cytology was performed using 400 mL Normal saline (Otsuka pharmaceutical Co. Ltd. Osaka, Tokyo, Japan). In all patients with gastric cancer, a Billroth II reconstruction was performed after gastrectomy. One cycle of HIPEC as adjuvant treatment was delivered after surgery for prevention of peritoneal recurrence. For patients with peritoneal carcinomatosis or ascites, 3–4 cycles of HIPEC were administered every 2 days after the operation [23]. In patients that received more than one cycle the catheters were left in place. HIPEC treatment was initiated after closure of the abdominal cavity. Two inflow drainage tubes were placed in the upper abdomen and two outflow tubes for perfusion in the lower abdomen. Subsequently, 3–4.5 L heated normal saline was circulated at a rate of 600 mL/min for 60 min using the BR-TRG-I hyperthermic perfusion intraperitoneal treatment system (Bright Medical Tech, Guangzhou, China). Perfusion was carried out at 43 °C with 1500 mg 5-fluorouracil (5-FU) and 100 mg cisplatin as chemotherapeutic agents. Patients were transferred to the intensive care unit for recovery, and relocated to the surgical oncology ward following stabilisation of their condition.
Statistical analysis
Tumour markers measured before and after treatment were evaluated using paired t-tests. Overall survival was determined based on time (months) between surgery and death or the last follow-up, and overall survival curves obtained using the Kaplan–Meier method with the log-rank test. Differences were considered statistically significant at P < 0.05. Multivariate analysis was performed with Cox’s proportional hazards regression model. All data were analysed with the statistical package for social sciences (SPSS, IBM, Armonk, NY), v 15.0.
Results
Patient demographics and descriptive data
A total of 231 patients treated using CRS combined with HIPEC at our centre between January 2009 and December 2014 participated in the study, including 162 men (70.1%) and 69 women (29.9%) with a median age of 55.1 years (range, 18 to 78). The majority of patients (70.0%) were diagnosed with poorly or undifferentiated adenocarcinoma, 65 (28.1%) with well or moderately differentiated adenocarcinoma, and 20 (8.6%) with both of cancer types. Ascites was detected in 48 patients (20.8%), while 142 (61.5%) had no observable ascites and 41 (17.7%) had no data record of ascites. All patients received HIPEC treatment (mean of 2.4 cycles, range, 1 to 4 cycles). A high rate of intra-abdominal spread was additionally observed in this series. We recorded 23 (9.9%) peritoneal invasion, six (2.6%) ovarian invasion, six (2.6%) colonic invasion, seven (3.0%) pancreatic invasion, 11 (4.8%) invasion of the gullet, and eight (3.5%) liver invasion cases ().
Table 1. Patient demographics and descriptive data.
Characteristics of the combination HIPEC procedure
All patients underwent surgical intervention and treatment with CRS combined with HIPEC. Palliative and curative procedures were conducted in 98 (42.4%) and 133 (57.6%) patients, respectively. Mean estimated blood loss was 242.1 mL (range, 5 to 3500) and mean length of hospital stay was 19.1 days (range, 5 to 90). The majority of patients underwent gastrectomy, as well as a few patients with colectomy, pancreatectomy and splenectomy before CRS. Overall, 131 (56.7%) patients achieved optimal cytoreduction (CCR-0), 60 (26.0%) were classified as CCR-1, 5 (2.2%) as CCR-2, and 25 (10.8%) as CCR-3. No record of the CCR score was found for the remaining 10 (4.3%) patients ().
Table 2. Procedure characteristics.
Effectiveness and safety of the combination procedure
Serum samples were collected both pre- and post-operatively just before patients left the hospital, usually within 30 days after treatment with the CRS–HIPEC combination. The pre- and post-operative levels of three tumour markers, specifically CEA, CA199 and CA125, were evaluated. Post-operative CEA levels were measured in 196 (92.5%) of the 212 patients for whom preoperative carcinoembryonic antigen (CEA) levels were obtained. Among the 136 patients displaying elevated preoperative CEA levels, 108 had normal post-operative CEA levels while 28 failed to normalise post-operatively despite complete cytoreduction. Further analysis of CEA before and after treatment in the 196 patients disclosed a significant decrease in the CEA serum level after treatment with the CRS–HIPEC combination in the CCR-0 and CCR-1 groups. No significant changes were observed in the CCR ≥2 group ().
