Abstract
Purpose: To determine the incidence, risk factors and prognosis associated with needle track seeding after percutaneous radiofrequency ablations (RFA) for hepatocellular carcinoma (HCC) with a long-term follow-up.
Materials and methods: A total of 741 HCC patients undergoing percutaneous RFA were retrospectively analysed. Mean follow-up interval was 34.3 ± 26.8 months. All seeding neoplasms were diagnosed by imaging modalities with or without pathological evaluation. Risk factors, including Child–Pugh grading, tumour size, number, location, serum alpha-fetoprotein (AFP) level, track number, biopsy before RFA and electrode type were performed by univariate analysis. Further therapy and survival of seeding after RFA were assessed. Survival analysis was analysed by Kaplan–Meier method.
Results: Twelve patients (12 tumours) were diagnosed as seeding. It corresponds to an incidence of 1.6% (12/741) per patient and 0.9% (12/1341) per tumour. Seeding developed an average of 14.0 ± 8.1 months (6–33 months). Significant risk factors included tumour >3 cm (p = 0.031), subcapsular tumour (p = 0.031), biopsy before RFA (p = 0.001) and non-cool-tip electrode (p = 0.034). Eight patients received local therapy and four cases only received systematic therapy for uncontrolled advanced hepatic tumour or distal metastasis. Of eight patients receiving local therapy, one patient had local recurrence 16 months later and other seven patients did not have local recurrence for 3–73 months. The cumulative survival rates after seeding were 55.6%, 27.8%, 9.3% at 1, 3 and 5 years, respectively.
Conclusion: Needle track seeding is a rare delayed complication after percutaneous RFA. Tumour >3 cm, subcapsular tumour, biopsy before RFA and non-cool-tip electrode are potential risk factors for seeding. Local therapies are effective methods for seeding patients.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignant tumours of the liver. Historically, curative therapies for HCC were surgical resection and transplantation [Citation1]. However, in recent years, radiofrequency ablation (RFA) has become an alternative curative therapy for early HCC [Citation2,Citation3]. In addition, RFA also plays an important role in the treatment of unresectable tumours [Citation4–6]. Even though RFA is a minimally invasive technique, various complications have been reported [Citation7–10]. Among those, needle track seeding is a rare major complication. Most studies reported the occurrence rate of seeding was at a lower incidence of 0–5.1% [Citation11–16] and the likely risk factors included poorly differentiated tumour, larger tumour, subcapsular tumour, a high serum alpha-fetoprotein (AFP) value, biopsy before RFA and repeated insertion [Citation11–14,Citation17]. However, risk factors for seeding remain poorly understood because of lower incidence. Furthermore, it is not clear whether seeding affect patients long-term outcome. In this study, we retrospectively summarised our 14 years’ experience on post-RFA needle track seeding with a large patients samples. We conducted this study to evaluate the incidence, risk factors and prognosis of needle track seeding associated with percutaneous RFA in HCC patients. Also, we collected the 1-, 3-, 5-year survival data after post-RFA needle track seeding to assess its effect on long-term outcomes of HCC patients.
Materials and methods
Patients
From January 2000 to December 2013, 772 consecutive HCC patients undergoing ultrasound-guided percutaneous RFA were retrospectively analysed in this study. The diagnosis of HCC was obtained by core needle biopsy or non-invasive criteria defined by American Association for the Study of Liver Diseases practice guidelines [Citation18]. The treatment was approved by the institutional review board and written informed consents were obtained from all patients before treatment.
All patients met the following criteria for RFA: (1) tumours visible on ultrasonography or contrast-enhanced ultrasonography, (2) without direct invasion of adjacent organs, (3) the absence of portal venous thrombosis, (4) platelet count >50 × 103/mm3 and prothrombin activity >50%, (5) for radical treatment, single tumour size ≤5 cm in maximum diameter, while multiple tumours with a maximum diameter ≤3 cm and tumour number ≤3, (6) for palliative treatment, patients with larger tumours or more tumour number were also included for the aim of reducing tumour burden and prolonging the survival.
