Abstract
Objectives: To present the clinical effect of microwave ablation (MWA) on renal cell carcinoma (RCC) of the patients with renal dysfunction, mainly focussing on the extent of renal tumour control and damage to the residual kidney function.
Methods and materials: From 2006 to 2014, 19 tumours of 18 patients with renal dysfunction underwent percutaneous ultrasound-guided MWA in our institution. The tumour diameters range from 1.9 to 5.0 cm. The serum creatinine and urea levels of each patient pre-MWA, one day after MWA and the most recent occasion on record at our institution were collected. After MWA all the patients were followed up using contrast enhanced ultrasound (CEUS) and computed tomography (CT) or magnetic resonance imaging (MRI) at the first 1, 3 and 6 months and every six months thereafter. Patients were available for clinical and laboratory evaluations at a median follow-up time of 24.9 months (range from 3.5 to 85.9 months). The technical success, survival rates and complications were accessed.
Results: Complete ablation was achieved in 19/19 (100%) lesions after 1 or 2 MWA sessions; 2/18(11.1%) patients died of other diseases. No severe complications occurred during MWA. After MWA no significant elevation of renal function was observed either in patients of CKD stage 1–3 or in patients of CKD stage 4–5.
Conclusions: MWA is an effective and relatively safe treatment option for patients with renal tumour who also suffered from renal dysfunction. The complication rate is low, and excellent tumour control can be achieved with acceptable loss of residual renal function.
Introduction
Widespread cross-sectional imaging has resulted in a 2.3%–4.3% annual increase in renal cell carcinoma (RCC) detection [Citation1]. Radical nephrectomy which removes the tumour-bearing kidney used to be the standard recommended curative therapy. However, for the patients with renal tumour being operated by this surgery means permanent loss of one kidney and half of renal function which increases the risk of renal dysfunction. Nephron-sparing surgery, open and laparoscopic partial nephrectomy have been more widely utilised to preserve residual kidney function. But a small number of patients with renal dysfunction and renal tumour should think twice before they receive surgery, as during all the above-mentioned surgery, renal blood supply has to be blocked for a short or longer while, and this procedure may eventually lead to deterioration of kidney function. This situation causes a dilemma for the surgeon and the patients with renal dysfunction when choosing the optimal method for treating renal tumour.
For the past few years, minimally invasive percutaneous thermal ablation techniques, such as radiofrequency (RFA), cryoablation and microwave ablation (MWA), have already been suggested as acceptable alternatives for the treatment of RCC [Citation2–6]. Image-guided ablative techniques can achieve excellent mid-term outcomes that are equivalent to those of open partial nephrectomy [Citation3,Citation5–7]. Cryoablation caused cell death by application of extremely low temperatures and ice crystal formation in the tumour tissue. RFA and MWA had something in common, that is, they both produced frictional heat and coagulation necrosis of tumour cell. Compared with RFA, the chief benefit of MWA is that microwaves can propagate through desiccated and charred tissue during the ablation to form a large ablation zone in less ablation time with higher intratumoral temperatures [Citation8], and it also has been reported to play an effective role in treating small RCC [Citation9,Citation10]. However, there is no evidence to indicate the safety of percutaneous MWA of RCC for patients with renal dysfunction. As patients suffered from both RCC and renal dysfunction are rare, all the patients with RCC and renal dysfunction who received MWA from 2006 to 2014 in our institution were included in the study. In the present retrospective study, we aimed to assess the safety and clinical outcomes of percutaneous MWA in treating RCC in patients with renal dysfunction with respect to tumour control and the residual renal function.