Table 3. Tumour marker measurement before and after treatment.
The carbohydrate antigen 199 (CA199) level was measured in 182 patients before and after treatment. Among the 116 patients with elevated pre-operative CA199, 83 displayed normalised levels following CRS and HIPEC therapy. To ascertain whether the CA199 level was suppressed as a result of the combination regime, pre- and post-treatment levels of the marker were further analysed using the paired t-test. Consistently, the serum level of CA199 was markedly decreased after exposure to CRS combined with HIPEC in the CCR-0 and CCR-1 groups, while no significant changes were observed in the CCR ≥2 group.
Finally, carbohydrate antigen 125 (CA125) levels were assessed both pre- and post-operatively in 176 patients. Among 82 patients with elevated preoperative CA125 levels, 66 experienced normalisation after exposure to CRS combined with HIPEC. Further analysis using the paired t-test revealed a significant decrease in the serum level of CA125 after treatment in the CCR-0 group, but no significant changes in the CCR ≥1 group ().
We additionally examined the survival times of patients. As shown in , the median follow-up period was 16.2 months (range 1 to 63 months). A total of 54 (23.4%) patients died from disease progression or recurrence, while 138 (59.7%) were still alive. Thirty-nine (16.9%) patients were lost to follow-up and thus excluded from analysis. The overall 1-, 2- and 3-year survival rates were 83.4%, 68.5%, and 38.7%, respectively. Median overall survival was 37.0 months (95% confidence interval (CI) 33.2–40.8). Our results indicated better survival rates of patients subjected to curative procedures compared to those treated with palliative intent (). Median overall survival was 28.0 months (95% CI 11.4–44.6) in the palliative arm, and 46.0 months (95% CI 21.2–70.6) in the curative arm. The overall 1-, 2- and 3-year survival rates for the palliative and curative arms were 73.9%, 52.4% and 28.6%, and 93.3%, 79.9% and 57.0%, respectively.
Figure 1. Kaplan–Meier survival curves after treatment with CRS combined with HIPEC. (A) Overall survival of gastric cancer patients subjected to HIPEC. (B) Overall survival of gastric cancer patients subjected to curative and palliative procedures. (C) Overall survival of gastric cancer patients with or without ascites. (D) Overall survival of gastric cancer patients with stages III and IV (P < 0.01, Stage IV vs. Stage III). (E) Overall survival of gastric cancer patients with CCR-0, 1 and -3 (P = 0.002, CCR-0 vs. CCR >0).
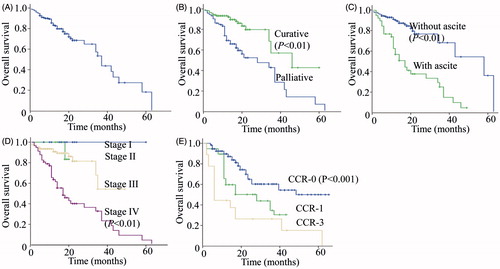
In terms of the impact of ascites on survival, patients without ascites had better survival rates (). The median overall survival was 58.0 months (95% CI 31.9–84.0) in the ascites-negative arm, compared to 17.0 months (95% CI 10.3–23.7) in the ascites-positive arm. The overall 1-, 2- and 3-year survival rates of the ascites-negative and ascites-positive arms were 90.4%, 76.9% and 68.4%, and 57.1%, 38.0% and 25.3%, respectively.
The previous finding that cancer stage has a significant impact on overall survival was further explored. Owing to the limited number of stage I and stage II gastric cancer cases, only stage III and stage IV patients were included in the current analysis. Our results showed that higher-grade disease results in poorer overall survival (). Mean overall survival was 38.3 months (95% CI 32.7–43.8) in the stage III arm and 24.2 months (95% CI 18.7–29.6) in the stage IV arm (P < 0.001). The overall 1-, 2- and 3-year survival rates for stages III and IV were 90.7%, 81.5% and 54.3%, and 63.6%, 40.2% and 32.9%, respectively.