Among 772 patients, 31 patients who lost follow-up were excluded. Finally, 741 patients with 1341 HCC tumours were included in the study. There were 596 males and 145 females. The mean age of patients was 59.7 ± 11.5 years (24–88 years). The mean follow-up interval was 34.3 ± 26.8 months (1–161 months). According to Child–Pugh grading, there were 993 Child–Pugh A patients, 320 Child–Pugh B patients and 28 Child–Pugh C patients. The mean tumour size was 2.9 ± 1.4 cm (0.6–8.6 cm).
RFA procedure
Three kinds of RFA system were used in this study, including multitined expandable (RITA-1500 RFA system, Mountain View, CA), internally cool-tip Monopolar (Cool-tip RF ablation system, Boulder, CO) and internally cool-tip multipolar (CelonLab POWER system, Teltow, Germany) electrodes. Aloka SSD-4000, SSD-5500, α-10 (Tokyo, Japan) and GE Logic-9 (CT) ultrasound systems were used for scanning and guidance.
All RFA procedures were performed by two radiologists with more than 5 years of experience in ultrasound-guided interventional procedures. During the procedure, intravenous moderate sedation anaesthesia was induced with 2.5–5.0 mg of midazolam (Roche; Basel, Switzerland) and 50–100 μg of fentanyl (Fentaini, YiChang, China). Local infiltration anaesthesia was induced by 1% lidocaine (Liduokayin, Beijing, China). For tumours adjacent to the diaphragm, hepatic hilum or liver surface, an intravenous bolus of propofol (Diprivan, Macclesfield, United Kingdom) 1–2 mg was given to enhance anaesthesia.
Patients were placed in the supine or oblique position depended on tumour location. Electrodes were inserted into the tumour under ultrasound guidance. For tumours larger than 3 cm, overlapping ablations were used. During the procedure, vital sign of the patients was continuously monitored. After treatment, track ablation was performed. The track ablation mode was selected according to equipment instruction. The electrode was withdrawn step by step and each step was around 1–2 cm distrance until leave the patients skin. During the procedure, the temperature of needle tip was kept being above 70 °C.
Follow-up and diagnosis of needle track seeding
Treatment response was evaluated by contrast-enhanced computed tomography (CECT) or magnetic resonance imaging (MRI) at 1 month after RFA procedure. Then, serum AFP (alpha-fetoprotein) test, abdominal ultrasound and CECT or MRI were repeated every 3 months in the first year, and every 4–6 months in the following years.
Needle track seeding was defined as new neoplastic nodule occurring along the RFA needle track outside the liver. All neoplasms were demonstrated by imaging modalities, including ultrasonography, CT and MRI. Final diagnosis can be confirmed by pathological evaluation from biopsy or resection tissues. For those without pathological evaluation, the final diagnosis was based on imaging examination.
Statistical analysis
Continuous variables were expressed as mean ± SD. Comparisons of continuous variables were performed by Student’s t-test. Comparisons of categorical variables were performed by Fisher exact test. Risk factors, including Child–Pugh grading, tumour size, number, location, serum AFP level, track number, biopsy before RFA and electrode type were analysed with univariate analysis. To elucidate tumour location as a possible risk factor for seeding, we divided tumours into two groups: subcapsular tumour and deep tumour. The subcapsular tumour was defined as the tumour was located under the surface of liver and electrode was directly inserted into the tumour without passing through normal liver parenchyma. Survival analysis was analysed by Kaplan–Meier method. The level of significant was set at 0.05 for all tests. SPSS19.0 software (SPSS Inc., Chicago, IL) was used to perform the analysis.
