Abstract
Purpose: To evaluate the effectiveness of whole-pelvic hyperthermia (HT) added to standard chemoradiotherapy (CRT) in locally advanced cervical cancer (CC), by investigating the clinical response and survival of patients treated with cisplatin-based CRT vs. CRT with HT (CRT + HT).
Materials and methods: This study was conducted at five hospitals in Japan between September 2001 and March 2015 in patients with the International Federation of Gynecology and Obstetrics stage IB (bulky)–IVA CC undergoing definitive CRT. After giving a written informed consent, patients were randomly allocated to two treatment groups: CRT and CRT + HT group. Overall survival (OS), disease-free survival (DFS), local relapse-free survival (LRFS), complete response (CR) rate and tolerability were evaluated.
Results: In total, 101 patients were treated. Patient characteristics, total dose of cisplatin and radiotherapy were similar for both groups. Although not statistically significant, the 5-year OS, DFS and LRFS in the CRT + HT group (77.8%, 70.8% and 80.1%, respectively) were better than those in the CRT group (64.8%, 60.6% and 71.0%, respectively). CR was significantly more likely to be achieved in patients in the CRT + HT group than in the CRT group (88% vs. 77.6%; adjusted odds ratio, 3.993; 95% confidence interval, 1.018–15.67; p = .047). CRT + HT was well tolerated and caused no additional acute or long-term toxicity compared with CRT alone.
Conclusions: HT combined with CRT improved the CR rate of CRT in patients with locally advanced CC, however, could not improve survival outcomes. Further studies in larger samples are warranted.
Introduction
Cervical cancer (CC) is one of the most common types of cancer, both in incidence and mortality, affecting women worldwide [Citation1]. In patients with early stage CC, radical surgery can be an effective treatment modality, whereas in patients with more advanced stage CC, cisplatin-based concomitant chemoradiotherapy (CRT) is generally administered. So far, five large-sample randomised studies have demonstrated the efficacy of CRT [Citation2–6].
Hyperthermia (HT) has also been shown to be effective in the treatment of CC [Citation7]. Two randomised phase III trials comparing patient groups receiving radiotherapy (RT) or RT + HT as a primary treatment for locally advanced CC showed higher complete response (CR) rates and better overall survival (OS) in the combination therapy group [Citation8,Citation9]. Furthermore, it was reported that HT enhanced the activity of chemotherapy, particularly that with cisplatin [Citation10]. The combination of cisplatin chemotherapy and loco-regional HT improved response rates and survival in patients with recurrent CC with no additional toxicity [Citation11,Citation12].
The findings of these studies suggest that a combination of all three treatments (RT, chemotherapy and HT) could further yield better clinical outcomes in patients with locally advanced CC. According to Westermann et al. [Citation13], in 68 patients prospectively registered in the USA, Norway and the Netherlands and treated with a combination of RT, weekly cisplatin and loco-regional HT, 5-year relapse-free survival and 5-year OS were similar or better compared with those in patients treated with standard CRT. However, there has been no randomised clinical trial comparing CRT + HT with CRT alone in patients with advanced CC.
Here, we report a Japanese multicentre randomised clinical trial of concurrent CRT + HT vs. CRT alone to evaluate the effectiveness and safety of this combination therapy in the daily clinical settings in patients with locally advanced CC.
Materials and methods
Patients
The present study was a prospective, multicentre, randomised, parallel-group study conducted at five Japanese institutions by the Japanese Society of Hyperthermic Oncology (JASHO) group between September 2001 and March 2015.
The study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was approved by the Kansai Medical University Review Board. All participants signed an informed consent form approved by each centre’s institutional review board.