Previous studies have indicated that the CCR score has a significant influence on overall survival. In our study patients in the CCR-0 group had better overall survival outcomes than those classified as CCR >0 (P < 0.001). However, no significant differences in survival were evident among patients classified as CCR-1, -2 or -3 (). Mean overall survival was 49.6 months (95% CI 31.1–59.2) in the CCR-0 arm, 35.9 months (95% CI 30.1–42.5) in the CCR-1 arm, and 17.4 months (95% CI 3.6–31.2) in the CCR-3 arm. The overall 1-, 2- and 3-year survival rates were 91.3%, 64.7%, and 60.1% in the CCR-0 arm, 64.7%, 51.4%, and 34.3% in the CCR-1 arm, and 42.9%, 23.2%, and 23.2% in the CCR-3 arm, respectively.
Multivariate analysis using Cox’s regression model identified TNM stage, ascites condition and CCR score as independent predictors for survival, but not intended curative or palliative surgery, repeated HIPEC therapy, post-operative chemotherapy, gender or age ().
Table 4. Summary of multivariate analysis of overall survival duration in all patients.
Finally, we analysed the complication profiles of patients following treatment with the CRS-HIPEC combination. The overall post-operative morbidity rate was 6.9% (16/231). The most commonly encountered side effect was hypoproteinaemia (nine cases, 3.9%), followed by electrolyte disturbance, vomiting, and ileus. Two patients (0.9%) died from multiple organ failure ().
Table 5. Complication profiles of patients subjected to cytoreductive surgery combined with HIPEC treatment.
Discussion
This retrospective study summarises our preliminary results on the efficacy of CRS combined with HIPEC for patients with gastric cancer. Data obtained from 231 patients subjected to the combination treatment showed a median overall survival rate of 37.0 months, and 1-, 2- and 3-year survival rates of 83.4%, 68.5% and 38.7%, respectively. Serum levels of CEA and CA199 were significantly decreased in the CCR-0 and CCR-1 groups after treatment with CRS plus HIPEC. The post-operative morbidity and mortality rates were 6.9% and 0.9%, respectively. Multivariate analysis revealed that low TNM tumour stage, ascites condition and CCR score are independent predictors for better survival. Our findings clearly suggest that CRS combined with HIPEC provides an effective and safe treatment modality for patients with advanced gastric cancer, in particular, when optimal cytoreduction is achieved before the HIPEC procedure.
Recent studies have focused on the relationship between gastric cancer and different tumour markers. A number of tumour-associated antigens, including CEA, CA199, and CA125, are elevated in the sera of patients with advanced gastric cancer [Citation24]. In the current study we measured the levels of these three tumour markers prior to treatment with CRS combined with HIPEC and post-treatment. Earlier, the group of Taflampas [Citation25] confirmed that preoperative tumour marker (CEA, CA125 and CA199) status can effectively predict recurrence and survival after CRS and HIPEC for appendiceal pseudomyxoma peritonei. Wagner et al. [Citation26] suggested that measurement of CEA levels following CRS-HIPEC combination therapy may allow the identification of a subset of patients with high risk of recurrence. Our results demonstrated a significant decrease in the CEA and CA199 levels after therapy with CRS combined with HIPEC in the CCR-0 and CCR-1 groups. In view of these differences, it is advisable to measure tumour marker levels both before and after treatment, since the majority of patients display normalisation of serum levels post-operatively, particularly in the CCR-0 and CCR-1 groups. For gastric cancer, complete cytoreduction is accepted as the strongest predictor of long-term survival following CRS and HIPEC. However, a number of patients develop recurrent disease. Thus, evaluation of the changes in tumour marker levels after exposure to CRS combined with HIPEC may aid in determining the appropriate treatment options and provide an opportunity for early detection of disease recurrence and timely intervention. However, at this time, insufficient numbers of patients are available to determine the usefulness of changes in CEA and CA125 levels before and after combination therapy as an independent predictor of recurrence or death. Ongoing studies are aimed at determining whether CA199 and CEA carry prognostic information.