Results
Incidence of needle track seeding
Twelve patients (12 tumours) were diagnosed as needle track seeding after RFA procedures (. It corresponds to an incidence of 1.6% (12/741) per patient and 0.9% (12/1341) per tumour. Needle track seeding developed an average of 14.0 ± 8.1 months (6–33months) after RFA (). The site of seeding included abdominal wall in seven patients, peritoneal cavity in two patients and thoracic wall in three patients. Among these patients, four cases were diagnosed based on pathological examination and the other eight cases were diagnosed based on imaging findings. All neoplasms were firstly detected by ultrasonography. High-frequency ultrasonography and colour Doppler ultrasonography were very sensitive for the diagnosis of seeding. Ultrasonography demonstrated neoplastic nodules along the needle track with hyperechogenicity or hypoechogenicity. Colour Doppler ultrasound showed arterial or venous signal in 10 lesions and no blood signal in two lesions.
Figure 1. A 48-year-old man with well-differentiated hepatocellular carcinoma underwent percutaneous RFA. (A) Twenty-month follow-up ultrasonography shows a 10 mm seeding nodule (arrow) with hypo echo in abdominal wall. (B) CECT shows seeding nodule (arrow) and subcapsular ablation zone (arrowhead) in portal phase.
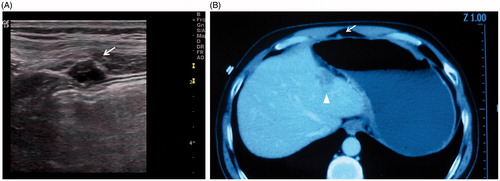
Table 1. Clinical feature of 12 HCC patients associated with neoplastic seeding.
Risk factors analysis
The clinical feature of two groups was summarised in . There was significant difference between seeding and without seeding group in tumour >3 cm (p = 0.031), subcapsular tumour (p = 0.031), biopsy before RFA (p = 0.001) and non-cool-tip electrode (p = 0.034), while no significant difference in Child–Pugh grading, tumour number, serum AFP level and track number.
Table 2. Clinical feature of 741 HCC patients with 1341 tumours treated by percutaneous RFA.
Therapy and prognosis
In 12 seeding patients, eight patients without uncontrolled advanced hepatic tumour received local therapy, including resection (n = 3), high-intensity focussed ultrasound therapy (HIFU) (n = 4) and external radiotherapy (n = 1). One patient with HIFU had local recurrence 16 months later and other seven patients did not have local recurrence. Four patients with uncontrolled advanced hepatic tumour only received systematic therapy. The mean follow-up period of seeding nodules after detection was 23.6 ± 22.1 months (3–73 months). The overall survival rates after seeding were 55.6%, 27.8%, 9.3% at 1, 3 and 5 years respectively (mean survival: 25.6 ± 6.4 months). The survival rates in patients without uncontrolled advanced hepatic tumour who received local therapy at 1, 3 and 5 years were 85.7%, 42.9%, 14.3%, respectively, while the survivals in patients with uncontrolled advanced hepatic tumour were only 3–8 months.
Discussion
RFA has been considered as a relatively safe and effective technique for small HCC in recent years. Although as a minimally invasive approach, RFA is associated with some major complications including uncontrolled bleeding, bowel perforation, bile fistula and needle track seeding [Citation7–10]. In the early stage, a small sample study by surgeons reported that needle track seeding occurred up to 12.5% [Citation17]. However, most subsequent studies reported seeding occurred 0–5.1% [Citation11–16]. In our centre, the incidence of seeding was 0.9% per tumour, which was at a low level of incidence compared with previous studies. One possible reason may be track ablation performed in our study. Track ablation can destroy potential tumour cell dissemination along the track during the procedure. According to previous studies, the incidence of seeding with track ablation was lower than those without track ablation (0.7–3.2% vs 5.1–12.5%) [Citation11–17]. Therefore, to our knowledge, track ablation is encouraged as a routine technique when RFA is terminated.
Needle track seeding developed an average of 14 months (6–33 months) after RFA. Some studies reported seeding neoplasms were detected even 5 years after RFA [Citation14,Citation15]. This indicates that neoplastic seeding is a delayed complication usually occurring several months after RFA. Accordingly, seeding should be carefully evaluated during follow-up, especially in the first 3 years.