Material and methods
Patients and clinical characteristics
This retrospective study was approved by the review board of Chinese PLA General Hospital. Written informed consent for the procedure was obtained from each enrolled patient. From August 2006 to December 2014, 19 tumour lesions of 18 patients with renal dysfunction were included and underwent percutaneous MWA at our department (16 males, 2 females; age range from 35 to 80 years; mean age 62.1 years). Of all patients, 4/18(22.2%) had a solitary kidney due to a previous radical nephrectomy for RCC. The patients either were high risk surgical candidates for their renal dysfunction or had refused surgery. The MWA procedure was proposed to these patients as an alternative treatment to surgical procedures. All patients had to meet the following criteria: each renal tumour lesion had to be less than 5.0 cm; a prothrombin coagulation time of less than 25 s; prothrombin activity higher than 40%; platelet count higher than 40 × 109/L; the absence of renal vein thrombosis and distant metastases beyond the kidney; the presence of an appropriate route for percutaneous puncture with US guidance; diagnosed as chronic kidney disease (CKD) according to the definition of Kidney Disease Outcomes Quality Initiative (K/DOQI) [Citation11].
Twelve tumour lesions were located in the right kidney, and seven lesions were in the left kidney. The tumour diameters range from 1.9 to 5.0 cm (average diameter: 3.1 cm). Four lesions located adjacent to kidney pelvis and one lesion was found to be near to the intestinal tract. Before MWA, with US and contrast enhanced ultrasound (CEUS), the operator can make an accurate operation plan of antenna placement according to the size and location of tumour lesion. The RCC diagnosis was largely made through an intraoperative tumour biopsy before ablation with US guidance. The main pathological characteristics of the patients and MWA treated tumours are summarised in .
Table 1. Pathologic characteristic of tumour lesions and ablation procedure.
Renal function and CKD stages
The serum creatinine and urea nitrogen levels were measured at the following time points: before MWA, 1 day after MWA, and the most recent occasion on record at our institution. Before MWA, glomerular filtration rate (GFR) of all patients was estimated by using Cockcroft Gault equation. The classification of patients was performed according to stages of chronic renal insufficiency which is in accordance with the criteria of K/DOQI [Citation11].
Cockcroft Gault equation (CG-CrCl): (ml/min)
MWA system
A KY2000 MW ablation system (Kangyou Medical Instruments, Nanjing, China) consists of two MW generators, two flexible coaxial cables and two cooled shaft antennae. One to 100 W of power at 2450 MHz can be produced by the generator. The cooled shaft antenna has a 15-gauge shaft coated with polytetrafluoroethylene to prevent adhesion, which can be observed on US in real-time. Inside the antenna shaft are dual channels through which distilled water is circulated by a peristaltic pump that continuously cools the shaft and prevents overheating. The MW machine is also equipped with a thermal monitoring system that can measure temperatures in real time during ablation to prevent unwanted injury to collecting system and intestinal tracts. During MWA, the real-time temperature of tumour margins or kidney proximal to the important adjacent organ was monitored. The temperature cut-off of MWA was set at 54 °C in patients without a history of prior laparotomy or 50 °C in patients with history of laparotomy. The emission of microwave was reactivated after the temperature decreased to 45 °C.
MWA procedure
After local anaesthesia with 1% lidocaine, US-guided biopsy was performed using an automatic biopsy gun with an 18-gauge cutting needle; 2 or 3 separate punctures were performed. Subsequently, the antenna was percutaneously inserted with US guidance into the tumour and placed in the aimed location. One antenna was inserted for lesions less than 2.0 cm, and 2 antennae were inserted for lesions measuring 2.0 cm or greater with an interantenna distance of no more than 1.8 cm. After all insertions, intravenous anaesthesia was administered using a combination of propofol (Diprivan; Zeneca Pharmaceuticals, Wilmington, DE, USA) and ketamine (Shuanghe Pharmaceuticals, Beijing, China) via the peripheral vein during standard hemodynamic monitoring. A power output of 50 W for 6–10 min was routinely used during MWA. If the heat-generated hyperechoic water vapour did not encompass the entire tumour completely, a prolonged microwave emission was applied until the expected temperature was reached. When the antenna was being withdrawn, the applicator track was heated with sufficient microwave energy by stopping the cooling-shaft water dump.