Consecutive patients with CC were screened for enrolment in the study. The eligibility criteria were as follows:
International Federation of Gynecology and Obstetrics (FIGO) stages [Citation14] IB bulky (≥4 cm diameter), IIA, IIB, IIIA, IIIB or IVA;
Eastern Cooperative Oncology Group (ECOG) performance status 0–2;
no history of RT, chemotherapy or surgery;
para-aortic lymph nodes negative for metastasis;
no double cancer;
no pacemaker;
adequate haematologic function: leukocyte count ≥3000/μL, platelet count ≥100 × 103/μL and haemoglobin level ≥10.0 g/dL;
adequate renal, hepatic and cardiac functions as well as levels of glutamic oxaloacetic transaminase and glutamic pyruvic transaminase ≤35 IU/L, total bilirubin ≤1.5 mg/dL and serum creatinine ≤0.8 mg/dL, with creatinine clearance ≥60 mL/min and normal electrocardiogram or electrocardiographic changes (not requiring treatment);
feasibility of cisplatin treatment;
feasibility of HT;
written informed consent.
Exclusion criteria included the following:
pregnancy or lactation;
distant metastasis;
serious, uncontrolled concurrent medical or neurologic conditions;
chronic heart failure or heart failure in the previous 3 months;
cerebral vascular disorder in the previous 3 months;
active infection.
Pre-randomisation evaluation included taking a medical history and physical examination, blood chemistry profile, chest radiograph, computed tomography (CT), magnetic resonance imaging (MRI), fluorodeoxyglucose-positron emission tomography/contrast-enhanced CT and biopsy.
Treatment
Chemoradiotherapy
Whole-pelvis radiotherapy was provided using 6- or 10-MV high-energy linear accelerators. An additional dose was given to the parametria with central shielding. The radiation was delivered to the tumour in fractions of 1.8–2 Gy daily, 5 days per week, with a standard 2- or 4-technique. Intensity-modulated radiotherapy was not permitted in this study. The patients additionally received 192Ir high-dose-rate intracavitary brachytherapy. The 192Ir brachytherapy was given to point A (2 cm lateral to the central canal of the uterus and 2 cm above the mucous membrane of the lateral fornix in the axis of the uterus) [Citation15] at a dose of 5–6 Gy per session once per week. Additionally, patients were treated weekly with cisplatin at a dose of 30–40 mg/m2 for 3–5 cycles.
Hyperthermia
Regional whole-pelvis HT was administered on a weekly basis concurrent with cisplatin-CRT, but not with brachytherapy. Treatment was given in succession in the following order: cisplatin, RT and HT. For all HT treatments, HT was delivered via a radiofrequency capacitive heating device (Thermotron RF-8, Yamamoto Vinita Com, Osaka, Japan), which uses 8 MHz radiofrequency electromagnetic waves as a source of heat. The output power ranged from 800 to 1500 W. Deep regional HT was applied within 30 min of external RT for 60 min once a week during an external beam RT course. For thermometry, a 4-point micro-thermocouple sensor was placed in the rectum and vagina. The heating procedure was carried out as previously described [Citation9]. The maximum tumour temperature (Tmax) was defined as the maximum temperature obtained in the rectum and vagina during the steady state at the end of treatment. All parameters were determined for each treatment session, and the averages of these parameters (Tave) were calculated over all treatments for a given tumour. In addition, the cumulative equivalent minutes at 43 °C for T90 (CEM43T90) were obtained as described by Fatehi et al. [Citation16] during all sessions with temperature measurements.
Evaluation of treatment
The primary endpoint was 5-year OS. The secondary endpoints included CR rate, 5-year disease-free survival (DFS), 5-year local relapse-free survival (LRFS) and acute- or late-phase toxicity. OS (all-cause death as an outcome event), DFS (all-cause death, local relapse or distant metastasis as outcome events) and LRFS (local relapse as an outcome event) were estimated as months from the enrolment to the date of occurrence of the outcome events or to the date of the last observation for patients who were censored. Regarding DFS and LRFS, non-CR of the tumour was considered as local relapse on the day the patient completed study treatment.
CR was achieved when no tumour was detected on physical examination or MRI, with cytology or biopsy results negative for malignant cells for at least 1 month after the treatment. CR rate was calculated using the number of patients who achieved CR as the numerator and the number of evaluated therapeutic responses as the denominator.