Previous studies have consistently confirmed that CRS combined with HIPEC is an effective treatment modality for patients with cancer. Stamou et al. reported that CRS combined with HIPEC is the most effective treatment for peritoneal mesothelioma [Citation27]. The groups of Yonemura [Citation19] and Canbay [Citation28] reported improved survival in patients treated with the CRS-HIPEC combination, with a 5-year overall survival rate of ∼30%. Another earlier phase III randomised clinical trial showed a median overall survival of 11.0 months for patients with advanced gastric cancer after combined therapy with CRS and HIPEC [Citation29]. Median overall survival of 24 months for patients who underwent gastrectomy combined with HIPEC has also been reported [Citation9,Citation30]. However, differences in overall survival between the current and the three earlier studies were observed, which may be attributed to the fact that patients with peritoneal carcinomatosis accounted for ∼25% of the patient populations in our study, compared to almost 100% in the other investigations. Accordingly, HIPEC was used as adjuvant treatment for the prevention of peritoneal recurrence after radical surgery for the majority of primary gastric carcinoma cases in our study. Disease dissemination and extent of the procedures required to achieve complete cytoreduction are the most important prognostic factors for both perioperative surgical outcome (mortality and morbidity) and long-term oncological outcome. Our results additionally indicate that cancer stage and CCR score have a significant influence on survival. Overall survival of patients with stage III gastric cancer was considerably better than that of patients with stage IV disease (40.0 months vs. 24.9 months, P < 0.001). Consistently, significant improvement in 5-year overall survival of patients with stage III gastric cancer was observed in a randomised trial (stage III vs. stage IV, 49.1% vs. 18.4%) [Citation31]. The differences in overall survival between stages III and IV may due to intrinsic limitations of HIPEC, in that maximum tissue penetration of the therapeutic agents is estimated as maximum of 3–5 mm [Citation32]. Patients with advanced gastric cancer normally display deep tissue invasion or distant metastases, which cannot be treated efficiently by chemotherapeutic drugs delivered via intraperitoneal chemoperfusion. Our results clearly support the utility of CCR as independent prognostic indicator. Patients in the CCR-0 group had better overall survival outcomes than those in the CCR >0 groups. Consistent with our findings, a recent review suggested that CRS combined with HIPEC may improve long-term survival rates in a select group of patients with peritoneal carcinomatosis of gastric origin for whom CCR-0 is achieved [Citation10]. Ovarian cancer studies additionally confirmed that 1-, 2-, 3-, 4-, and 5-year overall survival rates following therapy with HIPEC combined with CRS were progressively lower with decreasing in CCR scores [Citation33]. At a certain point thermal treatment enhances the cytotoxicity of drugs rather than killing tumour cells. Drug penetration and the residual tumour nodules size are key factors for successful treatment. Having the advantage of synergistic anti-tumour effects of thermal and chemotherapeutic drugs, combination therapy with CRS and HIPEC can facilitate the eradication of residual tumour micronodules (estimated to be a maximum of 3–5 mm). Thus, the outcome of HIPEC is highly dependent on the ‘cleanliness’ of the peritoneal cavity. Complete cytoreduction is therefore highly recommended prior to the HIPEC procedure in patients, especially those with large nodules.
In addition to significant survival benefits, CRS combined with HIPEC has also been shown to be effective in ascites control. In 48 patients, ascites of different volumes were resolved, leading to a 100% efficacy rate for ascites control (CR and PR). Furthermore, overall post-operative morbidity and mortality rates were satisfactory (6.9% and 0.9%, respectively), consistent with previous reports that morbidity and mortality after combination treatment with CRS and HIPEC ranged from 12–56% and 0–8%, respectively [Citation34,Citation35]. Adverse events included wound infection and sepsis, respiratory failure, gastrointestinal bleeding, severe bone marrow suppression, and intestinal obstruction. Common complications, such as bleeding, post-operative bowel obstruction, anastomotic leakage and wound infection were commonly related to surgery. Neutropenia, leucopenia, anaemia, thrombocytopenia, and organ toxicity are common side effects associated with chemotherapy drugs. In our experiments the most commonly encountered complication after treatment was hypoproteinaemia (3.9%), which may be caused by the rapid loss of large volumes of ascites. A high rate of hypoproteinaemia (40%) was similarly reported in a previous study [Citation35]. The other three complications were electrolyte disturbance, vomiting, and ileus, which were likely to be a consequence of chemotherapy drug toxicity. Notably no surgery- or HIPEC-related complications were observed validating the safety of this multimodality regime.
