Abstract
Purpose: Breast-conserving surgery is effective for breast cancer treatment but is associated with morbidity in particular high re-excision rates. We performed a systematic review and meta-analysis to assess the current evidence for clinical outcomes with minimally invasive ablative techniques in the non-surgical treatment of breast cancer.
Methods: A systematic search of the literature was performed using PubMed and Medline library databases to identify all studies published between 1994 and May 2016. Studies were considered eligible for inclusion if they evaluated the role of ablative techniques in the treatment of breast cancer and included ten patients or more. Studies that failed to fulfil the inclusion criteria were excluded.
Results: We identified 63 studies including 1608 patients whose breast tumours were treated with radiofrequency (RFA), high intensity focussed ultrasound (HIFU), cryo-, laser or microwave ablation. Fifty studies reported on the number of patients with complete ablation as found on histopathology and the highest rate of complete ablation was achieved with RFA (87.1%, 491/564) and microwave ablation (83.2%, 89/107). Short-term complications were most often reported with microwave ablation (14.6%, 21/144). Recurrence was reported in 24 patients (4.2%, 24/570) and most often with laser ablation (10.7%, 11/103). The shortest treatment times were observed with RFA (15.6 ± 5.6 min) and the longest with HIFU (101.5 ± 46.6 min).
Conclusion: Minimally invasive ablative techniques are able to successfully induce coagulative necrosis in breast cancer with a low side effect profile. Adequately powered and prospectively conducted cohort trials are required to confirm complete pathological ablation in all patients.
Introduction
Breast cancer is now diagnosed at an earlier stage due to the wider use of breast cancer screening and the use of more advanced imaging modalities including magnetic resonance imaging (MRI) [Citation1,Citation2]. In view of this, more patients are suitable for breast conserving surgery [Citation3,Citation4]. Although breast-conserving surgery is effective, it is associated with high re-excision rates of 20% in the United Kingdom due to its dependence on clear margins and the surgeon’s inability to visualise the tumour extent intra-operatively [Citation5]. Furthermore, it can be associated with poor cosmetic outcome [Citation6,Citation7]. There is thus a clinical need to develop non-operative techniques in order to treat patients with both tissue and volume preservation. Potential advantages of a non-operative approach to breast cancer treatment are the ability to image the tumour intra-operatively, reducing the surgical excision rate, reducing treatment cost and thereby potentially improving patients’ quality of life. Additional associated potential advantages include reducing the rate of general anaesthesia, reducing the complication rate and severity of these, reducing recovery time and reducing scarring [Citation5,Citation8]. In addition, adjuvant therapy may be administered faster after ablative treatment, in the absence of a wound-requiring healing.
Numerous articles have evaluated novel ablative techniques for the non-operative treatment of breast cancer and it is clinically important to evaluate the evidence in order to identify the most promising techniques for further clinical evaluation [Citation9]. We performed a systematic review and meta-analysis to assess the current evidence on clinical outcomes of minimally invasive ablative techniques for the non-operative treatment of breast cancer.
Methods
Study selection
A systematic review of the literature was performed using PubMed and Medline library databases to identify all studies published between 1994 and May 2016 that evaluated the role of ablative techniques for the treatment of breast cancer. The MESH terms used were ablative techniques, ablative interventions, ablative therapy, thermal ablation, high intensity focussed ultrasound (HIFU), radiofrequency ablation (RFA), laser ablation, cryo-ablation, stereotactic radiotherapy and microwave ablation in combination with breast and cancer. Except for reports in the English language and human subjects, there were no further restrictions. The related articles function was used to broaden the search and identify alternative ablative techniques. References of articles acquired were also searched by hand. The last search was conducted on 7 June 2016.
Inclusion criteria
Studies were considered eligible if they addressed the following: (1) studies performed on human subjects with breast cancer, (2) studies evaluating the role of a minimally invasive ablative technique and (3) studies with 10 or more patients included.
Exclusion criteria
Studies that failed to fulfil the inclusion criteria were excluded. Conference articles, letters, editorials and case reports were excluded from the study. Studies using laser as a surgical scalpel (without ablation), or studies using an ablative technique after surgical excision of the tumour were excluded. In the case of studies with overlapping populations, the most recent study with histopathological outcomes was included. Abstracts of studies that are as yet unpublished (full text not available) were excluded.
Data extraction
Each study was initially evaluated for either inclusion or exclusion. One reviewer (M. P.) extracted data for all selected studies and a second reviewer (M. A.) verified the accuracy of the extracted data. In case of a disagreement, the senior author (M. D.) made the final decision.
Risk of bias in individual studies
The “Risk of bias” tool presented in the Cochrane Handbook [Citation10] was used to determine the suitability of randomised control trials (RCT). The study quality of cohort studies was assessed according to the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [Citation11]. Seven items of the STROBE statement were considered relevant for quality evaluation. These included clearly reported objectives and inclusion criteria, usage of a standardised technique, standardised histopathology and standardised imaging, patient follow-up and reporting of any withdrawals from the study. Studies with a score of less than four were excluded. Two reviewers (M. P. and M. A.) performed the assessment independently. In case of a disagreement, a consensual decision was reached.
Statistical analysis
All extracted data were tabulated and presented as means, standard deviations (SD) and percentages. Numerators and denominators were provided to assess outcomes of included studies.
A meta-analysis was undertaken using a random effect logistic model given the wide variation in complete ablation percentages between studies. Parameter estimation was performed by a maximum-likelihood fit. To check the methodology, a parametric bootstrap technique was used [Citation12] to correct bias using maximum-likelihood estimates. From the bootstrapped solutions, standard errors (s.e.), significance tests and confidence intervals (CI) were calculated. In addition, an analysis of covariance was performed to determine any correlation between the treatment time and treated tumour size.
Results
Selected studies
A total of 2044 articles published between 1994 and May 2016 were identified from the literature search (). Three additional articles were identified by searching the references of selected articles. After reviewing the title and abstracts, 1930 articles were not deemed relevant and were excluded leaving 114 articles for full text examination. Several studies using techniques such as stereotactic radiotherapy and Gadolinium enhanced RODEO laser ablation were excluded as they did not meet the inclusion criteria (less than ten patients included). A total of 63 articles matched the selection criteria. The 63 articles included 16 feasibility studies [Citation13–28], 12 phase I studies [Citation29–40], nine phase II studies [Citation41–49], three comparative studies [Citation50–52], one retrospective study [Citation53] and four randomised controlled trials [Citation54–56]. In 18 studies the type of study was not reported [Citation8,Citation57–73]. One article [Citation54] contained the results of four studies of which two were previously published [Citation39,Citation41]. All four studies (one phase I [Citation39], one phase II [Citation41] and two randomised controlled trials [Citation54]) were included in this systematic review.