Acute-phase treatment-related toxicity was graded according to a modification of the Radiation Therapy Oncology Group (RTOG) morbidity scale [Citation17]. Clinical items and graded adverse events included haemoglobin, white blood cell count, platelet count, nausea, vomiting, diarrhoea, urinary, weight loss, fatigue, nephrotoxicity, and HT-related blistering and fat necrosis.
Patients were examined every month during 1 year of treatment and every 2 months thereafter. CR rate and acute-phase toxicity were evaluated 1 month after the completion of protocol treatment, and OS, DFS, LRFS and late-phase toxicity were assessed during the follow-up period until March 2015.
Study design
Eligible participants were randomised at the central data centre before the initiation of treatment according to a computer-generated random number list into two treatment groups: those treated with CRT alone and those treated with CRT + HT. The sample size was calculated to detect a difference in CR rates between the groups, assuming a CR rate of 50% for the CRT group and 80% for the CRT + HT group based on the results of our previous study [Citation9]. Fisher’s exact test with a 0.05 two-sided significance level would have an 80% power if the sample size in each group was 44. Allowing for a certain number of dropouts, a sample size of 50 patients for each group was determined.
Statistical analysis
To evaluate the effectiveness and safety of the protocol treatments in a daily clinical setting, the intent-to-treat approach was applied for statistical analysis. Patient characteristics were summarised as mean (range) for continuous variables and as number (%) for categorical variables. Clinical items for tabulation were as follows: age, tumour size, FIGO stage, histology, treatment duration, total dose of RT (external RT and brachytherapy), frequency of chemotherapy, total dose of cisplatin, number of HT treatments and thermometry results (Tmax, Tave and CEM43T90) for the CRT + HT group, and follow-up duration. Survival rates were estimated using the Kaplan–Meier method [Citation18] and differences in the 5-year survival between the treatments were examined using the log-rank test [Citation19]. Further analyses were conducted for survival outcomes by using multivariable Cox proportional hazards models [Citation20] adjusting for known clinical factors (age, FIGO stage and histology). Adjusted hazard ratio (HR) and 95% confidence interval (CI) of the CRT + HT group (reference: CRT group) for each outcome was estimated and tested for significance.
The association of CR rate with treatment groups was examined using cross tabulation and Fisher’s exact test. Further analysis was conducted for CR rate using a multivariable logistic regression model [Citation21] with treatment group as an independent variable and age, FIGO stage and histology as adjusting covariates. Adjusted odds ratio (OR) and 95% CI of the CRT + HT group for CR were estimated and tested for significance.
Acute-/late-phase treatment-related toxicity was summarised as the number (%) of adverse events.
All statistical analyses were performed using IBM SPSS Statistics 23.0 (IBM Corp., Armonk, NY). A two-sided p value below .05 was considered significant for all statistical tests.
Results
Patient characteristics
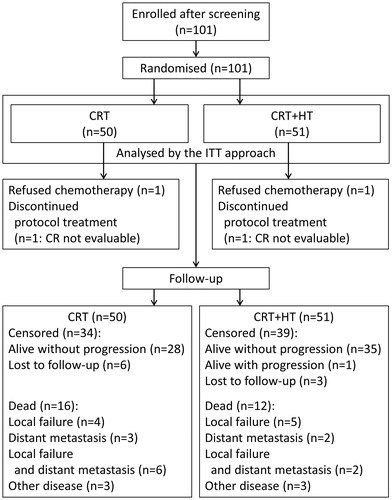
In total, 101 Japanese patients with CC with a mean age of 61.0 years (range, 24–87 years) fulfilled the eligibility criteria and were enrolled in the study from 3 September 2001 to 10 September 2013. Fifty patients (mean age, 62.1 years; range, 24–82) were randomised into the CRT group and 51 (mean age, 59.9 years; range, 30–87) were randomised into the CRT + HT group. Patients’ demographic characteristics, total dose of cisplatin and RT were similar in both groups (). Median follow-up durations for the CRT and CRT + HT groups were 47.1 and 63.1 months, respectively.