In the past, the adjuvant cases with no visible disease after gastrectomy that received HIPEC would seem to be excessive and potentially harmful. Limited survival benefit and the rates of morbidity and mortality after such a risky procedure have led to this conclusion. Although this retrospective study includes only a limited number of patients with prophylactic HIPEC, our results suggest that prophylactic HIPEC after radical surgery for gastric cancer presents a promising means to reduce mortality due to peritoneal seeding. Consistently, several authors have reported a potential benefit from using HIPEC as a complement to curative surgery in the absence of carcinomatosis. Recently, Kang et al. [Citation36] confirmed that there was no significant difference in the rate of complication between the group that underwent surgery alone and the group that underwent surgery with HIPEC. Curative surgery with HIPEC had a better prognosis for advanced gastric cancer with serosal invasion [Citation36]. Yarema et al. [Citation9] demonstrated that HIPEC was a well-tolerated and effective method of adjuvant therapy for gastric cancer with high risk of intraperitoneal progression. Costa et al. [Citation37] indicated that perioperative chemotherapy and HIPEC for high-risk gastric cancer patients is a feasible multimodality treatment with acceptable morbidity. Moreover, Yan et al. [Citation12] published a meta-analysis which also demonstrated that using HIPEC as an adjuvant treatment significantly improved the survival rates of patients with gastric cancer. This meta-analysis suggested that HIPEC was an effective approach. Another meta-analysis also showed the potential benefit of using HIPEC for patients with an advanced gastric cancer in an adjuvant setting [Citation38]. A meta-analysis included ten randomised controlled trials and a total of 1062 patients with gastric cancer demonstrated that HIPEC improved the overall survival rate for patients who received resection for advance gastric cancer, and help to prevent peritoneal local recurrence among patients with serosal invasion in gastric cancer [Citation39]. In short, the benefit of using HIPEC as an adjuvant treatment for gastric cancer has also been reported in several randomised studies and several meta-analyses [Citation40]. Although the safety and effectiveness of HIPEC needs more clinical research, our results and several other reports indicate that prophylactic HIPEC after radical surgery for high-risk gastric cancer patients with serosal invasion (T3 or T4), lymph node metastasis (N1) or positive peritoneal cytology, is a feasible multimodality treatment with acceptable morbidity.
Generally, choosing the appropriate cytotoxic agent is very important to delivering safe and effective HIPEC therapy. Certain characteristics of the medication should be considered, such as its direct local toxicity, efficacy, and metabolism. Currently, different drug regimens including single-agent and combination regimens have been used for HIPEC. Cisplatin and mitomycin C were the most widely used anti-cancer drugs in HIPEC. However, cisplatin and 5-FU combination regimens were used in the present study. This may be explained by their pharmacokinetic advantage of the intraperitoneal route of drug administration and their efficacy for gastric cancer. Cisplatin is among the most effective agents for gastrointestinal and gynaecological cancers. The drug is compatible with multiple other agents. It was also confirmed that hyperthermia had the potential to sensitise tumours to cisplatin [Citation41]. 5-FU is frequently used as post-operative intraperitoneal chemotherapy for carcinomatosis from gastric cancer. It was also shown to be effective as intraperitoneal chemotherapy and used as early post-operative intraperitoneal chemotherapy following HIPEC in recent trials [Citation37]. Recently, combination regimens including mitomycin C–5-FU–oxaliplatin combination, mitomycin C–cisplatin combination, doxorubicin–cisplatin combination and so on have been used for HIPEC. However, few articles are available regarding cisplatin and 5-FU combination regimens through the intraperitoneal route with heat. Fortunately, the current study has validated the safety and efficacy of CRS combined with HIPEC using cisplatin and 5-FU combination regimens in the treatment for gastric cancer, with acceptable rates of morbidity and mortality. As far as we know, this is the first report to be published using cisplatin and 5-FU combination regimens in HIPEC.
