Abstract
Prostate cancer is the most common malignancy amongst American men. However, the majority of prostate cancer diagnoses are of low risk, organ-confined disease. Many men elect to undergo definitive treatment, but may benefit from focal therapy to maintain continence and potency. This review reports the mechanism of action and outcomes of emerging focal therapies for prostate cancer. We report the mechanism of action of focal cryotherapy, high intensity focused ultrasound, focal laser ablation, and irreversible electroporation. In addition, we reviewed the largest studies available reporting rates of urinary incontinence, erectile dysfunction, biochemical recurrence-free survival (ASTRO), and post-operative adverse events for each procedure. Each treatment modality stated has a unique mechanism in the ablation of cancerous cells. Genito-urinary symptoms following these studies report incontinence and erectile dysfunction rates ranging from 0–15% and 0–53%, respectively. Biochemical disease-free survival was reported using the ASTRO definition. Some treatment modalities lack the necessary follow-up to determine effectiveness in cancer control. No focal therapy studies reported serious adverse events. These minimally invasive procedures are feasible in a clinical setting and show promising functional and disease control results with short to medium-term follow-up. However, each treatment requires additional robust prospective studies as well as its own unique domain to determine biochemical recurrence free survival to properly determine their role in treatment of organ-confined prostate cancer.
Introduction
Prostate cancer (PCa) is the most common malignancy specific to men, and it is estimated that over 200,000 men were diagnosed with prostate cancer in 2015 [Citation1]. In the past PCa diagnoses were associated with high mortality as cancer was typically not detected early on and at the time of presentation men had progression to the lymphatics or bones. However, the introduction of the prostate-specific antigen (PSA) test in 1991 caused a marked increase in prostate cancer incidence, particularly in that of low-risk, organ-confined disease [Citation2–4]. Nevertheless, management of indolent PCa remains ambiguous. Prostate carcinomas are known to be slow growing, as autopsy studies report prostate cancer cells found in up to 70% of men by age 80 [Citation5]. Often men diagnosed with PCa are counselled to undergo radical prostatectomy or radiation therapy in order to completely eradicate the entire gland. These radical therapies may damage bodily structures such as the urethra, external-striated sphincter or the neurovascular bundle, all of which play a pivotal role in a man’s quality of life. However, since the majority of organ-confined malignancies classified in the current era are now low risk, many of these cancers will remain clinically insignificant. Active surveillance is known to be an effective option for these men; however, only about 10% of PCa patients elect this treatment regimen, with nearly 90% of newly diagnosed PCa patients receiving definitive treatment [Citation6,Citation7]. Thus, many men who undergo radical treatment risk adverse effects such as urinary incontinence, erectile dysfunction, and bowel toxicity, with minimal survival benefits.
For patients with localised unilateral disease numerous treatment modalities exist to focally ablate cancerous tissue. Focal cryoablation, high intensity focused ultrasound (HIFU), irreversible electroporation (IRE), and MRI-guided focal laser ablation (FLA) are viable emerging treatment options in men with low to intermediate risk cancer. These targeted therapies ablate tumours while leaving healthy tissue intact to decrease treatment-related complications. This article will report on each of these treatment modalities. Our aim is to discuss the mechanism of action as well as clinically reported outcomes.
Minimally invasive treatment options
Focal cryotherapy
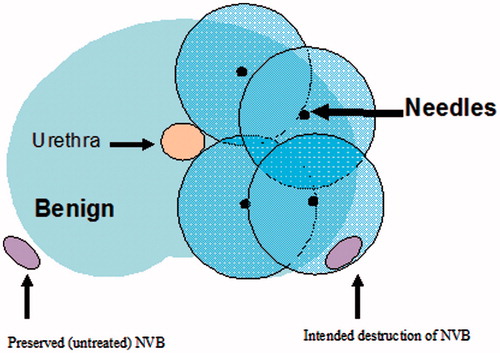
Focal cryotherapy is the controlled freezing of the prostate. A percutaneous approach and a trans-rectal ultrasound allow for real-time monitoring, and ‘cryo-needles’ are inserted into the prostate though a brachy-style template (). The area is rapidly frozen and thawed using argon gas, and two freeze–thaw cycles are completed. During the freeze cycle, decreasing the temperature within the prostate results in ice ball formation from the anterior to posterior end of the gland. The subsequent decrease in temperature results in protein denaturation, loss of blood supply, and apoptosis of prostate cells. In addition, the process of cryotherapy results in the formation of extracellular ice crystals and a hyperosmolar extracellular environment. The concentration gradient across the cellular membrane forces water to rush out of the cell, causing dehydration and metabolic failure [Citation8]. Thawing causes fluid to move from the extracellular space into the intracellular space, causing the cells to swell and burst. Thermocouples placed in and around the area of the cancer are used to monitor intra-operative temperature changes in real time to ensure that complete cell death has occurred. Most of the literature has documented that the temperatures need to reach a minimum of less than −40 °C, although temperatures during prostate cryoablation may drop as low as −80 °C [Citation9]. In men with unilateral disease, cryosurgery will ablate only one side of the prostate. Hemi-ablation of the prostate leaves one side of the neurovascular bundle and remaining healthy prostatic tissue intact to preserve continence and sexual function.