Table 1. Patient characteristics.
A patient flow diagram is presented in . One patient each in the CRT and CRT + HT groups rejected chemotherapy; both patients were treated with RT + HT. One patient in each group discontinued the treatment, and their therapeutic response could not be analysed (they were censored in the survival analyses). Of 101 randomised patients, 28 died (CRT + HT, n = 12; CRT, n = 16) during the follow-up period. In the CRT + HT group, 5 died due to local relapse, 2 due to distant metastasis, 2 due to local relapse and distant metastasis, and 3 due to other diseases, whereas in the CRT group, 4 died due to local relapse, 3 due to distant metastasis, 6 due to local relapse and distant metastasis, and 3 due to other diseases. Regarding the censored cases, at the time of the last observations, in the CRT + HT group, 35 patients were alive without progression, 1 was alive with progression and 3 were lost to follow-up, whereas in the CRT group, 28 were alive without progression and 6 were lost to follow-up.
Thermometry results
In the CRT + HT group, the thermometry results demonstrated a Tmax of 42.2 °C ± 0.9 °C (range, 40.1–44.6 °C), Tave of 41.1 °C ± 0.7 °C (range, 39.6–42.5 °C) and a CEM43T90 of 3.8 min (range, 0.1–46.6 min) ().
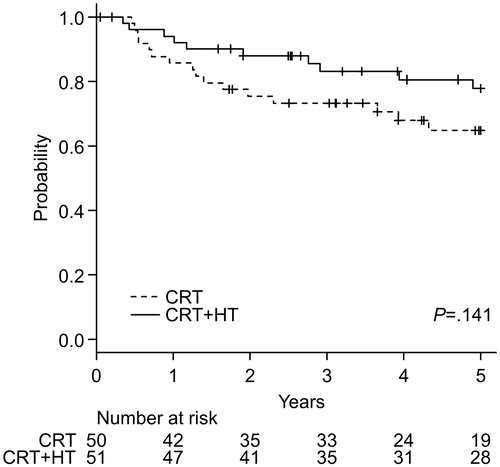
Overall survival
shows Kaplan–Meier curves for OS of both groups. In the CRT + HT group, the 5-year survival rate was 77.8% (95% CI, 62.3–87.5%), whereas that in the CRT group was 64.8% (95% CI, 48.7–77.0%). The log-rank test showed no significant difference between the two groups (p = .141). Adjusted HR (95% CI, p value) of the CRT + HT group for OS was 0.485 (0.217–1.082, p = .077).
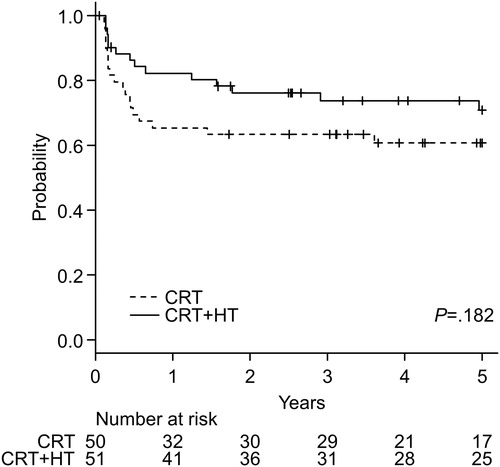
Disease-free survival
shows Kaplan–Meier curves for the DFS of both treatment groups. In the CRT + HT group, the 5-year survival rate was 70.8% (95% CI, 55.5–81.7%), whereas that in the CRT group was 60.6% (95% CI, 45.3–72.9%). The log-rank test showed no significant difference between the two groups (p = .182). Adjusted HR (95% CI, p value) of the CRT + HT group for DFS was 0.517 (0.251–1.065, p = .073).
