Abstract
Purpose: To evaluate the feasibility, safety and technical efficacy of ultrasound-guided percutaneous microwave ablation with artificial ascites for adenomyosis.
Materials and methods: Between May 2015 and May 2016, a total of 25 patients with symptomatic adenomyosis who underwent ultrasound-guided percutaneous microwave ablation with artificial ascites were included in this retrospective study. A matching cohort of 50 patients underwent ultrasound-guided percutaneous microwave ablation without artificial ascites as controls. The technical efficacy, complications and short-term treatment effectiveness were assessed and compared with the controls.
Results: Artificial ascites was successfully achieved in all of the 25 patients with the administration of a median of 550 mL (range, 250–1200 mL) of solution. There was substantial improvement in achieving a better antenna path in 100% (20/20) of the cases with a poor antenna path. The complete separation was achieved in 23 of 25 patients. The mean ablation time was 26.5 ± 7.3 min and the median non-perfusion volume ratio was 76% which was similar to the control group (p > .05). No serious complications were observed. Patient pain scores for dysmenorrhoea showed a statistically significant decline from the baseline of 6.71 ± 0.96 to 2.92 ± 0.79 and the symptom severity score declined statistically significantly from 21.8 ± 5.5 to 16.4 ± 4.8 at 3 months follow-up.
Conclusions: Percutaneous microwave ablation with artificial ascites is feasible, safe and can be effective in improving access for treatment of adenomyosis.
Hysterectomy is a mainstay treatment for the adenomyosis. However, most patients are not willing to lose their uterus. Various conservative treatment options are available, but the optimal method for the treatment of adenomyosis is controversial [Citation1]. The levonorgestrel-releasing intrauterine system appears to be effective, but the expulsion rate is relatively high due to uterine enlargement and regular heavy bleeding [Citation2,3]. Uterine artery embolisation (UAE) has been demonstrated to be effective and safe. However, the effect of this treatment modality upon fertility and ovary function remains to be determined [Citation4–6]. Recently, image-guided tumour ablation techniques have been introduced for the treatment of adenomyosis [Citation7–14]. The safety and efficacy of this relatively new strategy for adenomyosis are currently under evaluation. Various ablative energies are available including microwave (MW), radiofrequency (RF) and high-intensity focussed ultrasound (HIFU). MW has gained increasing attention due to its high thermal efficiency. In the case of uterine adenomyosis, the microwave ablation (MWA) can provide symptomatic relief and uterine volume reduction. It also has no influence on ovary function [Citation13–15].
The depth of adenomyosis may vary throughout the uterine wall. When adenomyosis penetrates deeply into the myometrium of the uterus and is near heat-vulnerable organs such as the intestinal tract, the successful performance of MWA is not straightforward due to the risk of collateral thermal damage. On the other hand, percutaneous approach is technically challenging due to bowel interference, especially for patients with a history of pelvic surgery or pelvic endometriosis, which can cause pelvic adhesions. Artificial ascites is a safe and effective technical measure for protecting non-target structures and improving the electrode/antenna path during percutaneous hepatic thermal ablation, as demonstrated by several experimental and clinical research studies [Citation16–19]. Given this success, we decided to apply this measure along with MWA for the treatment of the adenomyosis. The purpose of this study was to assess the feasibility, safety and technical efficacy of ultrasound-guided percutaneous MWA with artificial ascites for adenomyosis.
Materials and methods
Patients
Between May 2015 and May 2016, 109 premenopausal women with symptomatic uterine adenomyosis were treated with ultrasound-guided percutaneous MWA at our institution. Among the treated patients, 26 underwent MWA assisted by artificial ascites because either the planning ultrasound examination had shown that the antenna path for MWA was poor or the part of the uterus affected by the adenomyosis was <5 mm from the pelvic organ. A cohort of 25 patients who underwent MWA assisted by artificial ascites were enrolled in the study. A separate cohort of 50 women who underwent MWA without artificial ascites served as controls. Data from the patients were extracted from our maintained registry database (ratification no. 20100930–004, registration no. ChiCTRTRC-10001119). These patients were matched for adenomyoma diameter (focal adenomyosis) or uterine volume (diffuse adenomyosis). The mean adenomyoma diameter bands used for matching patients with focal adenomyosis were 3–5 cm and 5–7 cm. The uterine volume bands used for matching patients with diffuse adenomyosis were 150–250 cm3, 250–350 cm3, 350–450 cm3, 450–550 cm3 and 550–650 cm3. All of the procedures were performed by the same operator to avoid introducing interoperator variability. The two groups of patients met the following inclusion criteria: (1) adenomyosis diagnosed by ultrasound and MRI with a clinical syndrome, such as secondary dysmenorrhoea and menorrhagia; (2) patients with no desire for future pregnancy; (3) MWA was not combined with other treatments such as ethanol injection; and (4) patients with MRI examinations within 7 days before and after MWA. The exclusion criteria included the coexistence of other pelvic or uterine diseases (e.g., pelvic inflammatory diseases, ovarian endometriomas or uterine fibroids) or previous treatment such as UAE, radiofrequency ablation (RFA) or HIFU. The local institutional review board approved the study.