Study characteristics
In total, 63 studies with 1608 patients and 1627 breast cancers were included in the systematic review and meta-analysis. The characteristics of the studies are summarised per technique in table’s 1a-e. Radiofrequency ablation (RFA) was used in 27 studies (657 patients) [Citation13–17, Citation26,Citation27,Citation29–32,Citation42–48,Citation51,Citation64–71], in which a needle electrode is percutaneously inserted under ultrasound (US) guidance to deliver an alternating current that generates ionic agitation, localised tissue heating and cell death [Citation32,Citation46,Citation74]. Twelve studies used high-intensity focussed ultrasound (HIFU) (227 patients) [Citation8,Citation23,Citation24,Citation38,Citation52,Citation55–60,Citation72], a completely non-invasive ablative technique in which a focussed US beam propagates through tissue as a high-frequency pressure wave causing the temperature to rise, leading to protein denaturation and coagulative necrosis [Citation55,Citation75–77]. Ten studies used cryo-ablation (269 patients) [Citation19–22,Citation35–37,Citation49,Citation61,Citation73], in which a probe is inserted into the tumour under US guidance. The ablation process involves two phases: freezing and thawing with four mechanisms destroying the tumour cells: direct damage by intracellular ice formation and osmotic dehydration and indirect damage due to ischaemia and immunologic response [Citation36,Citation78]. Seven studies used laser-ablation (231 patients) [Citation18,Citation28,Citation33,Citation34,Citation50,Citation62,Citation63], in which lesions are ablated due to direct heating with low-power laser light energy delivered percutaneously via thin optical fibres. Upon absorption in the tissue, heat is produced, inducing lethal thermal injury. Six studies used microwave ablation (144 patients) [Citation25,Citation39–41,Citation54], which uses localised heating caused by water molecules which move within tissues, and externally applied focussed microwaves to cause tissue necrosis. This technique can heat and damage high-water-content tumour cells, while tissues with lower-water-content such as adipose and breast glandular tissues remain unharmed [Citation41,Citation79]. One study [Citation53] compared cryo-ablation with RFA (80 patients).
All but two studies treated patients with malignant breast tumours: one study treated newly diagnosed breast cancer and breast recurrences [Citation21] and one treated breast recurrences only [Citation43]. For image guidance of the ablative techniques, US was used by all five ablative techniques in 44 studies [Citation13–20,Citation22,Citation24–27,Citation29–36,Citation39,Citation40,Citation42–51,Citation53,Citation55,Citation56,Citation64–71], magnetic resonance imaging (MRI) in 11 studies using HIFU and cryo-ablation [Citation8,Citation23,Citation37,Citation38,Citation52,Citation57–61,Citation72]. Two cryo-ablation studies [Citation21,Citation73] and one laser ablation study [Citation28] used computer tomography (CT) in combination with US and two laser ablation studies [Citation62,Citation63] used stereotactic guidance. In three microwave studies, the imaging modality is not known [Citation41,Citation54]. The ablative treatments were performed by the surgeon in six studies [Citation25,Citation26,Citation35,Citation48,Citation56,Citation62], radiologist in four studies [Citation15,Citation31,Citation45,Citation64], a combination of both in one study [Citation40] and 52 studies did not report on who performed the treatment.
There are some important differences between the ablative techniques. The benefit of HIFU is that insertion of a probe is not required as this technique is completely non-invasive and scar less. Cryo-ablation and microwave ablation require the insertion of a single probe and RFA and laser ablation require the insertion of multiple probes. Furthermore, cryo-ablation is the only technique to use freezing rather than heat to cause tumour necrosis.
Quality assessment
Seven items of the STROBE statement [Citation11] were used for quality assessment of the included cohort studies (). All studies included specified study objectives and all but eight [Citation28,Citation33,Citation37,Citation39,Citation50,Citation51,Citation60,Citation61] had clear inclusion criteria. A standardised technique was used in all but five studies [Citation23,Citation26,Citation27,Citation36,Citation57], all but seven studies reported standardised histopathology [Citation19,Citation23,Citation33,Citation38,Citation52,Citation58,Citation60] and standard imaging was performed in 30 studies [Citation8,Citation16,Citation17,Citation21–24,Citation26–28,Citation31,Citation32,Citation34,Citation37,Citation38,Citation40,Citation46,Citation47,Citation50,Citation52,Citation53,Citation57–60,Citation66–68,Citation72,Citation73]. Patient follow-up, in the case of no surgical excision or after surgical excision of breast cancer, was undertaken in 17 studies [Citation21,Citation22,Citation24,Citation28,Citation30,Citation31,Citation33,Citation34,Citation42,Citation44,Citation52,Citation60,Citation66–68,Citation70,Citation73]; however, 14 studies [Citation17,Citation18,Citation26,Citation27,Citation40,Citation45–47,Citation49,Citation50,Citation53,Citation62,Citation63,Citation72] reported follow-up only until delayed surgical excision. In four studies [Citation16,Citation32,Citation65,Citation69], a group of patients underwent immediate surgical excision of the tumour and the remaining patients were followed up until surgical excision. In five studies [Citation21,Citation24,Citation49,Citation59,Citation63], patients withdrew from the study during or after treatment and in another six studies [Citation15,Citation26,Citation44,Citation48,Citation49,Citation72] patients withdrew before the start of the treatment. The overall STROBE score ranged from four to seven (mean 5.4 ± 0.9).
For the four included RCTs [Citation54–56], the Cochrane checklist [Citation10] was used (). All studies had unspecified sequence generations (selection bias) and allocated concealment (selection bias). All studies did not perform a power calculation or any blinding of the participants or personnel (performance bias) or outcome assessment (detection bias, patient-reported outcomes and mortality). The second study by Dooley et al. [Citation54] included incomplete data addresses (attrition bias; short- and longer-term outcomes missing) and all studies were free of selective reporting (selection bias) or other biases.
