Abstract
Purpose: Hyperthermia (HT), an adjuvant therapy for variable cancers, may cause physiological changes in the patients, which may lead to cardiovascular problems. Among various HT treatments, the physiological effects of deep regional HT are still unclear. We examined the physiological alterations throughout deep regional HT to improve the HT safety.
Materials and methods: Thirty-one patients (age: 61 ± 12 years) with cancer received HT in the thoracic or upper abdominal regions using an 8-MHz radiofrequency-capacitive-device for 50 min. Rectal temperature (Trec), systolic and diastolic blood pressures (SBP and DBP), pulse rate (PR), respiratory rate (RR), percutaneous oxygen saturation (SpO2) and sweating volume were evaluated throughout HT.
Results: At 50 min after starting HT, Trec, PR and RR were significantly increased compared with the baseline values (Trec: 38.2 ± 1.4 vs. 36.3 ± 0.8 °C, p < 0.001, PR: 104 ± 15 vs. 85 ± 16 bpm, p < 0.05, RR: 23 ± 3 vs. 21 ± 3/min, p < 0.05). Although the average SBP and DBP were both stable during HT in a recumbent position, these values dropped significantly in a standing position (SBP: 113 ± 16 vs. 127 ± 18 mmHg, p < 0.001, DBP: 70 ± 12 vs. 75 ± 13 mmHg, p < 0.01). The total amount of sweating was 356 ± 173 g/m2 on average.
Conclusions: Deep regional HT increased the deep body temperature and resulted in an increase of sweating with peripheral vasodilatation. Consequently, a significant reduction in BP would be induced on standing after HT. Careful attention is needed for patients receiving HT, especially when standing after HT.
Introduction
Hyperthermia (HT) is an adjuvant therapy for advanced, recurrent or refractory cancer patients receiving chemotherapy or radiotherapy. It is classified into local or regional HT for isolated metastatic cancers and whole-body HT for metastatic cancers that are spread throughout the body. Compared with whole-body HT performed under anaesthesia, local or regional HT using microwaves or radiofrequency (RF) waves has often been applied for various cancers, except for brain and optic tumours. Since deep regional HT is less invasive than whole-body HT, it can be performed in outpatient settings and has become the mainstream HT in Japan, especially since gaining coverage under the Japanese national health insurance program in 1990. The number of cancer patients receiving local or regional HT has increased approximately 3.8-fold in the past 5 years [Citation1]. Combination chemotherapy or radiotherapy with deep regional HT has been reported to diminish gastrointestinal, intrathoracic or intrapelvic tumour size more effectively than chemotherapy or radiotherapy alone and improved the survival rate [Citation2–7]. Ostapenko [Citation8] and Ohguri [Citation9] also showed that continuation of deep regional HT activated the immune function or relieved pain, resulting in an improvement in the quality of life (QOL) of cancer patients.
With the widespread application of deep regional HT, its adverse effects have also been examined. Pain caused by hotspots and/or burning is the most common adverse effect of deep regional HT [Citation10]. Significant increases in the body temperature, pulse rate (PR) and respiratory rate (RR) and a decrease in the arterial oxygen partial pressure after heating have also been reported [Citation11]. However, the precise alterations in the physiological parameters throughout HT are still unclear. The majority of advanced cancer patients with chemotherapy or radiotherapy suffer from a low metabolism and malnutrition, which exacerbate the adverse reactions of HT in a vicious cycle [Citation12,Citation13]. We should therefore consider the possibility that the adverse effects of cancer patients might experience more severe adverse reactions to HT than healthy people.
The aim of this study was to clarify the physiological changes in cancer patients receiving deep regional HT to improve the safety of it.