Follow-up
After 1–2 sessions of MWA, 1–3 days after the last ablation session, contrast-enhanced imaging (US and computed tomography [CT] or MR imaging) was performed to evaluate the immediate treatment effectiveness. Technique effectiveness, namely complete ablation, was defined as the absence of enhancement in any areas of the mass on enhanced images obtained at the one-month follow-up after MWA. If complete ablation was achieved, the routine contrast-enhanced US, CT or MR imaging was repeated to monitor for recurrence or metastasis at three months after MWA and then at six-month intervals. After MWA complications were detected by using US and CT or MR imaging and placed on the record, the patients were followed up one month after ablation. To evaluate renal function after MWA, the serum creatinine and urea nitrogen levels were measured at the one month, three months after MWA and then at six-month intervals.
Statistical methods
Data analysis was performed using SPSS (version 16.0.01 for Windows, SPSS, Chicago, IL, USA). The time of follow-up was expressed as the median. Differences were analysed by using Student’s t-test and p values less than 0.05 were considered significant.
Results
Pre-treatment clinical parameters and pathologic characteristics
Nineteen lesions in 18 patients with renal dysfunction had previously been diagnosed as RCC via contrast-enhanced imaging. Before MWA, percutaneous US-guided biopsy was performed in 17 patients, and one patient refused the biopsy for worrying about needle track seeding. Pathological examination indicated clear cell carcinoma in 16 patients and papillary carcinoma in one patient as shown in .
Clinical outcomes and complications
In our study, 19 lesions underwent 21 MWA sessions, and complete ablation was achieved in 19 of 19 lesions (100%). Among the 19 lesions, 17 lesions necrosed after the first ablation; the other two incompletely destroyed tumours were ablated successfully in a second MWA session. The median follow-up time was 24.9 months (range from 3.5 to 85.9 months). During the follow-up, there was no local tumour recurrence in 19 of 19 completely ablated tumour lesions, and of the two patients lost their life, one patient died of acute digestive tract haemorrhage 3.5 months after MWA, and the other patient died of heart failure 37.1 months after MWA. The overall and cancer specific survival rates were 88.9% and 100%, respectively.
According to the Clavien–Dindo Classification of Surgical Complications, Grade I complications were observed and recorded [Citation12]. A total of 2/18 (11.1%) patient complained of mild pain at the ablation site, which regressed with analgesics; 1/18 (5.6%) patient experienced vomiting after MWA; 4/18 (22.2%) patients had microscopic haematuria on the day following MWA. All patients’ symptoms were alleviated after suitable treatment.
Renal function
Before MWA, all 18 patients had abnormal initial serum creatinine values, which were identified by laboratory testing. Immediately after ablation, 14/18 patients had slightly elevated serum creatinine and urea nitrogen levels, while 4/18 patients had slightly decreased levels. The mean serum creatinine level of all 18 patients was 232.2 μmol/L before the initial procedure, 253.2 μmol/L one day after MWA and 300.5 μmol/L of the last follow-up (). The mean serum urea nitrogen level was 10.5 mmol/L before the initial procedure, 11.2 mmol/L 1 day after MWA and 11.9 mmol/L of the last follow-up (). However, there was no statistical difference between the pre-MWA and post-MWA serum creatinine and urea nitrogen levels.
Figure 1. The changes of renal function varied in patients of different CKD stages. (A) The serum creatinine concentration pre-MWA, one day after MWA and at the final follow-up; (B) The serum urea nitrogen concentration pre-MWA, one day after MWA and at the final follow-up.
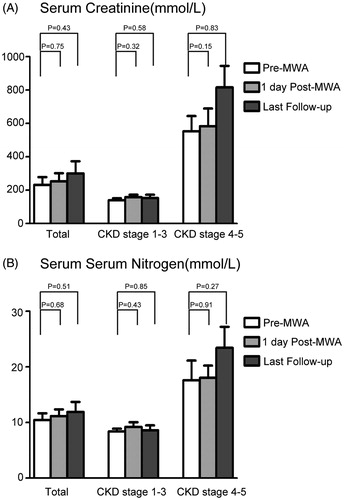
After MWA treatment, no obvious changes of renal function were observed in patients of CKD stage 1–3, and the renal function in patients of CKD stage 4–5 did not significantly deteriorate. As shown in , pre-MWA and one day after MWA and in the last follow-up, the mean serum creatinine levels were 553.2 μmol/L, 584.1 μmol/L and 816.0 μmol/L, respectively. Pre-MWA, one day after MWA and in the last follow-up, the mean serum urea nitrogen levels were 17.6 mmol/L, 18.0 mmol/L 23.4 mmol/L, respectively. However, the elevation of serum creatinine and urea nitrogen levels was not statistically significant. Renal function of 13 CKD stage 1–3 patients did not worsen enough to necessitate dialysis until the last follow-up. One patient of CKD stage 3 and 4 patients of CKD stage 4–5 who have received dialysis before MWA, survived depend on long-term dialysis.