Outcomes
Histopathology
Post-treatment surgical excision of tumours was performed in 52 studies (1339 patients) in which immediate surgical excision was performed in 16 studies (387 patients, most often with RFA) [Citation13–15,Citation18,Citation25,Citation26,Citation29,Citation35,Citation42–45,Citation48,Citation51,Citation64,Citation71], delayed surgical excision in 33 studies (853 patients) [Citation8,Citation17,Citation19,Citation20,Citation22,Citation27,Citation28,Citation33,Citation34,Citation36–41,Citation46,Citation47,Citation49,Citation50,Citation52–59,Citation61–63,Citation67,Citation70,Citation72] and a combination of immediate and delayed surgical excision in three studies (99 patients).[Citation16,Citation65,Citation69] A combination of follow-up and immediate or delayed surgical excision was performed in two studies (49 patients) [Citation32,Citation33]. Follow-up with imaging alone or imaging and core biopsies was performed in nine studies (220 patients) [Citation21,Citation23,Citation24,Citation30,Citation31,Citation60,Citation66,Citation68,Citation73]. Reasons for performing a treat and resect study or a follow-up study were often not reported. Follow-up was performed in studies with patients unsuitable or not willing to have surgical excision. Immediate surgical excision was performed to determine the true zone of necrosis and delayed surgical excision was performed to determine the degenerative changes over time.
Delayed surgical excision was performed within a week of treatment in four studies [Citation19,Citation33,Citation50,Citation72] (most often following laser ablation), within 2 weeks of treatment in ten studies [Citation8,Citation20,Citation27,Citation34,Citation36,Citation38,Citation55,Citation56,Citation58,Citation59] (most often following HIFU), within 3 weeks of treatment in eight studies [Citation17,Citation28,Citation39,Citation41,Citation46,Citation47,Citation54,Citation62] (most often following RFA), within 4 weeks of treatment in four studies [Citation37,Citation40,Citation49,Citation67] and longer than four weeks of treatment in six studies [Citation22,Citation52,Citation53,Citation61,Citation63,Citation70]. In two studies, the timing of surgical excision in relation to treatment was not reported [Citation54,Citation57]. In the three combination studies [Citation16,Citation65,Citation69] (all RFA studies), delayed surgical excision was performed after a longer period ranging from 1 to 40 months.
Complete ablation on histopathology was reported in 50 studies. Considering RFA, in 87.1 ± 12.8% (491/564) of all patients who underwent surgical excision, complete ablation of the tumour was achieved [Citation13–17,Citation26,Citation27,Citation29,Citation32,Citation42–48,Citation51,Citation53,Citation64,Citation65,Citation67,Citation69–71] (). For laser ablation, 52.2 ± 29.2% (48/92) of all patients had complete ablation post-treatment [Citation18,Citation34,Citation63] () and for cryo-ablation, complete ablation was achieved in 74.1 ± 28.9% (186/251) of all patients [Citation19,Citation20,Citation22,Citation35–37,Citation49,Citation53,Citation61] (). With HIFU, complete ablation was achieved in 47.6 ± 29.9% (60/126) of all patients [Citation8,Citation38,Citation52,Citation55,Citation57–59] () and in microwave ablation 83.2 ± 11.6% (89/107) of patients obtained complete ablation [Citation25,Citation41,Citation54] ().
Table 1. Study characteristics and outcomes for (a) radio-frequency, (b) laser, (c) cryo, (d) high intensity focussed ultrasound and (e) microwave ablation.Table 1(a). Study characteristics and outcomes for radiofrequency ablation (RFA).
Table 2. Quality assessment (yes/no) for (a) cohort studies and (b) randomised controlled trials.
Table 1(b). Study characteristics and outcomes for laser ablation.
Table 1(c). Study characteristics and outcomes of cryo-ablation.
Table 1(d). Study characteristics and outcomes for high intensity focussed ultrasound (HIFU).
Table 1(e). Study characteristics and outcomes for microwave ablation.
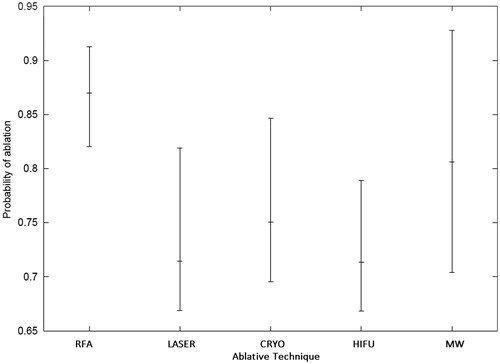
Using a random effect logistic model given the wide-variation in complete ablation rates between studies, the probabilities of success to achieve complete pathological response for the five ablative techniques with 95% CI were calculated. The highest estimate was for RFA (0.87 (0.82, 0.91)), followed by microwave ablation (0.81 (0.70, 0.93)), cryo-ablation (0.75 (0.70, 0.85)), laser ablation (0.71 (0.67, 0.82)) and HIFU (0.71 (0.67, 0.79)) ().
Figure 2. Probabilities of success to achieve complete pathological response with 95% confidence intervals calculated using a random logistic effect model. RFA: radiofrequency ablation; LASER: laser ablation; CRYO: cryo-ablation; HIFU: high intensity focused ultrasound; MW: microwave ablation.
When looking at complete ablation rates of RFA studies that followed patients up with core needle biopsy (CNB) and imaging but without surgical excision, complete ablation was achieved in 98.0 ± 4.0% (100/102) [Citation30,Citation32,Citation66,Citation68], in follow-up studies using cryo-ablation 100% (6/6) of patients had complete ablation [Citation21] and in follow-up studies using HIFU 89.1 ± 14.8% (41/46) of patients had complete ablation [Citation23,Citation24].
Follow-up
Follow-up was performed in all [Citation21,Citation23,Citation24,Citation30,Citation31,Citation60,Citation66,Citation68,Citation73] or in a cohort of patients [Citation32,Citation33] in 11 studies. Follow-up was performed with MRI (n = 3), US (n = 1) and cytology (n = 1) in RFA studies [Citation30–32,Citation66,Citation68] with a mean follow-up period of 28.1 ± 15.6 months; with CT (n = 1) and MRI (n = 2) in cryo-ablation studies [Citation21,Citation73] with a follow-up of 18.7 ± 5.8 months; with MRI (n = 3), US (n = 2), SPECT (n = 1) and core biopsies (n = 1) in HIFU studies [Citation23,Citation24,Citation60] with a mean follow-up period of 29.7 ± 22.0 months and with US (n = 1), CT (n = 1) and core biopsies (n = 1) in laser ablation studies with a mean follow-up of 20.5 ± 0 months [Citation33].
Treat and resect studies also undertook patient follow-up. RFA studies used MRI (n = 6), US (n = 4), mammography (n = 2) or CT (n = 1) for follow-up up to 1 month after surgical excision, laser ablation studies used MRI (n = 2), US (n = 1), CT (n = 1) and mammography (n = 1) for follow-up up to 2 weeks after surgical excision and every 3 months thereafter, cryo-ablation studies used MRI (n = 3) or US (n = 1) for follow-up up to 1 month after surgical excision, HIFU studies used MRI (n = 5) for follow-up up to 3 weeks after surgical excision, and microwave ablation studies used MRI (n = 1) or US (n = 1) up to 3 weeks prior to surgical excision.