Materials and methods
Patient characteristics
We recruited 31 cancer patients (mean age: 61 ± 12 years) receiving weekly deep regional HT at the outpatient ward of Tobata-Kyoritsu Hospital in this study. Their demographics and clinical characteristics are shown in . The original cancers were lung (22.6%), colon (22.6%) and stomach cancer (19.4%). The average body mass index (BMI) was 21.1 ± 3.5 kg/m2, which was not markedly lower than the average BMI of the general same-age Japanese population (males: 23.6 ± 3, females: 22.8 ± 3.6 kg/m2). 28 patients were treated with chemotherapy and three with radiotherapy, and chemotherapy had been implemented for more than 6 months before the date of the experiment. Among these patients, four patients were temporarily withdrawn for 1–2 months before the day of the experiment, while the remaining 24 were receiving the treatments just before HT on the same day shown in .
Table 1. Patient characteristics.
The values of the Eastern Cooperative Oncology Group (ECOG) Performance Status Scale [Citation14], which is widely used to evaluate the functional status of patients with cancers quantitatively, are also shown in . 25 (80.6%) were status 1, 5 (16.2%) were status 2 and 1 (3.2%) was status 0. The positions during deep regional HT were prone in 23 patients (74.2%) and supine in 8 (25.8%). The heating areas were the chest in 13 patients (41.9%) and the abdomen in 18 (58.1%).
This study was approved by both the Ethics Review Boards of Tobata-Kyoritsu Hospital (Approval No. 13–14) and Kyushu University (Approval No. 26–214) in Japan. All of the patients agreed to voluntarily participate in this study and gave their written informed consent. We did not recruit advanced cancer patients to undergo sham heating in a control group based on the recommendation of the Ethics Review Board.
Thermotherapy
The room temperature and relative humidity during deep regional HT were maintained at a mean 26 ± 0.6 °C and 50 ± 7%, respectively. The protocol and principles of the heating device have been previously reported [Citation15]. Briefly, all patients were heated for 50 min using an RF capacitive heating device (Thermotron RF-8; YAMAMOTO VINITA Co., Ltd., Osaka, Japan) that generated a self-excited oscillation circuit at 8 MHz RF and 1.5 kW output at maximum. The RF energy was transmitted from a generator via two coaxial cables to two disc electrodes. Patients placed the target regions between a pair of parallel-opposed circular electrodes measuring 30 cm in diameter in either a prone or supine position. The treatment goal was at least 30 min of continuous heating after the RF output was increased to the patient’s tolerance threshold. Patients were carefully instructed to report any unpleasant sensations. The RF output was increased to the maximum level tolerable by the patient after appropriately adjusting the treatment setting. To reduce any preferential heating of subcutaneous fat tissue, overlay boluses were applied in addition to the regular boluses attached in front of the metal electrodes. The liquid (0.5% NaCl) inside the overlay boluses was cooled by the RF-8 circulatory system during heating.
The maximum RF output was 1026 ± 307 W during deep regional HT. A comparison between the deep heating areas showed no significant differences in the maximum RF output between the heating areas: 973 ± 277 W at chest heating and 1067 ± 323 W at upper abdomen heating. The estimated heat energy delivered throughout deep regional HT was 10 138 ± 3865 J/m2.
Study protocol
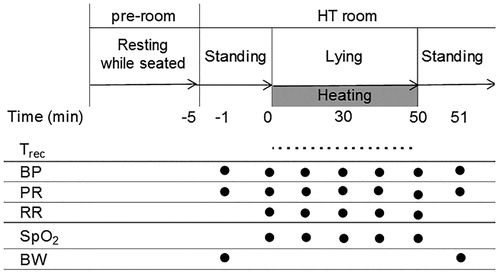
Twenty-four of 28 patients (excluding four patients in whom chemotherapy was ceased on the day of the experiment) underwent chemotherapy and three patients received radiotherapy on the same day (prior to HT). After urination, all patients were weighed on a scale while wearing a laboratory gown and then rested by sitting in a chair for approximately 5 min in the preparation room. On the HT table, they maintained a supine or prone position while being overlaid with boluses and a pair of circular electrodes for 50 min. There were no patients treated with deep regional HT in the pelvic region, which would have directly influenced the measurement of the rectal temperatures.