High intensity focused ultrasound
The foundations of HIFU are to utilise thermal energy produced from ultrasound waves to destroy cancerous cells with heat. Ultrasound waves are absorbed by the prostate and converted to heat, raising the temperature of the prostate to between 70–80 °C [Citation10]. Heating prostatic tissue disrupts the continuity of the cells’ lipid membranes, causes enzyme degradation and decreases oxygen supply to the prostate. The enzyme denaturation and hypoxia causes subsequent coagulative necrosis of tumours while avoiding exposure of the prostate to any instrument or needle [Citation11,Citation12]. Increasing the temperature beyond the boiling point of the tissue forms gas and creates air pockets within the tissue. This may be achieved through either raising the temperature past the tissue’s boiling point, or via either inertial or stable cavitation. Inertial cavitation involves a rapid increase in temperature of gas-micro bubbles that may disperse into the surrounding environment as a shock wave [Citation10]. Due to the unpredictable nature of inertial cavitation, it is avoided during HIFU. In stable cavitation, micro-bubble oscillations within the tissue may result in sheering forces and viscous-damping heating. Although there is some evidence that stable cavitation improves tissue destruction, it is currently avoided in clinical practice.
Focal laser ablation
Focal laser ablation (FLA) is defined as the thermal destruction of tissue by laser. In FLA, a focused beam of electromagnetic radiation delivers photons to rapidly elevate prostatic tissue temperature. The prostate is an organ well suited for FLA due to its optimal absorption rate, water content, and absence of excess vascularity, which may limit ablation size [Citation13]. Laser with a wavelength between 590 and 1064 nm is administered to induce thermal tissue damage [Citation14,Citation15]. Temperatures >60 °C cause protein denaturation, coagulative necrosis, and cell death [Citation16]. Subsequent rises in temperature >100 °C cause destruction of cellular organelles, gland shrinkage, and dehydration. Unique to focal laser ablation is the real-time monitoring of tissue destruction via proton resonance-free MR thermometry and contrast-enhanced ultrasonography (CEUS) [Citation17]. Following shrinking and desiccation of the prostate, subsequent laser energy causes drastic elevations in temperature. Temperatures greater than 300 °C can burn the prostate and cause carbonisation, attenuating the depth of laser penetration and limiting the size of the lesion produced. However, MR thermometry allows surgeons to visualise changes in temperature intraoperatively, ensuring minimal damage to surrounding tissues while adequately ablating the tumour. In addition, the ablated area will experience a marked reduction in blood perfusion, and no uptake of microbubbles. This will cause an absence of CEUS signal, allowing for accurate differentiation of ablated and healthy tissue.
Irreversible electroporation
Irreversible electroporation utilises short (microsecond to millisecond) high voltage pulses up to 3 kV via the Nanoknife™ system (manufactured by Angiodynamics, Albany, NY) to ablate cancerous cells without inducing thermal damage [Citation18]. Using electric field strengths greater than the threshold value of the cell membrane (20–40 A), a high voltage electrical current manipulates the permeability of the cell membrane. In a phenomenon called ‘poration’, the electric fields generated create nanopores in the cell’s lipid bilayer, allowing normally impermeable matter to freely move into the cytoplasm [Citation19]. The foundations of electroporation have been applied in clinical practice to facilitate the effect of chemotherapy drugs bleomycin and cisplatin [Citation13,Citation19]. The exact mechanism by which IRE induces cell death is currently unknown; however, initial results from both in vivo and in vitro IRE studies show promising results in destruction of various solid organ cancers [Citation18,Citation20,Citation21]. Studies on focal IRE of canine prostates found that the procedure spared the animal’s urethra, urethral sphincter, and neurovascular bundles from damage, a desired outcome in patients with low-grade disease who still wish to undergo definitive treatment [Citation22].