Margins
Fifteen studies reported on treating an additional margin of normal breast tissue around the tumour. Six RFA studies treated a margin of 5 mm (n = 3) [Citation29,Citation42,Citation44] or more than 5 mm (n = 3) [Citation13,Citation27,Citation45]. One laser ablation study treated an additional 5 mm of normal breast tissue [Citation62]. Two cryo-ablation studies treated an additional margin of 5–10 mm of normal tissue [Citation35,Citation37]. Six HIFU studies treated a margin of 5 mm (n = 3) [Citation8,Citation38,Citation52] or 15–20 mm (n = 3) [Citation24,Citation55,Citation56].
Axillary lymph nodes
The type of axillary treatment was reported in 40 studies. Axillary treatment was performed prior to ablative treatment (n = 17), after ablative treatment (n = 2), along with surgical excision (n = 14) or the timing was not specified (n = 7). Sentinel lymph node biopsy (SLNB) in immediate surgical excision studies was often performed just prior to the ablative treatment, at the same sitting, which was then followed by surgical excision of the tumour. For delayed surgical excision, SLNB was often performed along with surgical excision of the tumour or prior to the ablative treatment in order to perform an axillary clearance (if necessary) simultaneously with the surgical excision. For follow-up studies, SLNB was performed prior to ablative treatment. In the case of clinically or radiologically positive nodes or positive nodes after SLNB, an axillary node clearance was performed instead or after involved nodes were removed during SLNB.
A total of 187 positive nodes were found in the ablative studies, 103 with RFA, 20 with cryo-ablation, 17 with laser and microwave ablation and 15 with HIFU and the combined study (RFA and cryo ablation).
Local recurrence
Local recurrence was reported in 24 patients from nine studies (24/570, 4.2%). Most local recurrences were reported with laser ablation (10.7%, 11/103) [Citation33,Citation34] at a mean follow-up time of 20.5 ± 0 months, and RFA (3.1%, 9/291) [Citation31,Citation42,Citation64,Citation69] at a mean follow-up time of 30.8 ± 16.9 months. No local recurrences were reported with microwave ablation, (0/144) one case of local recurrence was reported with cryo-ablation (1.4%, 1/74) [Citation73] at a mean follow-up time of 16.9 ± 2.0 months and three cases with HIFU (2.9%, 3/102) [Citation24,Citation60] at a mean follow-up time of 21.4 ± 19.3 months.
Recurrences were documented in two studies (n = 3) who performed immediate surgical excision [Citation42,Citation64], one study (n = 2) with delayed surgical excision [Citation34], one study (n = 3) with combined immediate and delayed surgical excision [Citation69], one study with combined delayed surgical excision (n = 8) and follow-up (n = 1) [Citation33] and four studies (n = 7) who performed follow-up only [Citation24,Citation31,Citation60,Citation73].
Post-treatment short-term complications
Complications were reported in 9.8% of all patients (123/1258). The most common complications were skin burns (3.5%, 44/1258), pectoralis major muscle damage (1.1%, 14/1258), seromata (0.6%, 8/1258), skin necrosis (0.6%, 7/1258) and ecchymosis (0.6%, 7/1258). Other reported complications were blistering (0.5%, 6/1258), haematoma (0.4%, 5/1258), coagulative changes to the skin (0.4%, 5/1258), nipple retraction (0.3%, 4/1258), pneumothorax (0.2%, 3/1258), flap necrosis (0.2%, 3/1258), fever (0.2%, 3/1258), infection (0.2%, 2/1258), skin puckering (0.2%, 2/1258), skin retraction (0.2%, 2/1258) and single cases (0.1%, 1/1258) of overreaction of the ablated zone, fistula, white lumps over the treated area, haemorrhage, arterial bleeding, tumour rupture and abscess. All complications were device related rather than cancer specific complications, and thus far, only one of the nine studies [Citation21,Citation23,Citation24,Citation30,Citation32,Citation60,Citation66,Citation68,Citation73] without surgical excision of the ablated tumour post-treatment, documented longer-term complications, one patient (0.1%, 1/1258) with skin retraction which turned into skin ulceration at 12 months follow-up.
With RFA, 10.5% of patients developed post-treatment complications (58/555) of which 23 were skin burns [Citation14–16,Citation26,Citation27,Citation29–32,Citation42,Citation47,Citation48,Citation66,Citation67], 12 muscle burns [Citation14,Citation15,Citation48], five cases of blistering [Citation51], four of coagulative changes to the skin [Citation51], three were cases of ecchymosis [Citation27,Citation46], three cases of nipple retraction [Citation31], two cases of pneumothorax [Citation15,Citation64], two incidences of skin puckering [Citation44], two infections [Citation42,Citation44] and single cases of overreaction [Citation30] and fistula [Citation47] (). With cryo-ablation 10.9% of patients (20/183) developed a complication, these were skin necrosis (n = 5) [Citation61], haematoma (n = 5) [Citation22, Citation73], ecchymosis (n = 4) [Citation22], skin retraction (n = 2) [Citation73], seromata (n = 2) [Citation19,Citation20], arterial bleeding (n = 1) [Citation20] and skin ulceration (n = 1). [Citation73] Several patients also developed skin burns and mastitis (number unreported) [Citation37] (). With laser ablation 6.3% of patients (12/191) developed complications, which included skin burns (n = 7) [Citation18,Citation33,Citation63], necrosis (n = 2) [Citation34], haemorrhage (n = 1) [Citation28], pneumothorax (n = 1) [Citation18] and rupture of the tumour (n = 1) [Citation33] (). With HIFU complications occurred in 6.5% of patients (12/185) which included skin burns (n = 8) [Citation8,Citation23,Citation38,Citation55,Citation57,Citation60], fever (n = 3) [Citation56] and white lumps over the treatment site (n = 1) [Citation72] (). Microwave ablation resulted in the most complications (14.6%, 21/144), which included skin burns (n = 6) [Citation41,Citation54], seromata (n = 6) [Citation54], flap necrosis (n = 3) [Citation39], muscle burns (n = 2) [Citation25], blistering (n = 1) [Citation39], coagulative changes to the skin (n = 1) [Citation25], abscess (n = 1) [Citation54] and nipple retraction (n = 1) [Citation54] ().