As shown in , we measured the rectal temperature (Trec) using the thermometer attached to the ThermotronRF-8, blood pressure (BP; ES-H55D; TERUMO CORPORATION, Tokyo, Japan), PR, RR, percutaneous oxygen saturations (SpO2; PULSOX-1; KONICA MINOLTA JAPAN, INC., Tokyo, Japan) and body weight (BW; accuracy ±50 g; BS-150; Dretic CO., LTD., Saitama, Japan). The Trec was monitored at 1 min intervals during deep regional HT. The BP and PR were obtained with the patient in the recumbent position at 10-min intervals during heating and immediately upon standing beside the table after deep regional HT. Similarly, the RR and SpO2 were measured at 10-min intervals during deep regional HT. The amount of sweating was calculated as the difference in the BW before and after HT plus the oral intake during HT, divided by the body surface area (Du Bois formula) [Citation16]. The oral intake was also measured during deep regional HT.
Statistical analyses
All of the data are presented as the mean value ± standard deviation. Baseline (0 min) was defined as the variables measured in the recumbent position before heating. An analysis of variance was used to determine the changes in the physiological parameters during deep regional HT. Spearman’s rank-order coefficient (rs) was used to evaluate the monotonic relationship between the BP changes during HT and at standing after HT and factors that are likely to induce a reduction in the BP. A statistical analysis of the BP and PR between the lying down and standing positions was conducted using a paired t test. All of the statistical analyses were conducted using the SPSS version 21.0 software program (SPSS Inc., Chicago, IL). Differences of p < 0.05 were considered statistically significant.
Results
Changes in the Trec
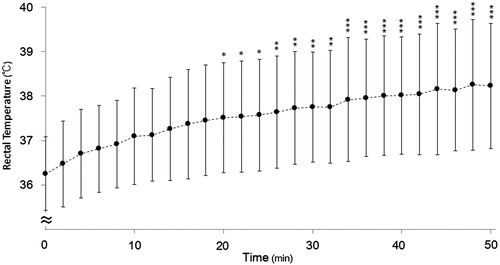
The changes in the Trec are shown in . A significant main effect was observed for the heating period (F (25 780) = 6.112, p < 0.001). The mean Trec of all patients increased gradually, reaching a significant difference from baseline from 20 min (37.5 ± 1.2 vs. 36.3 ± 0.8 °C, p < 0.001). At the end of HT (50 min), Trec was still significantly increased (38.2 ± 1.4 °C, p < 0.001) over that at baseline. Regarding the difference in the highest Trec between different heated regions at 50 min, there was no significant difference between the chest (37.5 ± 1.0 °C, n = 13) and upper abdomen (38.6 ± 1.5 °C, n = 18).
Changes in the PR and BP
The changes in the PR are shown in . A significant main effect was observed for the heating period (F (5180) = 7.41, p < 0.001). The mean PR of all patients increased gradually, reaching a significant difference from baseline from 20 min (99 ± 14 vs. 85 ± 16 bpm, p < 0.01). In the standing position after HT, the PR showed an increasing trend compared with the value obtained while lying down (107 ± 14 vs. 104 ± 15 bpm, p = 0.08), but the differences were not significant.
Figure 3. The serial changes in the (A) PR and (B) blood pressure during HT therapy. n = 31. The data are presented as the mean ± SD, **p < 0.01, ***p < 0.001 vs. 0 min. (C) The individual changes of the systolic and diastolic blood pressure on standing after HT. n = 31. ΔSBP means the difference value of SBP between the end of HT on recumbent position and upright position after HT. ΔDBP means the difference value of DBP between the end of HT on recumbent position and upright position after HT.