Clinical outcomes
Irreversible electroporation, focal laser ablation
Due to the novelty of both focal laser ablation and irreversible electroporation, all published studies to date consist of small sample sizes and short oncological follow-up. Nevertheless, these investigations reveal the side effects and possible adverse events following each of these procedures, respectively ().
Table 1. Pre-operative characteristics and clinical outcomes in an irreversible electroporation study.
A study by Valerio et al. exemplifies encouraging genito-urinary outcomes in patients following IRE [Citation23]. All patients continent before treatment maintained continence and only one (5%) reported new onset erectile dysfunction. Catalogued adverse events were haematuria and/or debris, dysuria, urinary tract infection, and urinary retention; however, all adverse events are classified as grade 1 or 2 according to the US National Cancer Institute’s common terminology criteria for adverse events (NCI-CTCAE). Follow-up in this study (median 6 months, range 1–24 months) is inadequate to determine cancer control rates following IRE. However, Valerio and colleagues reported four (16%) patients in this study moving on to secondary definitive therapy, with two receiving HIFU, one receiving IRE, and one undergoing radical prostatectomy.
Phase 1 clinical trials reported by Lepor et al. [Citation24], Lindner et al. [Citation25], and Oto et al. [Citation26] demonstrate the short term effectiveness of focal laser ablation in low risk patients. Perioperatively there were no complications in any of these series. Patients who underwent FLA in these studies reported no new-onset erectile dysfunction and no significant change in the Sexual Health Inventory for Men (SHIM) score, the International Index of Erectile Function (IIEF)-5 score, the American Urological Association Symptom Score (AUASS) or International Prostate Symptom Score (IPSS).
In Lepor’s cohort of 25 men, seven were unable to pass a trial of void immediately after the procedure and were subsequently catheterised. All of these men were able to spontaneously urinate within 3 days after laser ablation. Patients who underwent ablation experienced a mean PSA decrease of 2.3 ng/mL (44%). In total 28 sites were biopsied from the ablation zone 3 months after focal laser ablation, with only one (4%) showing Gleason 7 (3 + 4) disease.
In a cohort of 12 men, Linder and colleagues reported nine patients (75%) were discharged catheter free the same day, with the remaining three men (25%) discharged the next day uneventfully. Post-operative morbidities included perineal discomfort in three patients (25%), mild haematuria in two patients (17%), haematospermia in one patient (8%), and fatigue in one patient (8%), all of which spontaneously subsided without intervention. Re-biopsy of the ablated region 3–6 months after FLA revealed minimal disease in two patients (17%, Gleason 6 disease, ≤5% of core length, one core affected) and significant disease in two patients (17%, Gleason 6 disease, >50% of core length).
Oto et al.’s study [Citation26] demonstrated similar results, with only perineal abrasion and transient paraesthesia of the glans penis being reported as adverse events in one patient each, respectively. MR-guided re-biopsy of the ablated zone revealed Gleason 6 disease in only two patients (22%).
Similar to irreversible electroporation, all focal laser ablation studies published at the time of writing lack the follow-up and sample size necessary to truly determine its oncologic effectiveness. Nonetheless, these studies accurately depict the feasibility and safety of focal laser ablation in a clinical setting ().
Table 2. Pre-operative characteristics and clinical outcomes following focal laser ablation.
Table 3. Pre-operative characteristics and clinical outcomes in a large primary focal cryotherapy and primary HIFU study.