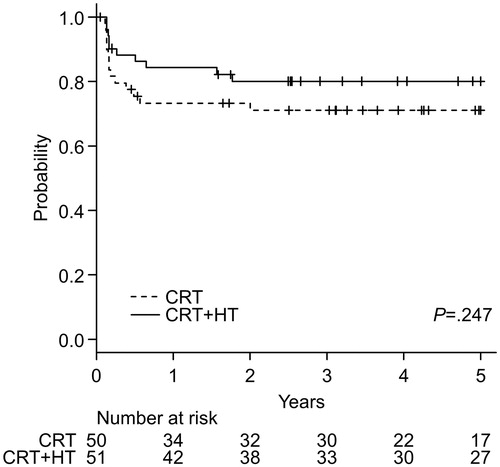
Local relapse-free survival
shows Kaplan–Meier curves for the LRFS of both treatment groups. In the CRT + HT group, the 5-year survival rate was 80.1% (95% CI, 66.1–88.8%), whereas that in the CRT group was 71.0% (95% CI, 56.0–81.7%). The log-rank test showed no significant difference between the two groups (p = .247). Adjusted HR (95% CI, p value) of the CRT + HT group for DFS was 0.475 (0.202–1.116, p = .087).
CR rate
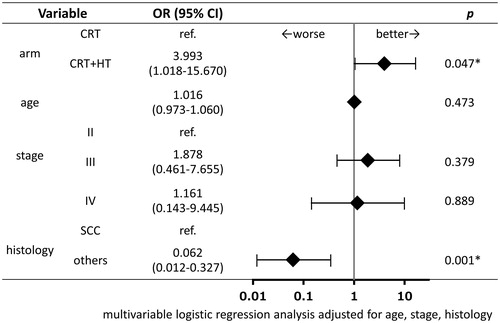
After the completion of the protocol treatment, a CR was achieved in 44 of 50 patients (88.0%) in the CRT + HT group and in 38 of 49 patients (77.6%) in the CRT group. Fisher’s exact test showed no significant association between CR rates and treatment groups (p = .192). Further analysis using a multivariable logistic regression model adjusted for relevant clinical factors (age, FIGO stage and histology) indicated that patients in the CRT + HT group were significantly more likely to achieve a CR than those in the CRT group (OR, 3.993; 95% CI, 1.018–15.670; p = .047; ).
Acute- and late-phase toxicity
Acute-phase toxicity is summarised in . In the analysed 101 patients, the presence of toxicity and RTOG grades were similar between the two groups. Except for haematologic toxicity related to standard CRT, only 1 patient in the CRT group experienced ≥ grade 3 adverse effects (grade 3 fatigue). Late-phase toxicity is summarised in , showing similar toxicity in both groups. No blistering or fat necrosis related to HT was observed in the CRT + HT group.
Table 2. Acute-phase adverse events.
Table 3. Late-phase adverse events.
Discussion
In the last decade, cisplatin-containing CRT has gained widespread acceptance in the treatment of advanced CC after the positive reports of five large randomised studies [Citation2–6]. Most reviews concluded that there was a beneficial effect of CRT at least in patients with FIGO stage I and II malignancies. However, in patients with a more advanced stage CC, it was rather difficult to achieve local control with CRT alone, raising a question whether the beneficial effect of CRT in early stage CC could extend to a more advanced stage CC. A recent systematic review confirmed the suspicion that the improvement in 5-year OS may only be 3% in patients with FIGO stage III–IVA CC [Citation22].
In 2010, Lutgens et al. [Citation7] published a systematic review comparing the combined use of HT and RT with RT alone for treating locally advanced CC. They reported that RT combined with HT yielded superior local tumour control rates (RR, 0.56; 95% CI, 0.39–0.79; p < .001) and OS (HR, 0.67; 95% CI, 0.45–0.99; p = .05) compared with those yielded by RT alone. With regards to the safety analysis, it was reported that the pooled data including 310 study patients showed no difference in the acute treatment-related toxicity between the two treatment groups (RR, 0.99; 95% CI, 0.30–3.31; p = .99). Similarly, pooled data including 264 study patients indicated no difference in the late toxicity between the two groups (RR, 1.01; 95% CI, 0.44–2.30; p = .98). It was concluded that RT combined with HT was more effective and caused no additional toxicity compared with RT alone for patients with locally advanced CC.