Treated tumour sizes
Considering the size of treated tumours, microwave ablation was used to treat the largest tumours, with a mean tumour diameter of 2.7 ± 1.1 cm (six studies) [Citation25,Citation39–41,Citation54]. HIFU was used to treat tumours of 2.1 ± 0.9 cm (seven studies) [Citation8,Citation23,Citation24,Citation38,Citation52,Citation55,Citation72], and cryo-ablation was used to treat tumours with a mean size of 1.6 ± 0.7 cm (eight studies) [Citation19,Citation21,Citation22,Citation35–37,Citation49,Citation61]. The smallest tumours were treated with laser-ablation (1.2 ± 0.2 cm, three studies) [Citation34,Citation62,Citation63] and RFA (1.5 ± 0.4 cm, 17 studies) [Citation13,Citation15,Citation29,Citation30,Citation42,Citation44–48,Citation51,Citation65–69,Citation71]. Only mean sizes were included in this analysis ().
Total treatment duration
Considering treatment duration, RFA had the shortest mean treatment time of 15.6 ± 5.6 min (20 studies) [Citation13–15,Citation29–31,Citation42–46,Citation48,Citation51,Citation53,Citation64–68,Citation70]. Laser ablation had a mean treatment time of 25.7 ± 6.1 min (two studies) [Citation18,Citation34] and microwave ablation had a mean treatment time of 19.0 ± 18.2 min (four studies) [Citation25,Citation39,Citation40,Citation54]. Cryo-ablation had a much longer mean treatment time of 50.3 ± 58.4 min (seven studies) [Citation19,Citation21,Citation35–37,Citation61,Citation73] and HIFU the longest mean treatment time of 101.5 ± 46.6 min (four studies) [Citation8,Citation52,Citation57,Citation72]. Only mean treatment times were included in this analysis ().
An analysis of covariance’s initially showed a significant increase in treatment time with tumour size. Correcting for tumour size showed that microwave ablation was the quickest technique, followed by RFA, laser ablation, cryo-ablation and HIFU. A purpose written Fortran programme and RFA as a baseline showed the following estimates (95% CI): microwave ablation 0.32 (0.15, 0.68), RFA 1.0 (1.0, 1.0), laser ablation 1.27 (0.76, 2.11), cryo-ablation 2.58 (1.69, 3.96) and HIFU 5.03 (3.15, 8.02). Unfortunately, on replacing tumour sizes by rank size, no significant relationship between the treatment time and tumour size was found. The apparent strong dependence of treatment time on tumour size was shown to be spurious, and driven by outlying studies with large tumour sizes and long treatment times.
Cosmetic outcome
Cosmetic outcome was reported in nine studies using RFA, HIFU and cryo-ablation. Seven studies [Citation30,Citation32,Citation42,Citation53,Citation66,Citation67,Citation70] using RFA reported an excellent cosmesis in 85.3% of patients (168/197), good cosmesis in 9.6% (19/197), acceptable cosmesis in 0.5% (1/197), fair cosmesis in 2.5% (5/197), poor cosmesis in 1.5% (3/197) and cosmesis was unknown in 0.5% (1/197). Cosmesis was collected using a 1–4 point grading scale (n = 4), a 1–10 point grading scale (n = 1) or it was not reported (n = 2). The cosmesis was evaluated by the consultant (n = 3), the patient (n = 2) or this was not reported (n = 2) and it was evaluated 4 weeks after treatment (n = 2), 1 year after treatment (n = 1), at 1, 3 and 6 months after treatment (n = 1) or not reported (n = 3). No surgical excision was performed in three studies [Citation30,Citation32,Citation66] and delayed surgical excision was performed in three studies [Citation53,Citation67,Citation70] and cosmesis in these six studies was evaluated prior to surgical excision. In one study [Citation42], immediate surgical excision was performed and cosmesis was evaluated after surgical excision (excellent (12), good (3) cosmesis).
With HIFU [Citation24,Citation38], 59.3% of patients (16/27) graded their cosmetic outcome as good and 7.4% (2/27) as acceptable and cosmesis was unknown in nine patients (9/27, 33.3%). One follow-up study [Citation24] evaluated the cosmesis using a 1–5 point scale at the last follow-up and cosmetic evaluation was undertaken by the consultant. The other study [Citation38] performed delayed surgical excision and did not report on the methods used to evaluate cosmesis. With cryo-ablation [Citation53], excellent cosmesis was reported in 92.5% (37/40) of patients, good cosmesis in 5.0% (2/40) and acceptable in 2.5% (1/40). This study performed delayed surgical excision and the cosmesis was evaluated by the consultant after 4 weeks, using a 1–4 point grading scale.
Discussion
The trials conducted to date demonstrate feasibility and potential benefits for minimally invasive ablative treatment of breast cancer. However, ablative techniques are generally being evaluated in small, often uncontrolled studies that are unlikely to change clinical practice or provide the basis for phase III trials. The trials in this systematic review also included four RCTs but none of these carried out adequate sample size calculations. Therefore, a deficiency of this systematic review is the limited quality of published studies in this field.
The most important outcome measures are completeness of ablation, complication rate and tumour recurrence. In terms of complete ablation, the best outcomes are reported with RFA (87.1%, 491/564), microwave ablation (83.2%, 89/107) and cryo-ablation (74.1%, 186/251). Limitations exist in the comprehensive recording of reported histopathological outcomes. The most reliable way to determine cell death (especially immediately post-surgical excision) is with nicotinamide adenine dinucleotide (NADH) staining. However, this type of staining was not always used [Citation16]. With respect to radio-pathological correlation, more concordance with imaging was observed with NADH assessment of necrosis compared to haematoxylin and eosin (H&E).
The histopathology results in almost all studies described the number of patients with complete ablation. In patients with partial ablation, the percentage of viable tumour seen within the ablated zone was only reported in three studies [Citation57,Citation58,Citation61]. Several studies used biopsies of the ablated zone to evaluate completion of tumour ablation. With respect to percentages of complete ablation and mean tumour size, no direct comparison can be made because of study heterogeneity. Rate of complete ablation cannot be controlled for lesion size, since lesion size was not consistently reported in each study.
The most common complications were skin burns which occurred in 3.5% of patients (44/1258, most in RFA) and damage to the pectoralis major muscle which was reported in 1.1% of patients (14/1258, most in RFA). With respect to treatment-related complications, laser ablation (6.3%, 12/191) and HIFU (6.5%, 12/185) have the fewest complications and most complications were reported with microwave-ablation (14.6%, 21/144). However, complications may be under-reported since some such as pain, oedema and erythema are not consistently reported in all studies. Some studies only report severe complications and others report all complications. Furthermore, not all studies evaluated the level of pain during and after treatment. Skin burns were the most serious complication described, and likely causation was not described in most studies; however, in some studies, the burn may have been caused by a short lump-skin distance or therapy was performed immediately after biopsies were taken. Only one longer-term complication was reported in the nine studies, without surgical excision of the ablated tumour post-treatment. All other studies included only short-term complications up until surgical excision. Large prospective trials with long-term follow-up of at least 5 years are required to determine the long-term complications reliably.