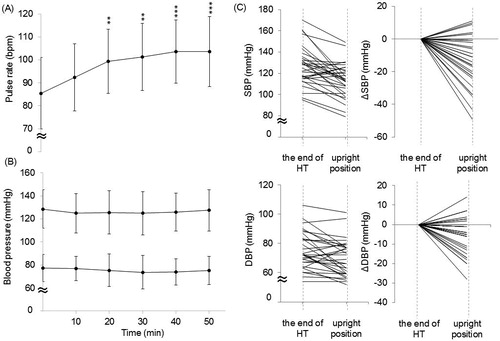
During deep regional HT, the main effect of the heating period on the average BP on recumbent position was not statistically significant (). However, the BP changes during HT were variable in each patient; SBP changed from −52 to +33 mmHg and DBP changed from −26 to +20 mmHg, respectively. We examined the correlation of the changes of SBP and DBP to BMI, Hb, PR and sweating during HT. Only BMI positively correlated with the DBP change (r = 0.406, p = 0.024) and tended to be correlated with SBP change (r = 0.355, p = 0.05) during recumbent position.
Especially, the average SBP and DBP significantly decreased in the standing position after HT than in the lying position (113 ± 16 vs. 127 ± 18 mmHg, p < 0.001, and 70 ± 12 vs. 75 ± 13 mmHg, p < 0.01, respectively), and shows the individual postural BP change in the 31 patients after HT. The number of patients in whom the BP decreased on standing after deep regional HT was 26 (83.9%), and in 16 (61.5%) of them, the SBP dropped more than 20 mmHg and/or the DBP more than 10 mmHg within 3 min after standing from lying down. In addition, 9 of 16 patients reported experiencing dizziness and/or light-headedness upon standing. In two of the nine symptomatic patients, the SBP dropped from 113 to 90 mmHg and from 132 to 98 mmHg, respectively.
The BP decrease occurring on standing after deep regional HT was not correlated with the Hb level, dehydration (amount of sweating) or BMI, all of which were presumed to influence the decrease in the BP on standing. (BMI vs. ΔSBPstanding-lying: r= −0.132, p = 0.478, BMI vs. ΔDBPstanding-lying: r= −0.285, p = 0.120, sweating vs. ΔSBPstanding-lying: r = 0.218, p = 0.240, sweating vs. ΔDBPstanding-lying: r = 0.276, p = 0.133, Hb vs. ΔSBPstanding-lying: r = 0.077, p = 0.681, Hb vs. ΔDBPstanding-lying: r = 0.057, p = 0.761). Additionally, the change of SBP on upright position was negatively correlated with the PR change on recumbent position during HT (r= −0.457, p = 0.01) but not that of DBP.
Changes in the RR and SpO2
The changes in the RR are shown in . A significant main effect was observed for the heating period (F (5180) = 3.085, p = 0.011). The mean RR of all patients was significantly higher than that at baseline from 40 min (23 ± 3 vs. 21 ± 3/min, p < 0.05). As for the respiratory status before deep regional HT, 12 patients suffered from lung cancer (primary in three patients and metastasis in nine patients). However, there was no significant difference in the change of the RR during HT between the patients with and without lung cancer (data not shown).
Figure 4. The serial changes in the (A) RR and (B) SpO2 during HT therapy. n = 31. The data are presented as the mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001 vs. 0 min.
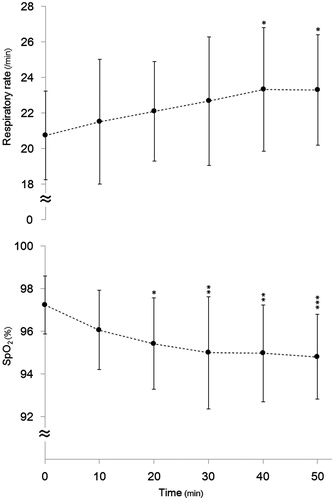
The changes in the SpO2 are shown in . A significant main effect was observed for the heating period (F (5180) = 5.94, p < 0.001). The mean SpO2 was significantly lower than that at baseline at 20 min (95 ± 2% vs. 97 ± 1%, p < 0.05). However, these decreases were within the normal ranges. As with the RR, the SpO2 was not significantly different between the patients with and without lung cancer (data not shown).