In conclusion, the current study has validated the safety and effectiveness of CRS combined with HIPEC in the treatment for gastric cancer, with acceptable rates of morbidity and mortality. It is worth noting that complete cytoreduction is important for long-term survival. HIPEC is an adjuvant modality used after surgery to clear residual tumour micro-nodules, and optimal cytoreduction is highly recommended before this procedure. Further research should focus on optimisation of drug penetration into tumour tissue with the aim of increasing the efficacy of HIPEC.
Acknowledgements
Yinuo Tu, Yunhong Tian, Zhiyuan Fang have joint first authorship.
Disclosure statement
This work was supported by grants from the PhD Start-up Funds of Guangzhou Medical College, Guangdong Province, China (Nos. 2012C66, 2014C45 and 2012C69); Guangdong Province Natural Science Fund (No. 2013010016662) and the National Natural Science Foundation of China (Nos. 81201932, 81502342 and 81372493). The authors have no conflict of interest and exclude any financial or personal relationship with other people or organisations that could inappropriately influence the results of this work. The authors alone are responsible for the content and writing of the paper.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29.
- Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439–49.
- Fang C, Hua J, Li J, Zhen J, Wang F, Zhao Q, et al. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymphadenectomy for advanced gastric cancer. Am J Surg 2014;208:391–6.
- Yonemura Y, Endou Y, Sasaki T, Hirano M, Mizumoto A, Matsuda T, et al. Surgical treatment for peritoneal carcinomatosis from gastric cancer. Eur J Surg Oncol 2010;36:1131–8.
- Coccolini F, Gheza F, Lotti M, Virzi S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol 2013;19:6979–94.
- Yonemura Y, Kawamura T, Bandou E, Tsukiyama G, Endou Y, Miura M. The natural history of free cancer cells in the peritoneal cavity. Recent results. Cancer Res 2007;169:11–23.
- Elias D, Goere D, Dumont F, Honore C, Dartigues P, Stoclin A, et al. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur J Cancer 2014;50:332–40.
- Kim KW, Chow O, Parikh K, Blank S, Jibara G, Kadri H, et al. Peritoneal carcinomatosis in patients with gastric cancer, and the role for surgical resection, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy. Am J Surg 2014;207:78–83.
- Yarema RR, Ohorchak MA, Zubarev GP, Mylyan YP, Oliynyk YY, Zubarev MG, et al. Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: Results of a single-centre retrospective study. Int J Hyperthermia 2014;30:159–65.
- Roviello F, Caruso S, Neri A, Marrelli D. Treatment and prevention of peritoneal carcinomatosis from gastric cancer by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Overview and rationale. Eur J Surg Oncol 2013;39:1309–16.
- Rettenmaier MA, Mendivil AA, Abaid LN, Brown Iii JV, Wilcox AM, Goldstein BH. Consolidation hyperthermic intraperitoneal chemotherapy and maintenance chemotherapy following laparoscopic cytoreductive surgery in the treatment of ovarian carcinoma. Int J Hyperthermia 2015;31:8–14.
- Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol 2007;14:2702–13.
- Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43.
- Cotte E, Glehen O, Mohamed F, Lamy F, Falandry C, Golfier F, et al. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: Prospective study of 81 patients. World journal of surgery. 2007;31:1813–20.
- Di Giorgio A, Naticchioni E, Biacchi D, Sibio S, Accarpio F, Rocco M, et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 2008;113:315–25.
- Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560–7.
- Duraj FF, Cashin PH. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal and hepatic metastases: A case-control study. J Gastrointest Oncol 2013;4:388–96.
- Magge D, Zenati M, Mavanur A, Winer J, Ramalingam L, Jones H, et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol 2014;21:1448–55.
- Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005;92:370–5.
- Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systematic review of survival, mortality, and morbidity. J Surg Oncol 2011;104:692–8.