In 2005, Vasanthan et al. [Citation23] published a negative result for HT in 110 patients with FIGO stage IIB–IV CC treated with RT; they reported no beneficial effects on either survival or local control at a 3-year follow-up. In their study, HT was applied using an 8-MHz capacitive system with intravaginal electrodes in most patients. In 2013, Zolciak-Siwinska et al. [Citation24] reported a phase III study to investigate whether interstitial HT combined with high-dose brachytherapy had any influence on local control, DFS or acute and late side effects in patients with advanced CC. They also concluded that HT did not significantly influence failure or interactions with potential prognostic factors for local control or DFS. It is possible that the HT procedure used in these two studies resulted only in heating the cervix and not the entire pelvis, presumably because intravaginal electrodes were used to heat up the tumour volume. Although very high temperatures can be achieved at the tumour’s intravaginal surface with this method, temperatures may rapidly decline at the tumour’s periphery.
From the point of view that concurrent CRT and concurrent whole-pelvic HT with RT both increased the effectiveness of RT for CC, we proposed a combination treatment for all three modalities: RT, chemotherapy and HT to further improve the outcomes. We conducted the present randomised clinical study to evaluate the effectiveness and safety of this combination therapy compared with CRT alone.
Our results suggest that with respect to CR rates, CRT combined with whole-pelvic HT is a potentially promising treatment compared with CRT alone in patients with locally advanced CC. Furthermore, our study suggests better OS and DFS for the CRT + HT group; however, the differences between groups were not significant. The most common side effects were haematologic toxicity, with no difference in the occurrence rates of grade 3 or worse adverse events in both groups. No thermotoxicity was observed in the CRT + HT group. Although cisplatin significantly increased the side effects of RT, HT did not change the toxicity profile of RT.
Van der Zee et al. [Citation8] previously reported the results of a randomised multicentre trial evaluating CR rate and duration of local control of RT + CT compared with RT alone in patients with cervical, bladder or rectal cancer. In their study, initially displayed significant difference in local control rate between groups disappeared during the long-term follow-up period in patients with bladder cancer. They discussed that RT could kill enough cells to establish a clinical CR but did not sterilise all clonogenic tumour cells, leading to such inconsistency between short- and long-term local control of tumours. In our study as well, initial significant difference in CR rate turned not significant in the long-term local control rate (LRFS). This phenomenon may be explained in the same way as a specific characteristic of RT.
It is important to discuss the results in the context of the study settings. The main limitation of this study was that the effectiveness of standard CRT observed was far higher than that assumed for the sample size calculation (CR rate of 77.6%, instead of 50%), requiring an unrealistically large difference in effect size or a much larger sample size in order to demonstrate significance. Furthermore, regarding survival rates, both the 5-year OS and DFS (64.8% and 60.6%, respectively) in the CRT group seemed quite high considering that 73.3% (74 of 101) of the enrolled patients had FIGO stage III–IVA CC. Such results may be due to the following two eligibility criteria of our study: excluding para-aortic node-positive patients and requiring haemoglobin levels ≥10 g/dL.
The presence of positive para-aortic lymph nodes is a known poor prognostic factor in patients with CC. Retrospective studies conducted on this population have demonstrated 5-year survival of about 30% [Citation25].
Another known poor prognostic factor in patients with CC having CRT is anaemia [Citation26]. Bishop et al. investigated the association between anaemia and clinical outcomes before and after the introduction of CRT in such patients. For the entire cohort, patients with haemoglobin concentrations higher than 10 g/dL had significantly better disease-specific survival rates than those with concentrations lower than 10 g/dL (p < .001) in a univariable analysis [Citation27].
Considering the ethical feasibility of the present interventional randomised controlled trial, we decided to set conservative criteria based on aortic lymph nodes and anaemia compared with other published single-arm studies.