Cryotherapy, high intensity focused ultrasound
A recent analysis by Ward and colleagues of the cryo-line database (COLD) registry represents the largest publication of primary focal cryotherapy outcomes to date [Citation27]. In this analysis, 1160 men with an average follow up of 21.1 months demonstrated favourable clinical characteristics following focal cryosurgery. Biochemical recurrence-free survival (ASTRO) rates at 6, 12, 24, and 36 months were 84.2%, 80.7%, 75.7%, and 75.7%, respectively; 14.1% of the study cohort experienced a rise in PSA substantial enough to warrant re-biopsy following cryosurgery. Only 26.3% of re-biopsies were positive, representing 3.7% of the entire cohort. In addition, eight (1.6%) experienced urinary incontinence, 122 (41.9%) had new onset erectile dysfunction, and six (1.2%) went into urinary retention following cryotherapy. One patient (0.1%) experienced recto-urethral fistula. Smaller studies have been published highlighting the effectiveness of cryotherapy in a primary setting, with incontinence and erectile dysfunction rates ranging from 0–3.6% and 0–42%, respectively [Citation28]. These publications reported biochemical disease-free survival rates between 71 and 93% at follow-up ranging from 9 to 70 months, with metastasis-free and cancer-specific survival rates reaching as high as 100%.
A study conducted by Uchida and colleagues from 1999 to 2012 reported long-term outcomes following treatment of prostate cancer using HIFU [Citation29]. This study of 918 men demonstrated similar functional outcomes to that of Ward and Jones [Citation27]. In total, 21 men (2.3%) experienced urinary incontinence, and 37 (34.9%) had erectile dysfunction 2 years after HIFU. One patient (0.1%) experienced recto-urethral fistula. Biochemical recurrence-free survival (ASTRO) rates at 5 and 10 years were 57.1% and 48.8%, respectively. Numerous HIFU studies report similar oncological outcomes, with biochemical disease-free survival rates at 5 and 10 years ranging from 46–84% and 32–71%, respectively [Citation29,Citation30]. In addition, urinary incontinence and erectile dysfunction rates in HIFU publications range from 1–15% and 13–53%, respectively.
An additional HIFU study prospectively monitored 42 patients following focal HIFU [Citation33]. This study used validated IIEF, Expanded Prostate Cancer Index Composite (EPIC), IPSS, and Functional Assessment of Cancer Therapy Prostate (FACT-P) questionnaires to assess genito-urinary function before and after thermal ablation. While there was an observed decline in genito-urinary function 1 month and 3 months following HIFU, 40 of these men maintained pad-free continence 12 months after the procedure. Of 35 men with erectile function satisfactory for penetration before HIFU, 31 maintained potency at the conclusion of the study. A total of 39 of these men were re-biopsied 6 months after treatment, revealing cancer in nine (23%) of these men. However, only 8% of these cancers, were clinically significant as classified by the Epstein criteria (Gleason >3 + 3, > 2 cores positive, > 2 mm cancer involvement [Citation34,Citation35]).
Discussion
This review establishes minimally invasive treatment modalities to be as feasible and safe as whole gland therapies in the treatment of men with focal prostate cancer, with several studies publishing minimal adverse events and treatment-related side effects post-operatively. Nevertheless, there are controversies regarding focal therapy as well as several limitations in this review.
At the time of writing there is no consensus on how to determine biochemical recurrence-free survival in a focal setting. Present-day investigators typically report biochemical recurrence using either Phoenix or American Society for Therapeutic Radiology and Oncology (ASTRO) criteria. These criteria were created to establish a definition of failure using PSA kinetics following external beam radiotherapy (EBRT). However, EBRT treats the entire prostate while focal therapies leave benign tissue intact, which continue to secrete PSA after treatment. Furthermore, the mechanism of cell death in each modality is fundamentally unique, and thus each form of therapy requires distinct definitions of biochemical recurrence.
In light of the emerging research surrounding the effectiveness of focal therapy, these treatments should only be administered to patients who will benefit from minimally invasive treatment. There has been a recent controversy surrounding the screening and overtreatment of clinically insignificant prostate cancers [Citation31,Citation32]. Active surveillance is safe for men harbouring indolent PCa. Although the focal treatments stated herein are associated with minimal genito-urinary effects, active surveillance may be appropriate for older men who may be unsuitable for definitive treatment, or men with very low risk of distant metastasis. In addition, leaving cancer untreated may result in significant anxiety in these men, and it is important to acknowledge that the treatment a man decides to undergo is ultimately their decision. Focal therapies should not be substituted for active surveillance when definitive treatment is unnecessary, nor should they replace radical whole-gland therapy in high risk patients. Focal therapies should only be recommended to patients with clinically significant, localised disease. These patients should subsequently be followed similarly to those on active surveillance, as tumorigenesis is still feasible and these men may still be harbouring clinically undetectable cancer.