- Younan R, Kusamura S, Baratti D, Cloutier AS, Deraco M. Morbidity, toxicity, and mortality classification systems in the local regional treatment of peritoneal surface malignancy. J Surg Oncol 2008;98:253–7.
- Losa F, Barrios P, Salazar R, Torres-Melero J, Benavides M, Massuti T, et al. Cytoreductive surgery and intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis from colorectal origin. Clin Transl Oncol 2014;16:128–40.
- Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681–5.
- Hwang GI, Yoo CH, Sohn BH, Shin JH, Park YL, Kim HD, et al. Predictive value of preoperative serum CEA, CA19-9 and CA125 levels for peritoneal metastasis in patients with gastric carcinoma. Cancer Res 2004;36:178–81.
- Taflampas P, Dayal S, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Pre-operative tumour marker status predicts recurrence and survival after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for appendiceal pseudomyxoma peritonei: Analysis of 519 patients. Eur J Surg Oncol 2014;40:515–20.
- Wagner PL, Austin F, Sathaiah M, Magge D, Maduekwe U, Ramalingam L, et al. Significance of serum tumor marker levels in peritoneal carcinomatosis of appendiceal origin. Ann Surg Oncol 2013;20:506–14.
- Stamou K, Tsamis D, Pallas N, Samanta E, Courcoutsakis N, Prassopoulos P, et al. Treating peritoneal mesothelioma with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. A case series and review of the literature. Int J Hyperthermia 2015;13(1):1–7.
- Canbay E, Mizumoto A, Ichinose M, Ishibashi H, Sako S, Hirano M, et al. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol 2014;21:1147–52.
- Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575–81.
- Saladino E, Fleres F, Mazzeo C, Pruiti V, Scollica M, Rossitto M, et al. The role of prophylactic hyperthermic intraperitoneal chemotherapy in the management of serosal involved gastric cancer. Anticancer Res 2014;34:2019–22.
- Randle RW, Swett KR, Swords DS, Shen P, Stewart JH, Levine EA, et al. Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Ann Surg Oncol 2014;21:1474–9.
- El-Kareh AW, Secomb TW. A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance. Neoplasia 2004;6:117–27.
- Huo YR, Richards A, Liauw W, Morris DL. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: A systematic review and meta-analysis. Eur J Surg Oncol 2015;41:12.
- Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: A multi-institutional study of 1,290 patients. Cancer 2010;116:5608–18.
- Yang XJ, Li Y, al-shammaa Hassan AH, Yang GL, Liu SY, Lu YL, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival in selected patients with peritoneal carcinomatosis from abdominal and pelvic malignancies: Results of 21 cases. Ann Surg Oncol 2009;16:345–51.
- Kang LY, Mok KT, Liu SI, Tsai CC, Wang BW, Chen IS, et al. Intraoperative hyperthermic intraperitoneal chemotherapy as adjuvant chemotherapy for advanced gastric cancer patients with serosal invasion. J Chin Med Assoc 2013;76:425–31.
- Costa WL, Jr., Coimbra FJ, Ribeiro HS, Diniz AL, de Godoy AL, Begnami M, et al. Safety and preliminary results of perioperative chemotherapy and hyperthermic intraperitoneal chemotherapy (HIPEC) for high-risk gastric cancer patients. World J Surg Oncol 2012;10:195.
- Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol 2014;40:12–26.
- Sun J, Song Y, Wang Z, Gao P, Chen X, Xu Y, et al. Benefits of hyperthermic intraperitoneal chemotherapy for patients with serosal invasion in gastric cancer: A meta-analysis of the randomized controlled trials. BMC Cancer 2012;12:526.
- Glehen O, Passot G, Villeneuve L, Vaudoyer D, Bin-Dorel S, Boschetti G, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: A randomized and multicenter phase III study. BMC Cancer 2014;14:183.
- Muenyi CS, States VA, Masters JH, Fan TW, Helm CW, States JC. Sodium arsenite and hyperthermia modulate cisplatin-DNA damage responses and enhance platinum accumulation in murine metastatic ovarian cancer xenograft after hyperthermic intraperitoneal chemotherapy (HIPEC). J Ovarian Res 2011;4:9.