Westermann et al. [Citation28] analysed prospective survival data for a combination of CRT and HT in 68 patients with stage IIB–IVA CC in 2005. They also reported the same patients’ long-term survival rates in 2012 [Citation13]. In their long-term follow-up study, a 5-year OS of 66.1% (95% CI, 55.1–79.3%) and 5-year relapse-free survival rate of 57.5% (95% CI, 46.6–71.0%) were observed. In our study, 5-year OS and DFS in the CRT + HT group were 77.8% and 70.9%, respectively, apparently demonstrating better survival outcomes than those reported in the Westermann et al. paper. However, para-aortic node-positive patients were included in their study. Moreover, in their study, low levels of haemoglobin were used as eligibility criterion compared with those used in ours (≥7.0 compared with ≥10.0, respectively). It seems pertinent to interpret the results in the light of the differences in patients’ characteristics. The CR rate in 68 patients with CC treated with CRT + HT reported by Westermann et al. was 90% [Citation28], which was almost the same as 88.0% (44 of 50 patients) achieved in the CRT + HT group in our study.
The relatively good survival outcomes in our study could also be interpreted in the context of the biological basis for combining HT and RT or chemotherapy, which includes two types of interactions: direct hyperthermic cytotoxicity (at temperatures of ≥42.5 °C) and radio- and/or chemosensitisation (at temperatures of <43 °C). Most of the tumour volume could be heated to radio- and/or chemosensitising temperatures in the range of 40–42 °C [Citation29]. In the present study, the average temperature was 41.1 °C ± 0.7 °C, which was in the radio- and/or chemosensitising range. The mechanism of tumour response to CRT + HT within this range of temperatures seems to involve the initiation of apoptosis through the activation of one of the BAX pathways that we have previously reported on [Citation30]. In addition, Eppink et al. [Citation31] reported that HT-induced DNA repair deficiency was enhanced by the inhibitors of the cellular heat-shock response. Moreover, recently, it has become clear that HT affects not only the cells in the treatment area but also the systemic immune response [Citation32]. These effects may as a whole play an important role in a combined treatment with CRT and HT.
As discussed above, the main limitation of the present study was a small sample size for detecting significance. However, it is difficult to enrol a large number of subjects in HT studies. Compared with the notably high numbers of randomised patients in chemotherapy studies [Citation2–6], those in HT studies are quite limited [Citation7]. This is because low availability of HT has so far resulted in quite a limited worldwide application of HT treatment to CC. Indeed, there are not many hospitals in Japan that use HT as a treatment modality for malignancies.
Our findings need to be validated in future phase III studies or meta-analyses consisting of larger sample sizes.
Conclusions
HT combined with CRT improved the CR rate of CRT in patients with locally advanced CC, however, it could not improve survival outcomes. Further studies in larger samples are warranted.
Disclosure statement
The authors report no conflicts of interest.
References
- Arbyn M, Castellsague X, de Sanjose S, . (2011). Worldwide burden of cervical cancer in 2008. Ann Oncol 22:2675–86.
- Rose PG, Bundy BN, Watkins EB, . (1999). Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 340:1144–53.
- Peters WA III, Liu PY, Barrett RJ II, . (2000). Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18:1606–13.
- Morris M, Eifel PJ, Lu J, . (1999). Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 340:1137–43.
- Keys HM, Bundy BN, Stehman FB 3rd, . (1999). Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 340:1154–61.
- Whitney CW, Sause W, Bundy BN Jr, . (1999). Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB–IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 17:1339–48.
- Lutgens L, van der Zee J, Pijls-Johannesma M, . (2010). Combined use of hyperthermia and radiation therapy for treating locally advanced cervical carcinoma. Cochrane Database Syst Rev CD006377.
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, . (2000). Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 355:1119–25.
- Harima Y, Nagata K, Harima K, . (2001). A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia 17:97–105.
- Urano M, Kahn J, Majima H, . (1990). The cytotoxic effect of cis-diamminedichloroplatinum(II) on cultured Chinese hamster ovary cells at elevated temperatures: Arrhenius plot analysis. Int J Hyperthermia 6:581–90.