Sweating during HT
The mean amount of sweating was 356 ± 173 g/m2 during HT. In addition, positive correlations were noted between sweating and ΔTrec (r = 0.48, p < 0.01) (data not shown). During deep regional HT, only two patients drank a small volume of water (approximately 20 ml per person).
Discussion
In the present study, we clarified the serial changes in the physiological parameters in patients with cancer during deep regional HT. This therapy increased the core body temperatures through RF energy, resulting in a reduction in the body fluid lost by sweating. Consequently, a significant decrease in the BP on standing after HT was induced. Careful attention is needed for patients receiving deep regional HT, especially when standing from the recumbent position after therapy.
The average Trec at 20 min after starting deep regional HT was significantly higher than at baseline (+1.0 to +2.5 °C) to the thorax or upper abdomen, and immediately before finishing HT (at 50 min), the Trec exceeded 38.2 °C, a finding similar to those noted in previous reports of local or regional HT [Citation17–19]. We previously reported the alterations in the physiological parameters during 30 min of whole-body heating using a domed sauna to treat patients with cardiovascular diseases without anaesthesia or sedation (intra-dome temperature: 70 °C), and the Trec slowly increased up to +0.6 °C from baseline [Citation20]. These present and previous results indicated that the deep regional HT increased the body temperature further than whole-body treatment with a domed sauna with relatively moderate temperatures.
The PR gradually increased during deep regional HT, ultimately differing significantly from that at baseline after 20 min of heating. However, the serial changes in the averaged BP of all patients were not significant in the recumbent position. Individually, the BP changes during HT were varied, as reported in previous studies [Citation21,Citation22]. Similarly, in this study, the variable BP changes in each patient were averaged, resulting in showing no significant change. Although it was difficult to conduct the sham experiment in advanced cancer patients due to the ethical reasons, the previous studies showed that BP and heart rates in healthy subjects without heating did not significantly change while keeping the recumbent position for several hours [Citation23–25]. Despite the significant increase in PR, the BP changed diversely during recumbent position for 50 min in this study, which could be influenced by heat stress. Generally, under heat exposure, marked cutaneous and non-cutaneous vasodilatation occurs, and thus, the cardiac output must increase for maintaining the BP. In order to increase the cardiac output, sympathetic nerve activities must increase, which results in a raised heart rate and visceral vasoconstriction [Citation26]. However, in deep regional HT, visceral vasoconstriction is difficult due to the powerful vasodilative effects of heating. Furthermore, the circulating blood volume might decrease due to sweating during HT. Thus, the BP changes during deep regional HT would depend on the balance between the strength of the sympathetic tone and the volume of venous return. Although many complex factors determined the BP changes, there was a positive correlation between the BP changes during recumbent position and the patients’ BMI.
The averaged BP was significantly decreased in the standing position after deep regional HT. Orthostatic hypotension (OH) was defined as an SBP reduction of at least 20 mmHg or a DBP reduction of at least 10 mmHg within 3 min of standing, and postural light-headedness or syncope is a symptom associated with OH [Citation27]. In this study, although the postural SBP dropped by more than 20 mmHg in 16 patients, 9 of the patients complained dizziness and/or light-headedness along with a decrease in BP upon standing after HT. Heat stress does not generally affect baroreflex control of the heart rate and sympathetic nerve activity while in a supine position; however, the baroreflex control of sympathetic vascular resistance may be impaired, perhaps due to attenuated vasoconstrictor responsiveness of the cutaneous circulation when standing [Citation28]. The dropped SBP on standing correlated with the increase in PR on recumbent position during HT, which was suggested that the SBP decrease tended to occur in the subjects whose sympathetic activation occurred stronger. The decrease of BP on standing position did not correlate with BMI, Hb concentrations and the amount of sweating. Consequently, we could not clarify the predictive factors for BP decrease on standing in this clinical observational study, and the further study would be needed.