Previous studies reported that risk factors for seeding may include poorly differentiated tumour, larger tumour, subcapsular tumour, a high serum AFP value, biopsy before RFA and repeated insertion [Citation11–14]. In the present study, Child–Pugh grading, tumour size, number, location, serum AFP level, track number, biopsy before RFA and electrode type were considered. The results showed that tumour size >3 cm, subcapsular tumour, biopsy before RFA and electrode type were potential risk factors for neoplastic seeding. Tumour size is an important factor for RFA effect. Complete necrosis is more likely to be achieved in tumour ≤3 cm than >3 cm [Citation2]. Once incomplete necrosis occurred, activated tumour cells may disseminate along the needle track and finally become seeding neoplasm. During RFA procedure of subcapsular tumours, intratumoral pressure rapidly increased by heat may lead to dissemination of tumour cells into peritoneal cavity [Citation14]. To prevent rapidly increased intratumoral pressure, gradually amplifying radiofrequency power may reduce the rate of seeding [Citation14]. Moreover, electrode should not be inserted into subcapsular tumour directly if possible. Instead, a path that passed through normal liver parenchyma was recommended [Citation13]. This approach created an intrahepatic path to reduce the risk of dissemination of tumour cells into peritoneal cavity.
Stigliano et al. reported the median risk of seeding was 0.61% (0–5.56%) for RFA without biopsy and 0.95% (0–12.5%) for RFA with biopsy [Citation19]. Although track ablation was performed during RFA, biopsy before RFA was not in the same track of RFA electrode and the incidence of seeding increased. Therefore, it is suggested that biopsy should be avoided if HCC can be diagnosed by imaging examination and serum test.
Electrode type is another potential factor of seeding. Comparison of different electrode type was not reported in previous studies. Seeding in non-cool-tip electrode group is more than cool-tip electrode group. A possible explanation for this is that carbonisation occurs much more in non-cool-tip electrode and carbonisation tissue may adhere to the hook of electrode. When withdrawing the hooks, carbonisation tissue is clipped between the hooks, which increases the risk of dissemination of tumour cells, especially for the condition of repetitious electrode placement. However, the incidence of seeding in non-cool-tip group was still at a low level of 1.4% (11/800). Due to a small number of seeding cases, further studies are needed to confirm whether cool-tip electrode is superior.
As a major complication, neoplastic seeding seemed to affect patients’ prognosis. However, there were few studies reported impact of seeding on the patients’ survival. Imamura et al. reported the cumulative survival rates in the patients with seeding at 1, 2 and 3 years were 81.3%, 45.4% and 26.9%, respectively [Citation14]. The authors considered seeding neoplasms themselves did not directly affect the patients’ survival. Shirai et al. reported the cumulative survival rates after seeding were 76.9% at 1 year, 41.0% at 3 years and 20.5% at 5 years [Citation15]. In the present study, post-seeding survival rates were 55.6%, 27.8%, 9.3% at 1, 3 and 5 years, respectively, which showed poorer prognosis than the former studies. The reason might be that there were more advanced tumours included in our centre for palliative therapy.
The therapy of seeding includes resection, external radiotherapy, RFA and HIFU according to the previous studies [Citation20–22]. In our study, three patients received resection, four received HIFU and one received external radiotherapy. About 87.5% (7/8) patients achieved local cure. The survival rate of those eight patients at 3 years was up to 42.9%. This result showed that the neoplastic seeding was a low-risk complication of percutaneous RFA of liver cancer and was considered acceptable in general after local therapy. Thus, once occurring track seeding without distant metastasis, seeding can be well controlled. However, patients with uncontrolled advanced hepatic tumour did not receive local therapy, and the survival was only 3–8 months. Therefore, local therapy should be considered, especially for those without uncontrolled advanced hepatic tumour.
The limitations of present study were as follows: (1) As a retrospective study, limited potential risk factors were analysed, and other factors which may result in seeding were not included, such as tumour differentiation. Further well-designed prospective studies are warranted to confirm. (2) Because of low incidence of seeding, a small number cases was another limitation. Thus, further multiple-centre studies or systems analysis are needed to well understand risk factors.