Discussion
It is a difficult problem for the urologists to treat renal tumours detected in patients who already suffered from their CKD. Even without the detection of renal tumour, there is a possibility for the patients with renal function insufficiency to receive dialysis. But neither the urologists nor the patients want to increase this possibility in the treatment of renal tumours. The goals of the treatment are the complete removal of the tumour and the maintenance of renal function. Currently, more and more urologists realised that laparoscopic partial nephrectomy was risky for patients who suffer from both renal dysfunction and renal tumour. Previous study illustrated percutaneous RFA is superior to open partial nephrectomy with respect to the preservation of renal function [Citation6]. Compared with RFA, MWA could form a larger ablation zone which may be a double-edge sword. The larger ablation zone allows a safer ablation margin, but sometimes it may also cause more kidney parenchyma destruction. Our previous study found that after MWA there was a slight elevation in the serum creatinine and urea nitrogen levels [Citation13]. Other studies demonstrated that during the follow-up, the decrease of estimated GFR after MWA was similar to that after the partial nephrectomy [Citation7]. On one hand, to our knowledge, there have been no reports specialised in the clinical outcomes and affect to renal function of percutaneous MWA in patients with renal dysfunction; on the other hand, we hope to identify the influence of MWA treatment to residual renal function in such patients using this newly rising minimal invasive thermal technique in renal tumour.
First, the percutaneous US-guided MWA is an effective and safe technique for treating RCC patients with renal dysfunction. In our study, under the guidance of US, complete ablation was successfully achieved in all the tumour lesions. The technical effectiveness was 100% and no local recurrence was detected in all the ablated lesions during the follow-up ( and ). In our study, four tumour lesions adjacent to kidney pelvis and 1 lesion close to intestinal tract were completely ablated with the help of the thermal monitoring system without posing injury to the adjacent organ. Two out of the 18 (11.1%) patients died of other diseases uncorrelated to RCC and renal dysfunction.
Figure 2. A 3.3 × 3.0 cm tumour in the solitary kidney of a 48-year-old woman treated with MWA. (A) Preablation enhanced-CT image shows the lesion adjacent to the renal pelvis (white arrow); (B) CEUS displays a heterogeneous hyperenhancement neoplasm (white arrow); (C) CEUS shows no hyperenhancement in the ablation zone (grey arrow) three days after MWA; (D) After MWA, a MR Image shows no residual tumour in the ablation zone (grey arrow).
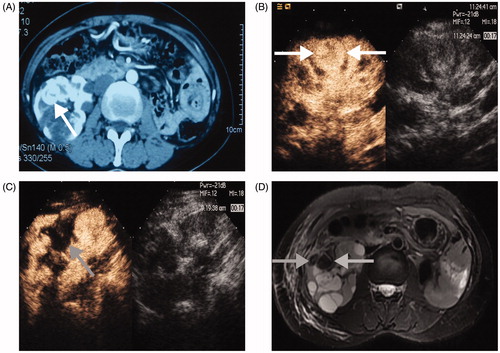
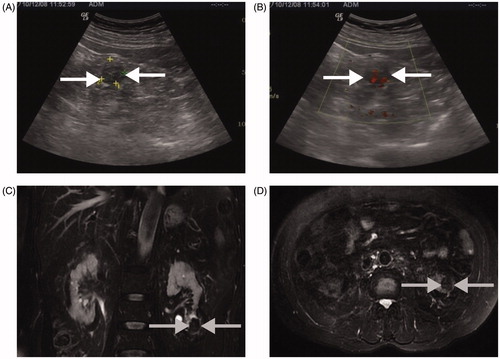
Figure 3. A 2.5 × 2.5 cm tumour lesion in the left kidney of a 76-year-old man treated with MWA. (A and B) A right kidney tumour is detected by conventional US at physical check-up (white arrow); (C and D) Five years after MWA MR image shows no recurrent tumour in the ablation zone (grey arrow).