Of note, whole gland treatments via radical prostatectomy or radiation therapy have been proven to be safe and offer strong oncological control. However, erectile dysfunction rates following these treatment modalities may reach 75% and 60% each, respectively [Citation36–40]. In addition, incontinence rates following radical prostatectomy and radiation therapy range from 9–15% and 4–5%, respectively [Citation40,Citation41]. Given the side effects often associated with whole gland therapy, men may elect to undergo focal treatment to eradicate cancer while preserving health-related quality of life.
A major limitation in this review is the short follow-up in focal laser ablation and irreversible electroporation studies, which was emphasised throughout. Unfortunately, clinicians cannot come to a consensus on which treatment provides the most effective long-term oncological control without comparing long-term mortality outcomes. Due to the novelty of focal laser ablation and irreversible electroporation, this data is not readily available. Prospective, comprehensive studies must be completed for each treatment modality to determine which focal therapy has the most encouraging outcome on an individualised basis.
Conclusion
Focal therapy’s intentions are to ablate cancerous tissue without damaging surrounding bodily structures in an attempt to maintain health-related quality of life. Emerging treatment modalities have strong short-term oncological control and biochemical disease-free survival rates; however, further prospective studies with longer follow-up are necessary to validate these findings. Nevertheless, the minimally invasive procedures stated herein are feasible and safe, with patients reporting minimal genito-urinary symptoms and adverse events post-operatively. Although these treatment options are enticing, they should not be recommended in place of active surveillance in men with clinically insignificant disease who will not benefit from definitive treatment.
Disclosure statement
The authors report no conflict of interest and are solely responsible for the writing of this article.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;1:5–29.
- Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection in the rising incidence of prostate cancer. JAMA 1995;273:548–52.
- Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA 1993;270:948–54.
- Jacobsen SJ, Katusic SK, Bergstralh EJ, Oesterling JE, Ohrt D, Klee GG, et al. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA 1995;274:1445–9.
- Haas GP, Delongchamps N, Brawley OW. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol 2008;15:3866–71
- Cooperberg M, Broering J, Carroll P. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol 2010;28:1117–23.
- Ganz PA, Barry JM, Burke W, Col NF, Corso PS, Dodson E, et al. National Institutes of Health State-of-the-Science Conference: role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med 2012;156:591–5.
- Nomura T, Mimata H. Focal therapy in the management of prostate cancer: an emerging approach for localized prostate cancer. Adv Urol 2012;2012:391437.
- Katz AE. The definitive guide to prostate cancer: everything you need to know about conventional and integrative therapies. New York: Rodale, 2011.
- Alkhorayef M, Mahmoud MZ, Alzimami KS, Sulieman A, Fagiri MA. High-intensity focused ultrasound (HIFU) in localized prostate cancer treatment. Pol J Radiol2015;80:131–41.
- Gelet A, Chaperon JY, Bouvier R, Souchon R, Pangaud C, Abdelrahim AF, et al. Treatment of prostate cancer with transrectal focused ultrasound: early clinical experience. Eur Urol1996;29:174–83.
- Uchida T, Nakano M, Hongo S, Shoji S, Nagata Y, Satoh T, et al. High intensity focused ultrasound therapy for prostate cancer. Int J Urol 2012;19:187–201.
- Lindner U, Lawrentschuk N, Trachtenberg J. Focal laser ablation for localized prostate cancer. J Endourol 2010;24:791–7.
- Huang GT, Wang TH, Sheu JC, Daikuzono N, Sung JL, Wu MZ, Chen DS. Low power laserthermia for the treatment of small hepatocellular carcinoma. Eur J Cancer 1991;27:1622–7.
- Colin P, Mordon S, Nevoux P, Marqa MF, Ouzzane A, Puech P, et al. Focal laser ablation of prostate cancer: definition, needs and future. Adv Urol 2012;2012:589160.
- Ritchie KP, Keller BM, Syed KM, Lepock JR. Hyperthermia (heat shock)-induced protein denaturation in liver, muscle and lens tissue as determined by differential scanning calorimetry. Int J Hyperthermia 1994;10:605–18.
- Lee T, Mendhiratta N, Sperling D, Lepor H. Focal laser ablation for localized prostate cancer: principles, clinical trials, and our initial experience. Rev Urol 2014;16:55–66.