Local recurrence occurred following 11 laser, nine RFA, three HIFU and one cryo-ablation treatment; however, only 22 studies looked at the recurrence rates, including all nine follow-up studies. With respect to cosmesis, patients treated with cryo-ablation and RFA seem to have a better cosmesis post-treatment compared with pre-treatment than patients treated with HIFU. However, HIFU is a completely non-invasive technique which requires no incision whilst all the other techniques do require a small incision. Therefore, HIFU is expected to achieve a better cosmesis. In addition, the only complications reported with HIFU were skin burns, while all other ablative techniques reported complications related to the insertion of the needle or probe.
Analysis of mean treatment duration demonstrated that RFA (15.6 ± 5.6 min), laser (25.7 ± 6.1 min) and microwave ablation (19.0 ± 18.2 min) have the shortest treatment time. Analysis of covariance was difficult due to inconsistent methods of reporting tumour sizes and treatment times. After replacing tumour sizes by their ranks, no significant relationship was found. Clearly, the choice of ablative technique in individual studies was based on access or availability of the technique rather than a conscious selection based on which ablative technique has the shortest treatment times or showed highest complete ablation rates.
The limitation of this study is that only four RCTs [Citation54–56] and one retrospective analysis comparing two techniques [Citation53] were included and, therefore, a comparative meta-analysis of these could not be performed. The RCTs compared HIFU and microwave ablation with breast conserving surgery [Citation54–56] or microwave ablation with neo-adjuvant chemotherapy or with chemotherapy alone [Citation54]. When considering histopathology, treatment time and complications, RFA demonstrated the most promise of any minimally invasive technique for the non-surgical treatment of breast cancer, but RFA is not included in the RCTs. More RCTs comparing ablative techniques with surgical excision or with each other (including RFA) are needed with sample size calculations to accurately evaluate differences between the techniques. However, initially adequately powered cohort trials should be conducted to confirm complete pathological ablation in all patients is feasible. This can be achieved by first developing a predictive tool for assessing complete ablation within treat and resect studies, by imaging the tumour post-treatment prior to surgical excision and verifying the extent of ablation on imaging with histopathological correlation. And second by using this predictive tool in follow-up studies to determine the amount of complete ablation. Once efficacy to achieve complete pathological ablation is confirmed, RCTs comparing the most promising ablative technique to surgical excision can be conducted to determine long-term treatment related and cancer specific complications.
Another limitation is that the cohort studies included have considerable heterogeneity. It is, therefore, not possible to perform a quantitative comparison between the studies. Compared to breast surgery, these techniques have the advantage of intra-operative imaging to improve accuracy during the treatment. Other potential benefits are the low and less severe complication rates, minimal invasiveness of the techniques resulting in a short hospital stay and recovery time which might lead to a reduction in treatment cost compared with breast surgery [Citation8,Citation9,Citation80]. Also adjuvant therapy may be administered faster after ablative treatment, in the absence of a wound requiring healing. All the trials treated patients with invasive breast cancer or breast recurrences, for the treatment of ductal carcinoma in situ the challenge is the lack of reliable imaging tools for real-time treatment planning and assessment of response to treatment. However, the disadvantage of all these techniques is that surgical axillary staging is still required in patients with early breast cancer so surgery cannot be avoided completely.
Conclusion
Minimally invasive ablative techniques are able to successfully induce coagulative necrosis with a low side-effect profile but complete ablation is not achieved consistently. The best response in terms of complete ablation was reported following RFA and the fewest complications were reported following HIFU treatment. Adequately powered and prospectively conducted cohort trials are required to confirm that complete pathological ablation is achievable in all patients and to develop a predictive tool for assessing complete ablation. Once this is confirmed, RCTs comparing the most promising ablative technique to surgical excision can be conducted to determine long-term treatment related and cancer specific outcomes.
Acknowledgements
Authors M. P., M. A., A. N. and M. D. have special interest in the HIFU technique. M. D. has some prior experience with RFA and laser ablation. None of the other ablative techniques mentioned in the manuscript have been used by the authors.
Disclosure statement
The authors report that they have no conflicts of interest.
Funding
The author would like to thank Theraclion Ltd (Malakoff, France) for an unrestricted educational grant.
References
- Hscic (2015). HaSCIC. Breast Screening Programme, England—2013–14. 2015(25-11).
- Ahmed M, Rubio IT, Klaase JM, Douek M. (2015). Surgical treatment of nonpalpable primary invasive and in situ breast cancer. Nat Rev Clin Oncol 12:645–63.
- Fisher B, Anderson S, Bryant J, et al. (2002). Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233–41.
- Moran MS, Schnitt SJ, Giuliano AE, et al. (2014). Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol 21:704–16.
- Jeevan R, Cromwell DA, Trivella M, et al. (2012). Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. BMJ: Br Med J 345:e4505.
- Al-Ghazal SK, Blamey RW, Stewart J, Morgan AA. (1999). The cosmetic outcome in early breast cancer treated with breast conservation. Eur J Surg Oncol 25:566–70.
- Nissen MJ, Swenson KK, Ritz LJ, et al. (2001). Quality of life after breast carcinoma surgery: a comparison of three surgical procedures. Cancer 91:1238–46.
- Furusawa H, Namba K, Thomsen S, et al. (2006). Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness. J Am Coll Surg 203:54–63.
- Zhao Z, Wu F. (2010). Minimally-invasive thermal ablation of early-stage breast cancer: a systemic review. Eur J Surg Oncol 36:1149–55.
- The Nordic Cochrane Centre CC. (2008). Review Manager (RevMan) [Computer programme]. Version 5.0.
- Von Elm E, Altman DG, Egger M, et al. (2007). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–7.
- Efron B, Tibshirani R. (1993). An introduction to the bootstrap. New York: Chapman and Hall.
- Noguchi M, Earashi M, Fujii H, et al. (2006). Radiofrequency ablation of small breast cancer followed by surgical resection. J Surg Oncol 93:120–8.
- Imoto S, Wada N, Sakemura N, et al. (2009). Feasibility study on radiofrequency ablation followed by partial mastectomy for stage I breast cancer patients. Breast 18:130–4.