Regarding the RR and SpO2, Hirano et al. similarly reported a decrease in the arterial oxygen pressures and an increase in the RR in cancer patients receiving deep regional HT [Citation11]. When the core body temperature increases to 38.0–39.0 °C through passive heating, the ventilatory volume is enhanced to twice that under normothermic conditions [Citation29]. In addition, it is well-known that an increase in the body temperature results in an increase in the oxygen consumption and a decrease in the SpO2 [Citation30]. However, the SpO2 decrease observed in our study remained above 95%, which was within the physiological range explained by the right side shift of the Hb-O2 dissociation curve during increases in body temperatures.
Study limitations
Several limitations associated with the present study. First, the sample size was relatively small. Since deep regional HT is not a generally used treatment for cancer patients in Japan, our study was performed at a single hospital. Multi-centred studies or longer study periods are needed to overcome this limitation. Thus, our results are not applicable to all cancer patients. Second, we were unable to control the fluid intake before HT, since all of the patients were outpatients. Third, the RF energy of deep regional HT differed on a case by case basis, as the protocol was tailored to each patient based on their condition. Finally, the present study did not include a control group because the study population was composed of advanced-stage cancer patients and the Ethics Review Board did not recommend it.
Conclusions
Deep regional HT using a capacitive device increased the core body temperature, resulting in body fluid loss and peripheral vasodilatation. As a result, a decrease in the BP was induced on standing after the therapy. The decrease in the BP on standing occurred even if the BP was stable during HT. Careful attention is needed for patients with HT, especially when standing after the therapy.
Acknowledgements
We appreciate the cooperation of the cancer patients who participated in our study and the medical staff at Tobata Kyoritsu Hospital.
Disclosure statement
The authors declare no conflicts of interest in association with this study.
References
- Terashima H, Ohguri T, Imada H, et al. (2014). Hyperthermia- gengyo-chosa (2012) houkokusyo [The current reports of hyperthermia in 2012]. Thermal Med 31:89–98. (in Japanese).
- Ohga S, Nakamura K, Yoshitake T, et al. (2012). A case of recurrent esophageal cancer treated with chemoradiation combined with long-term hyperthermia treatment. Thermal Med 28:17–22.
- Lee CK, Song CW, Rhee JG, et al. (1996). Clinical experience using 8 MHz radiofrequency capacitive hyperthermia in combination with radiotherapy: results of a phase I/II study. Int J Radiat Oncol 32:733–45.
- Nagata Y, Hiraoka M, Nishimura Y, et al. (1997). Clinical results of radiofrequency hyperthermia for malignant liver tumors. Int J Radiat Oncol Biol Phys 38:359–65.
- Ishikawa H, Nakayama Y, Sakurai H, et al. (2005). Challenge of hyperthermia combined with chemotherapy or chemo-radiotherapy for unresectable intrathoracic malignant tumors: a preliminary result. Jpn J Hyperthermic Oncol 21:159–69.
- Franckena M, Stalpers LJ, Koper PC, et al. (2008). Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: an update of the dutch deep hyperthermia trial. Int J Radiat Oncol 70:1176–82.
- Wust P, Hildebrandt B, Sreenivasa G, et al. (2002). Hyperthermia in combined treatment of cancer. Lancet Oncol 3:487–97.
- Ostapenko VV, Koshida S, Katahira A, et al. (2008). Quality of life (QOL) studies in patients with various malignancies treated with local or regional hyperthermia combined with chemo-and/or radiotherapy. Thermal Med 24:51–9.
- Ohguri T, Imada H, Kato F, et al. (2006). Radiotherapy with 8 MHz radiofrequency-capacitive regional hyperthermia for pain relief of unresectable and recurrent colorectal cancer. Int J Hyperthermia 22:1–14.