Second, the percutaneous US-guided MWA did not pose unacceptable harm to the residual renal function. Protecting renal function as much as possible is of paramount importance in the treatment of patients with renal dysfunction. As the renal function reservation vary in patients of different CKD stages, we analysed the change of renal function pre-MWA and post-MWA according to the CKD stage. Our data showed that no significant elevation was observed either in patients of CKD stage 1–3 or in patients of CKD stage 4–5. In the last follow-up, the serum creatinine and urea nitrogen levels of CKD stage 4–5 patients were higher than that of pre-MWA. One reason is that patients of CKD stage 4–5 had a worse renal function reservation than patients of CKD stage 1–3. The other reason is that primary CKD did harm to kidney function continuously. However, in this study there were only four patients of CKD stage 4–5, further intensive study should be performed to identify the exact reason in the future.
Based on our follow-up results, we conclude that percutaneous MWA is effective and safe for the management of RCC in patients with renal dysfunction and that it causes an acceptable loss of renal function. This thermal technique brings a feeble gleam of hope to the patients who lost the chance of surgery. Even for the patients of CKD stage 4–5 with RCC, MWA would not pose unacceptable damage to their residual function. As the patient with renal dysfunction and RCC is rarer, our current results are based on a relatively small sample size, and the follow-up data in our study were limited. The long-term clinical efficiency and safety of MWA for renal tumours of patients with kidney dysfunction required a well-designed prospective study, a larger sample size and dynamic observation of renal function after MWA.
Acknowledgments
This research was supported by the State Key Program of National Natural Science Foundation of China [Grant No. 81430039] and the National Science & Technology Pillar Program during the 12th Five-year Plan Period [Grant No. 2013BAI01B01].
Disclosure statement
The authors report no declarations of interest.
References
- Luciani LG, Cestari R, Tallarigo C. (2000). Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982–1997). Urology 56:58–62.
- Ljungberg B, Hanbury DC, Kuczyk MA, et al. (2007). Renal cell carcinoma guideline. Eur Urol 51:1502–10.
- Zondervan PJ, Buijs M, de la Rosette JJ, et al. (2016). Cryoablation of small kidney tumors. Int J Surg 2016. [Epub ahead of print]. doi: 10.1016/j.ijsu.2016.06.049.
- Weisbrod AJ, Atwell TD, Frank I, et al. (2010). Percutaneous cryoablation of masses in a solitary kidney. AJR Am J Roentgenol 194:1620–5.
- Shin BJ, Chick JF, Stavropoulos SW. (2016). Contemporary status of percutaneous ablation for the small renal mass. Curr Urol Rep 17:23
- Sung HH, Park BK, Kim CK, et al. (2012). Comparison of percutaneous radiofrequency ablation and open partial nephrectomy for the treatment of size- and location-matched renal masses. Int J Hypertherm 28:227–34.
- Guan W, Bai J, Liu J, et al. (2012). Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol 106:316–21.
- Simon CJ, Dupuy DE, Mayo-Smith WW. (2005). Microwave ablation: principles and applications. Radiographics 25:S69–S83.
- Carrafiello G, Mangini M, Fontana F, et al. (2010). Single-antenna microwave ablation under contrast-enhanced ultrasound guidance for treatment of small renal cell carcinoma: preliminary experience. Cardiovasc Interven Radiol 33:367–74.
- Chen CN, Liang P, Yu J, et al. (2016). Contrast-enhanced ultrasound-guided percutaneous microwave ablation of renal cell carcinoma that is inconspicuous on conventional ultrasound. Int J Hypertherm 32:607–13.
- National Kidney F. (2002). K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39:S1–S266.
- Dindo D, Demartines N, Clavien PA. (2004). Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–13.
- Yu J, Liang P, Yu XL, et al. (2012). US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology 263:900–8.