- Rietbroek RC, Schilthuis MS, Bakker PJ, . (1997). Phase II trial of weekly locoregional hyperthermia and cisplatin in patients with a previously irradiated recurrent carcinoma of the uterine cervix. Cancer 79:935–43.
- de Wit R, van der Zee J, van der Burg ME, . (1999). A phase I/II study of combined weekly systemic cisplatin and locoregional hyperthermia in patients with previously irradiated recurrent carcinoma of the uterine cervix. Br J Cancer 80:1387–91.
- Westermann A, Mella O, Van Der Zee J, . (2012). Long-term survival data of triple modality treatment of stage IIB–III–IVA cervical cancer with the combination of radiotherapy, chemotherapy and hyperthermia – an update. Int J Hyperthermia 28:549–3.
- Benedet JL, Bender H, Jones H III, . (2000). FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet 70:209–62.
- Bethesda MD. (1985). Dose and volume specification for reorting intracavitary therapy in gynecology. International Commission of Radiation Units and Measurements.
- Fatehi D, van der Zee J, de Bruijne M, . (2007). RF-power and temperature data analysis of 444 patients with primary cervical cancer: deep hyperthermia using the Sigma-60 applicator is reproducible. Int J Hyperthermia 23:623–43.
- Trotti A, Byhardt R, Stetz J, . (2000). Common toxicity criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 47:13–47.
- Kaplan E, Meier P. (1958). Nonparametric estimation from incomplete observations. J Am Statist Assoc 53:457–81.
- Mantel N. (1966). Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163–70.
- Cox DR. (1992). Regression models and life-tables. Breakthroughs in statistics. New York: Springer; p. 527–41.
- Cox D. (1958). The regression analysis of binary sequences (with discussion). J R Stat Soc B 20:215–42.
- Vale C. (2008). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol 26:5802–12.
- Vasanthan A, Mitsumori M, Park JH, . (2005). Regional hyperthermia combined with radiotherapy for uterine cervical cancers: a multi-institutional prospective randomized trial of the international atomic energy agency. Int J Radiat Oncol Biol Phys 61:145–53.
- Zolciak-Siwinska A, Piotrkowicz N, Jonska-Gmyrek J, . (2013). HDR brachytherapy combined with interstitial hyperthermia in locally advanced cervical cancer patients initially treated with concomitant radiochemotherapy – a phase III study. Radiother Oncol 109:194–9.
- Grigsby PW, Heydon K, Mutch DG, . (2001). Long-term follow-up of RTOG 92-10: cervical cancer with positive para-aortic lymph nodes. Int J Radiat Oncol Biol Phys 51:982–7.
- Grogan M, Thomas GM, Melamed I, . (1999). The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer 86:1528–36.
- Bishop AJ, Allen PK, Klopp AH, . (2015). Relationship between low hemoglobin levels and outcomes after treatment with radiation or chemoradiation in patients with cervical cancer: has the impact of anemia been overstated? Int J Radiat Oncol Biol Phys 91:196–205.
- Westermann AM, Jones EL, Schem BC, . (2005). First results of triple-modality treatment combining radiotherapy, chemotherapy, and hyperthermia for the treatment of patients with stage IIB, III, and IVA cervical carcinoma. Cancer 104:763–70.
- Feldmann HJ, Seegenschmiedt MH, Molls M. (1995). Hyperthermia – its actual role in radiation oncology. Part III: clinical rationale and results in deep seated tumors. Strahlenther Onkol 171:251–64.
- Harima Y, Nagata K, Harima K, . (2000). Bax and Bcl-2 protein expression following radiation therapy versus radiation plus thermoradiotherapy in stage IIIB cervical carcinoma. Cancer 88:132–8.
- Eppink B, Krawczyk PM, Stap J, . (2012). Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int J Hyperthermia 28:509–17.
- Frey B, Weiss EM, Rubner Y, . (2012). Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 28:528–42.