Conclusions
Needle track seeding is a rare delayed complication after percutaneous RFA. Tumour >3 cm, subcapsular tumour, biopsy before RFA and non-cool-tip electrode were potential risk factors for seeding. Careful follow-up should be done to detect seeding earlier. Local therapies are effective methods for seeding patients, especially those without uncontrolled intrahepatic recurrence.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Bruix J, Sherman M. (2005). Management of hepatocellular carcinoma. Hepatology 42:1208–36.
- Cucchetti A, Piscaglia F, Cescon M, et al. (2013). Systematic review of surgical resection vs radiofrequency ablation for hepatocellular carcinoma. World J Gastroenterol 19:4106–18.
- Lau WY, Lai EC. (2009). The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg 249:20–5.
- Wang W, Shi J, Xie WF. (2010). Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: a meta-analysis. Liver Int 30:741–9.
- Howard JH, Tzeng CW, Smith JK, et al. (2008). Radiofrequency ablation for unresectable tumors of the liver. Am Surg 74:594–600.
- Yan k, Chen MH, Yang W, et al. (2008). Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol 67:336–47.
- Kong WT, Zhang WW, Qiu YD, et al. (2009). Major complications after radiofrequency ablation for liver tumors: analysis of 255 patients. World J Gastroenterol 15:2651–6.
- Chen TM, Huang PT, Lin LF, Tung JN. (2008). Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: single center experience. J Gastroenterol Hepatol 23:445–50.
- Giorgio A, Tarantino L, de Stefano G, et al. (2005). Complications after percutaneous saline-enhanced radiofrequency ablation of liver tumors: 3-year experience with 336 patients at a single center. Am J Roentgenol 184:207–11.
- Fu Y, Yang W, Wu JY, et al. (2011). Intrahepatic biliary injuries associated with radiofrequency ablation of hepatic malignancies. Chin Med J 124:1957–63.
- Latteri F, Sandonato L, Marco VD, et al. (2009). Seeding after radiofrequency ablation of hepatocellular carcinoma in patients with cirrhosis: a prospective study. Dig Liver Dis 40:684–9.
- Livraghi T, Lazzaroni S, Meloni F, Solbiati L. (2005). Risk of tumor seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br J Surg 92:856–8.
- Jaskolka JD, Asch MR, Kachura JR, et al. (2005). Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol 16:485–91.
- Imamura J, Tateishi R, Shiina S, et al. (2008). Neoplastic seeding after radiofrequency ablation for hepatocellular carcinoma. Am J Gastroenterol 103:3057–62.
- Shirai K, Tamai H, Shingaki N, et al. (2011). Clinical features and risk factors of extrahepatic seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res 41:738–45.
- Cabibbo G, Craxi A. (2009). Needle track seeding following percutaneous procedures for hepatocellular carcinoma. World J Hepatol 1:62–6.
- Llover JM, Cilana R, Brú C, et al. (2001). Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology 33:1124–9.
- Bruix J, Sherman M. (2011). Management of hepatocellular carcinoma: an update. Hepatology 53:1020–2.
- Stigliano R, Marelli L, Yu D, et al. (2007). Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellularcarcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev 33:437–47.
- Yu J, Liang P, Yu XL, et al. (2012). Needle track seeding after percutaneous microwave ablation of malignant liver tumors under ultrasound guidance: analysis of 14-year experience with 1462 patients at a single center. Eur J Radiol 81:2495–9.
- Wang Y, Wang W, Wang Y, Tang J. (2010). Ultrasound-guided high-intensity focused ultrasound treatment for needle-track seeding of hepatocellular carcinoma: preliminary results. Int J Hyperthermia 26:441–7.
- Espinoza S, Briggs P, Duret JS, et al. (2005). Radiofrequency ablation of needle tract seeding in hepatocellular carcinoma. J Vasc Interv Radiol 16:743–6.