- Wiksell H, Lofgren L, Schassburger KU, et al. (2010). Feasibility study on the treatment of small breast carcinoma using percutaneous US-guided preferential radiofrequency ablation (PRFA). Breast 19:219–25.
- Ohtani S, Kochi M, Ito M, et al. (2011). Radiofrequency ablation of early breast cancer followed by delayed surgical resection – a promising alternative to breast-conserving surgery. Breast 20:431–6.
- Schassburger KU, Lofgren L, Leifland K, et al. (2014). Minimally-invasive treatment of early stage breast cancer: A feasibility study using radiofrequency ablation under local anaesthesia. Breast 23:152–8. doi:10.1016/j.breast.2013.12.007.
- Van Esser S, Stapper G, Van Diest PJ, et al. (2009). Ultrasound-guided laser-induced thermal therapy for small palpable invasive breast carcinomas: a feasibility study. Ann Surg Oncol 16:2259–63.
- Pfleiderer SO, Freesmeyer MG, Marx C, et al. (2002). Cryotherapy of breast cancer under ultrasound guidance: initial results and limitations. Eur Radiol 12:3009–14.
- Pfleiderer SO, Marx C, Camara O, et al. (2005). Ultrasound-guided, percutaneous cryotherapy of small (< or =15 mm) breast cancers. Invest Radiol 40:472–7.
- Littrup PJ, Jallad B, Chandiwala-Mody P, et al. (2009). Cryotherapy for breast cancer: a feasibility study without excision. J Vasc Interv Radiol 20:1329–41.
- Manenti G, Perretta T, Gaspari E, et al. (2011). Percutaneous local ablation of unifocal subclinical breast cancer: clinical experience and preliminary results of cryotherapy. Eur Radiol 21:2344–53.
- Gianfelice D, Khiat A, Boulanger Y, et al. (2003). Feasibility of magnetic resonance imaging-guided focused ultrasound surgery as an adjunct to tamoxifen therapy in high-risk surgical patients with breast carcinoma. J Vasc Interv Radiol 14:1275–82.
- Wu F, Wang ZB, Zhu H, et al. (2005). Extracorporeal high intensity focused ultrasound treatment for patients with breast cancer. Breast Cancer Res Treat 92:51–60.
- Zhou W, Zha X, Liu X, et al. (2012). US-guided percutaneous microwave coagulation of small breast cancers: a clinical study. Radiology 263:364–73.
- Singletary ES. (2003). Feasibility of radiofrequency ablation for primary breast cancer. Breast Cancer 10:4–9.
- Hayashi AH, Silver SF, Van Der Westhuizen NG, et al. (2003). Treatment of invasive breast carcinoma with ultrasound-guided radiofrequency ablation. Am J Surg 185:429–35.
- Harries SA, Amin Z, Smith ME, et al. (1994). Interstitial laser photocoagulation as a treatment for breast cancer. Br J Surg 81:1617–9.
- Izzo F, Thomas R, Delrio P, et al. (2001). Radiofrequency ablation in patients with primary breast carcinoma: a pilot study in 26 patients. Cancer 92:2036–44.
- Yamamoto N, Fujimoto H, Nakamura R, et al. (2011). Pilot study of radiofrequency ablation therapy without surgical excision for T1 breast cancer: evaluation with MRI and vacuum-assisted core needle biopsy and safety management. Breast Cancer 18:3–9.
- Palussiere J, Henriques C, Mauriac L, et al. (2012). Radiofrequency ablation as a substitute for surgery in elderly patients with nonresected breast cancer: pilot study with long-term outcomes. Radiology 264:597–605.
- Yoshinaga Y, Enomoto Y, Fujimitsu R, et al. (2013). Image and pathological changes after radiofrequency ablation of invasive breast cancer: a pilot study of nonsurgical therapy of early breast cancer. World J Surg 37:356–63.
- Akimov AB, Seregin VE, Rusanov KV, et al. (1998). Nd: YAG interstitial laser thermotherapy in the treatment of breast cancer. Lasers Surg Med 22:257–67.
- Haraldsdottir KH, Ivarsson K, Gotberg S, et al. (2008). Interstitial laser thermotherapy (ILT) of breast cancer. Eur J Surg Oncol 34:739–45.
- Tafra L, Smith SJ, Woodward JE, et al. (2003). Pilot trial of cryoprobe-assisted breast-conserving surgery for small ultrasound-visible cancers. Ann Surg Oncol 10:1018–24.
- Sabel MS, Kaufman CS, Whitworth P, et al. (2004). Cryoablation of early-stage breast cancer: work-in-progress report of a multi-institutional trial. Ann Surg Oncol 11:542–9.
- Morin J, Traore A, Dionne G, et al. (2004). Magnetic resonance-guided percutaneous cryosurgery of breast carcinoma: technique and early clinical results. Can J Surg 47:347–51.
- Zippel DB, Papa MZ. (2005). The use of MR imaging guided focused ultrasound in breast cancer patients; a preliminary phase one study and review. Breast Cancer 12:32–8.
- Gardner RA, Vargas HI, Block JB, et al. (2002). Focused microwave phased array thermotherapy for primary breast cancer. Ann Surg Oncol 9:326–32.
- Zhou W, Jiang Y, Chen L, et al. (2014). Image and pathological changes after microwave ablation of breast cancer: a pilot study. Eur J Radiol 83:1771–7.
- Vargas HI, Dooley WC, Gardner RA, et al. (2004). Focused microwave phased array thermotherapy for ablation of early-stage breast cancer: results of thermal dose escalation. Ann Surg Oncol 11:139–46.
- Medina-Franco H, Soto-Germes S, Ulloa-Gomez JL, et al. (2008). Radiofrequency ablation of invasive breast carcinomas: a phase II trial. Ann Surg Oncol 15:1689–95.
- Garbay JR, Mathieu MC, Lamuraglia M, et al. (2008). Radiofrequency thermal ablation of breast cancer local recurrence: a phase II clinical trial. Ann Surg Oncol 15:3222–6.
- Khatri VP, Mcgahan JP, Ramsamooj R, et al. (2007). A phase II trial of image-guided radiofrequency ablation of small invasive breast carcinomas: use of saline-cooled tip electrode. Ann Surg Oncol 14:1644–52.
- Fornage BD, Sneige N, Ross MI, et al. (2004). Small (< or =2-cm) breast cancer treated with US-guided radiofrequency ablation: feasibility study. Radiology 231:215–24.
- Burak WE Jr, Agnese DM, Povoski SP, et al. (2003). Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer 98:1369–76.
- Vilar VS, Goldman SM, Ricci MD, et al. (2012). Analysis by MRI of residual tumor after radiofrequency ablation for early stage breast cancer. AJR Am J Roentgenol 198:W285–91.