Induction of artificial ascites
After the optimal route was chosen (), an 18-gauge intravenous (IV) catheter (BD Angiocath; Sandy, UT) was inserted into the peritoneal cavity under ultrasound guidance. At times, it was very difficult to insert the catheter directly into the correct site due to the bowel interference. To allow for safe insertion, the catheter was inserted into a shallow site, and then, a small amount of fluid was injected to separate the bowel from the uterine surface allowing for tip visualisation. Next, the catheter was inserted deeper, and the process was repeated until the tip of the catheter was placed in the proper site (). Once ultrasound images showed that the catheter was inserted into the correct site, the inner stylet was removed. A sufficient amount of 0.9% saline solution was injected until a separation of at least 0.5 cm between the uterus and the adjacent structures was achieved (. We then performed the MWA procedure. The drip infusion was continued via the catheter during the MWA procedure.
Figure 1. (A) An intravenous catheter (arrow) was inserted into the shallow site (a), and then, a small amount of saline was injected to separate the intestinal tract (*) from the uterus surface. (B) The catheter was inserted deeper, and the process (A) was repeated until the tip of the catheter (arrow) was positioned between the uterus and the bowel (*) under ultrasound guidance. (C) After anechoic artificial ascites was introduced, the gap between the uterus and abutting bowel (*) was widened. We then found a better antenna path. (D) Artificial ascites fluid was added into the pelvis, separating the uterus from adjoining structures by more than 5 mm. The separation was still identifiable after 15 min of ablation (Arrow = antenna, a = ablation zone, b = artificial ascites). Complete separation was achieved in this patient.
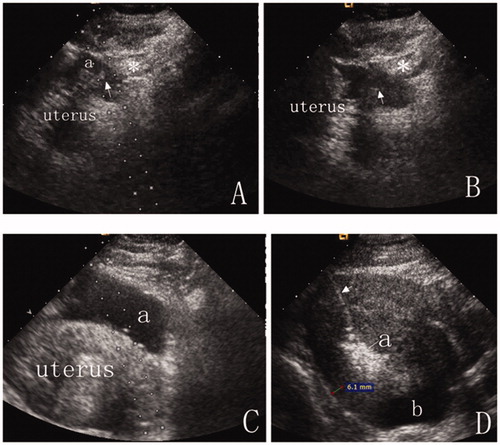
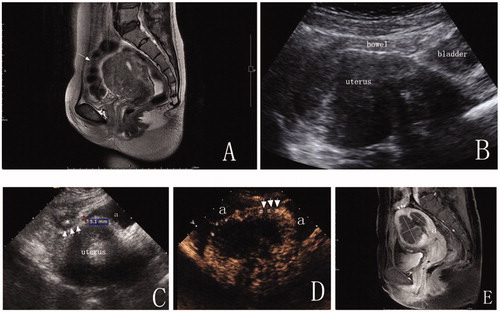
Figure 2. A 43-year-old woman with diffuse adenomyosis. (A) T2WI before MWA. The anterior uterine wall was extensively involved with adenomyosis. A bowel loop was close to the anterior uterine wall. (arrow = bowel). (B) A percutaneous approach was difficult due to bowel interference under ultrasound guidance. (C) After anechoic artificial ascites fluid was added, part of the uterus was separated from the bowel, and a distinct adhesion (arrow) was observed between the uterus and the adjacent bowel (*). (D) A CEUS image displays the ablative margin. The ablative margin (#) is more than 0.5 cm from the serosa where the uterus was not separated from the adjacent structures due to distinct pelvic adhesion (arrow). The ablative margin (*) was 0.3 cm from the uterine serosa where the uterus was completely separated from the adjacent organs by artificial ascites (a). (E) Enhanced T1WI at five days after MWA. The dark shadow indicates the area of lesion ablation (nonperfused volume, NPV). The percentage of NPV in this patient was 72%. The ablative margin was 0.7 cm from the distinct adhesion between the uterus and the bowel (arrow = distinct adhesion between uterus and bowel; a = uterine endometrium).
Microwave ablation
In this study, a cooled-shaft MW tumour coagulator (KY-2000; Kangyou Medical, Nanjing, China) consisting of a 15-gauge needle antenna with a 0.5 cm or 1.1 cm exposed tip was used. This MW tumour coagulator was capable of producing 100 W of power at 2450 MHz. A power output between 40 W and 60 W was used during MWA. The antenna was percutaneously inserted into the lesion under the guidance of transabdominal ultrasound. The entire ablation procedure was performed under real-time US guidance. The ablation was performed according to the “moving shot” technique; the lesion was ablated unit by unit by moving the antenna tip. Once the hyperechogenic signal covered the entire lesion or reached 3–5 mm from the margin of the serosa or uterine endometrium on real-time ultrasound, the energy was discontinued. The effectiveness of ablation was then immediately assessed using contrast-enhanced ultrasonography (CEUS, SonoVue, Bracco SinePharm, Milan, Italy). Non-enhanced regions on CEUS indicated necrotic areas (.