- Miller L, Leor J, Rubinsky B. Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat 2005;4:699–705.
- Long G, Bakos G, Shires PK, Gritter L, Crissman JW, Harris JL, Clymer JW. Histological and finite element analysis of cell death due to irreversible electroporation. Technol Cancer Res Treat 2014;13:561–9.
- Thomson KR, Cheung W, Ellis SJ, Federman D, Kavnoudias H, Loader-Oliver D, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 2011;22:611–21.
- Kasivisvanathan V, Thapar A, Oskrochi Y, Picard J, Leen EL. Irreversible electroporation for focal ablation at the porta hepatis. Cardiovasc Intervent Radiol 2012;35:1531–4.
- Tsivian M., Polascik T.J. Bilateral focal ablation of prostate tissue using low-energy direct current (LEDC): a preclinical canine study. BJU Int 2013;112:526–30.
- Valerio M, Stricker PD, Ahmed HU, Dickinson L, Ponsky L, Shnier R, et al. Initial assessment of safety and clinical feasibility of irreversible electroporation in the focal treatment of prostate cancer. Prostate cancer Prostatic Dis 2014;17:343–7.
- Lepor H, Llukani E, Sperling D, Futterer JJ. Complications, recovery, and early functional outcomes and oncologic control following in-bore focal laser ablation of prostate cancer. Eur Urol 2015;68:924–6.
- Lindner U, Weersink RA, Haider MA, Gertner MR, Davidson SR, Atri M, et al. Image guided photothermal focal therapy for localized prostate cancer: phase 1 trial. J Urol 2009;182:1371–7.
- Oto A, Sethi I, Karczmar G, McNichols R, Ivancevic MK, Stadler WM, et al. MRI imaging-guided focal laser ablation for prostate cancer: phase 1 trial. Radiology 2013;267:932–40.
- Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: a report from the national cryo on-line database (COLD) registry. BJU Int 2011;109:1648–54.
- Shah TT, Ahmed H, Kanthabalan A, Lau B, Ghei M, Maraj B, Arya M. Focal cryotherapy of localized prostate cancer: a systematic review of the literature. Expert Rev Anticancer Ther 2014;41:1337–47.
- Uchida T, Tomonaga T, Kim H, Nakano M, Shoji S, Nagata Y, Terachi T,. Improved outcomes with advancements in high intensity focused ultrasound for the treatment of localized prostate cancer. J Urol 2015;193:103–10.
- Warmuth M, Johansson T, Mad P. Systematic review of the efficacy and safety of high intensity focused ultrasound for the primary and salvage treatment of prostate cancer. Eur Urol 2010;58:803–15.
- Penson D. The pendulum of prostate cancer screening. JAMA 2015;314:2031–3.
- Jemal A, Fedewa S, Ma J, Siegel R, Lin CC, Brawley O, Ward EM. Prostate cancer incidence and PSA testing patterns in relation to USPSTY screening recommendations. JAMA 2015;314:2054–61.
- Ahmed H, Hindley R, Dickinson L, Freeman A, Kirkham AP, Sahu M, et al. Focal therapy for localized unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol 2012;13:622–32.
- Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994;271:368–74.
- Epstein JI. Prognostic significance of tumor volume in radical prostatectomy and needle biopsy specimens. J Urol 2011;186:790–7.
- Emanu J, Avildsen I, Nelson C. Erectile dysfunction after radical prostatectomy: prevalence, medical treatments, and psychosocial interventions. Curr Opin Support Palliat Care 2016;10:102–7.
- Ward JF, Slezak JM, Blute ML, Bergstralh EJ, Zincke H. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int 2005;95:751–6.
- Loeb S, Smith ND, Roehl KA, Catalona WJ. Intermediate-term potency, continence, and survival outcomes of radical prostatectomy for clinically high-risk or locally advanced prostate cancer. Urology 2007; 69:1170–5.
- Potosky A, Legler J, Albertsen P, Stanford JL, Gilliland FD, Hamilton AS, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the prostate cancer outcomes study. J Natl Cancer Inst 2000;92:1582–92.
- Salomon L, Droupy S, Yiou R, Soulié M. Functional results and treatment of functional dysfunctions after radical prostatectomy. Prog Urol 2015;25:1028–66.
- Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250–61.