- Kinoshita T, Iwamoto E, Tsuda H, Seki K. (2011). Radiofrequency ablation as local therapy for early breast carcinomas. Breast Cancer 18:10–17.
- Simmons RM, Ballman KV, Cox C, et al. (2016). A phase II trial exploring the success of cryoablation therapy in the treatment of invasive breast carcinoma: results from ACOSOG (Alliance) Z1072. Ann Surg Oncol 23:2438–45. doi:10.1245/s10434-016-5275-3.
- Mumtaz H, Hall-Craggs MA, Wotherspoon A, et al. (1996). Laser therapy for breast cancer: MR imaging and histopathologic correlation. Radiology 200:651–8.
- Hung WK, Mak KL, Ying M, Chan M. (2011). Radiofrequency ablation of breast cancer: a comparative study of two needle designs. Breast Cancer 18:124–8.
- Cavallo Marincola B, Pediconi F, Anzidei M, et al. (2015). High-intensity focused ultrasound in breast pathology: non-invasive treatment of benign and malignant lesions. Expert Rev Med Dev 12:191–9.
- Manenti G, Scarano AL, Pistolese CA, et al. (2013). Subclinical breast cancer: minimally invasive approaches. Our experience with percutaneous radiofrequency ablation vs. Cryotherapy. Breast Care (Basel) 8:356–60.
- Dooley WC, Vargas HI, Fenn AJ, et al. (2010). Focussed microwave thermotherapy for preoperative treatment of invasive breast cancer: a review of clinical studies. Ann Surg Oncol 17:1076–93.
- Wu F, Wang ZB, Cao YD, et al. (2003). A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br J Cancer 89:2227–33.
- Guan L, Xu G. (2016). Damage effect of high-intensity focused ultrasound on breast cancer tissues and their vascularities. World J Surg Oncol 14:153.
- Gianfelice D, Khiat A, Amara M, et al. (2003). MR imaging-guided focused US ablation of breast cancer: histopathologic assessment of effectiveness-initial experience. Radiology 227:849–55.
- Gianfelice D, Khiat A, Amara M, et al. (2003). MR imaging-guided focused ultrasound surgery of breast cancer: correlation of dynamic contrast-enhanced MRI with histopathologic findings. Breast Cancer Res Treat 82:93–101.
- Khiat A, Gianfelice D, Amara M, Boulanger Y. (2006). Influence of post-treatment delay on the evaluation of the response to focused ultrasound surgery of breast cancer by dynamic contrast enhanced MRI. Br J Radiol 79:308–14.
- Furusawa H, Namba K, Nakahara H, et al. (2007). The evolving non-surgical ablation of breast cancer: MR guided focused ultrasound (MRgFUS)). Breast Cancer 14:55–8.
- Pusztaszeri M, Vlastos G, Kinkel K, Pelte MF. (2007). Histopathological study of breast cancer and normal breast tissue after magnetic resonance-guided cryotherapy ablation. Cryobiology 55:44–51.
- Bloom KJ, Dowlat K, Assad L. (2001). Pathologic changes after interstitial laser therapy of infiltrating breast carcinoma. Am J Surg 182:384–8.
- Dowlatshahi K, Francescatti DS, Bloom KJ. (2002). Laser therapy for small breast cancers. Am J Surg 184:359–63.
- Waaijer L, Kreb DL, Fernandez Gallardo MA, et al. (2014). Radiofrequency ablation of small breast tumours: evaluation of a novel bipolar cool-tip application. Eur J Surg Oncol 2014;40:1222–9.
- Earashi M, Noguchi M, Motoyoshi A, Fujii H. (2007). Radiofrequency ablation therapy for small breast cancer followed by immediate surgical resection or delayed mammotome excision. Breast Cancer 14:39–47.
- Oura S, Tamaki T, Hirai I, et al. (2007). Radiofrequency ablation therapy in patients with breast cancers two centimetres or less in size. Breast Cancer 14:48–54.
- Manenti G, Bolacchi F, Perretta T, et al. (2009). Small breast cancers: in vivo percutaneous US-guided radiofrequency ablation with dedicated cool-tip radiofrequency system. Radiology 251:339–46.
- Nagashima T, Sakakibara M, Sangai T, et al. (2009). Surrounding rim formation and reduction in size after radiofrequency ablation for primary breast cancer. Jpn J Radiol 27:197–204.
- Motoyoshi A, Noguchi M, Earashi M, et al. (2010). Histopathological and immunohistochemical evaluations of breast cancer treated with radiofrequency ablation. J Surg Oncol 102:385–91.
- Noguchi M, Motoyoshi A, Earashi M, Fujii H. (2012). Long-term outcome of breast cancer patients treated with radiofrequency ablation. Eur J Surg Oncol 38:1036–42.
- Tsuda H, Seki K, Hasebe T, et al. (2011). A histopathological study for evaluation of therapeutic effects of radiofrequency ablation in patients with breast cancer. Breast Cancer 18:24–32.
- Merckel LG, Knuttel FM, Deckers R, et al. (2016). First clinical experience with a dedicated MRI-guided high-intensity focussed ultrasound system for breast cancer ablation. Eur Radiol. [Epub ahead of print]. doi:10.1007/s00330-016-4222-9.
- Cazzato RL, De Lara CT, Buy X, et al. (2015). Single-centre experience with percutaneous cryoablation of breast cancer in 23 consecutive non-surgical patients. Cardiovasc Interv Radiol 38:1237–43.
- Gillams AR. (2005). The use of radiofrequency in cancer. Br J Cancer 92:1825–9.
- Schmitz AC, Gianfelice D, Daniel BL, et al. (2008). Image-guided focused ultrasound ablation of breast cancer: current status, challenges, and future directions. Eur Radiol 18:1431–41.
- Kim SH, Jung SE, Kim HL, et al. (2010). The potential role of dynamic MRI in assessing the effectiveness of high-intensity focused ultrasound ablation of breast cancer. Int J Hyperthermia 26:594–603.
- Haar GT, Coussios C. (2007). High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia 23:89–104.
- Hahn M, Pavlista D, Danes J, et al. (2013). Ultrasound guided cryoablation of fibroadenomas. Ultraschall Med 34:64–8.
- Greenberg R, Skornick Y, Kaplan O. (1998). Management of breast fibroadenomas. J Gen Intern Med 13:640–5.
- Roubidoux MA, Yang W, Stafford RJ. (2014). Image-guided ablation in breast cancer treatment. Tech Vasc Interv Radiol 17:49–54.