- Terashima H, Yamashita S, Imada H, et al. (1992). Side effects is RF capacitive heating. Jpn. J. Hyperthermic Oncol 8:51–8.
- Hirano S, Tabeta H, Sugito K, et al. (2000). The influence on cardiopulmonary system in hyperthermia for intrathoracic tumor. Jpn J Hyperthermic Oncol 16:153–7.
- Hill A, Kiss N, Hodgson B, et al. (2011). Associations between nutritional status, weight loss, radiotherapy treatment toxicity and treatment outcomes in gastrointestinal cancer patients. Clin Nutr 30:92–8.
- Gudny Geirsdottir O, Thorsdottir I. (2008). Nutritional status of cancer patients in chemotherapy; dietary intake, nitrogen balance and screening. Food Nutr Res 52:1–6.
- Oken MM, Creech RH, Tormey DC, et al. (1982). Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–55.
- Abe M, Hiraoka M, Takahashi M, et al. (1986). Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer 58:1589–95.
- Du Bois D, Du Bois EF. (1916). A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–71.
- De Leeuw AA, Van Vulpen M, Van De Kamer JB, et al. (2003). Increasing the systemic temperature during regional hyperthermia: effect of a cooling strategy on tumour temperatures and side-effects. Int J Hyperthermia 19:655–63.
- Cho C, Wust P, Hildebrandt B, et al. (2008). Regional hyperthermia of the abdomen in conjunction with chemotherapy for peritoneal carcinomatosis: evaluation of two annular-phased-array applicators. Int J Hyperthermia 24:399–408.
- Samulski TV, Kapp DS, Fessenden P, et al. (1987). Heating deep seated eccentrically located tumors with an annular phased array system: a comparative clinical study using two annular array operating configurations. Int J Radiat Oncol 13:83–94.
- Sawatari H, Chishaki A, Miyazono M, et al. (2015). Different physiological and subjective responses to the hyperthermia between young and older adults: basic study for thermal therapy in cardiovascular diseases. J Gerontol a Biol Sci Med Sci 70:912–6.
- Matsuoka H, Furusawa M, Tomoda H, et al. (1995) Efficacy of indomethacin pretreatment with regional hyperthermia for treating upper abdominal malignancies. Int J Hyperthermia 11:169–71.
- Puchinger M, Meinitzer A, Stettin M, Rehak PH. (2009). Psychological and systemic stress reactions of patients during hyperthermia treatments. Int J Hyperthermia 25:488–97.
- MacWilliam JA. (1933). Postural effects on heart-rate and blood pressure. Exp Physiol 23:1–33.
- Inoue K, Sato Y, Niino M, et al. (2002). The influence of the fixation in supine position: a study on subjective complains and distress area. Yamagata J Health Sci 5:69–75. (in Japanese).
- Dohi K, Ishikawa Y, Nakanishi Y, et al. (1994). Effects of 8 hour-fixed supine position on autonomic nervous activities. Kobe J Med Sci 10:119–28. (in Japanese).
- Grandill CG, Gonzalez-Alonos J. (2010). Cardiovascular function in the heat-stressed human. Acta Physiol (Oxf) 199:407–23.
- Freeman R. (2011). Syncope. In: Longo DL, Kasper DL, Jameson JL, et al., eds. Harrison's principles of internal medicine. 18th ed. Vol. 1, New York: McGraw-Hill Medical, 171–78.
- Schlader ZJ, Wilson TE, Crandall CG. (2016). Mechanisms of orthostatic intolerance during heat stress. Auton Neurosci 196:37–46.
- Cabanac M, White MD. (1995). Core temperature thresholds for hyperpnea during passive hyperthermia in humans. Eur J Appl Physiol Occup Physiol 71:71–6.
- White MD. (2006). Components and mechanisms of thermal hyperpnea. J Appl Physiol (1985) 101:655–63.