Assessment of technical efficacy and safety
For the evaluation of technical efficacy, we assessed the time for selection of the peritoneal space (from skin anaesthesia to placement of the angiocath into the proper site), total ablation time, total ablation energy, non-perfused volume (NPV) and NPV ratio. The total ablation energy was calculated as ablation power × ablation time, which was recorded. The zone without contrast agent perfusion in the arterial phase and the parenchymal phase in the treated area was considered to be ablated necrotic tissue [Citation20]. The extent of the NPV was evaluated by a post-ablation enhanced MRI performed within five days after the procedure. The NPV, uterine volume and lesion volume were calculated as 0.5233 × superoinferior diameter × anteroposterior diameter × transverse diameter. The NPV ratio was calculated according to the following equation: NPV ratio = Necrotic tissue volume/lesion volume ×100%. To evaluate the effect of artificial ascites on the technical efficacy of MWA, we statistically compared the total ablation time, energy required per unit volume (EPV) and NPV ratio between the groups with and without artificial ascites. EPV = total energy of MWA/NPV volume. The NPV ratio demonstrated the ablative results of MWA. The EPV showed how much energy was required to ablate one unit of the adenomyotic lesion [Citation12,Citation15].
For the evaluation of safety, MRI imaging was performed within five days after the procedure to accurately evaluate the possible injury to the surrounding organs. Complications were obtained by reviewing the medical records. We classified complications according to the unified standardised Society of Interventional Radiology (SIR) grading system.
Assessment of treatment effectiveness
The patients returned to the hospital at the end of the third month after the procedure for a routine follow-up examination. All patients completed a symptom severity score (SSS) questionnaire and a visual analogue scale (VAS) questionnaire before and 3 months after the procedure. The SSS questionnaire included eight items: the amount of menstrual blood loss, menstrual blood clotting, prolonged menstruation, menstrual disorders, pelvic pressure, frequent urination during day and night times and fatigue. The VAS was used to assess the extent of dysmenorrhoea.
Statistical analysis
The statistical analysis was performed with SPSS 18 (SPSS Inc., Chicago, IL). Student’s t-test was used to compare normally distributed data. The Wilcoxon signed rank test was used to compare data with skewed distributions. Chi-square or Fisher’s exact tests were used to compare the proportions.
Results
Patient characteristics
The mean patient age was 41.4 ± 4.2 years (range, 35–50 years). The mean BMI was 23.0 ± 1.6 kg/m2. All women included in the study were premenopausal patients who had dysmenorrhoea (n =25, 100%), menorrhagia (n =17, 68%) or bulk pressure (n = 5, 20%). Ten of the women had diffuse adenomyosis, and 15 had focal adenomyosis. Twelve of the women had a history of pelvic surgery.
The baseline information of the patients is shown in . No significant difference was observed between the two groups concerning age, BMI, lesion volume, lesion position, uterine volume, uterine position and symptom (p > .05).
Table 1. Baseline information; means ± SD or median (P25-P75).
Technical feasibility and efficacy
Introduction of artificial ascites was successful in all 25 patients. The antenna path was poor in 20 cases. Among the 20 patients, nine had a history of pelvic surgery. A substantial improvement was observed in achieving a better antenna path after introducing artificial ascites in all 20 cases (). Complete separation was achieved in 23 of 25 patients. After injecting artificial ascites, distinct adhesions between the uterus and adjacent organs were observed using ultrasound in two patients. Partial separation was achieved in these two patients (). The amount of solution used for introduction of artificial ascites ranged from 250 mL to 1200 mL (median, 550 mL).
The mean ablation time was 26.5 ± 7.3 min (range, 10.0–38.0 min). The median NPV was 78.7 cm3 (interquartile range, 15.4–291.5 cm3) and the median NPV ratio was 76% (interquartile range, 71–93%) as assessed by enhanced MRI five days after the procedure (). The NPV ratio, total ablation time and EPV in the MWA with artificial ascites group were similar to those of the MWA without artificial ascites group ().
Table 2. Technical efficacy and therapeutic results at the 3-month follow-up.
Safety
The artificial ascites disappeared in 21 of 25 patients within five days after the procedure as confirmed by MRI. A minimal amount of ascites was noted in the Douglas pouch in four patients.
No haematuria was observed in the study group after the procedure. In contrast, eight cases of transient gross haematuria without urinary symptoms were observed in the control group immediately after the procedure. This phenomenon disappeared quickly after ablation without treatment. All eight patients had an ablation zone on the anterior uterine wall (five for diffuse adenomyosis, three for focal adenomyosis). In the study group, six patients developed lower abdominal pain, and one patient required analgesia. The pain spontaneously disappeared within three days. Vaginal discharge was noticed in eight patients, which spontaneously resolved within two weeks. All adverse events were classified as grade A or B according to the unified standardised SIR grading system. Other than post-procedural transient haematuria, no statistically significant differences were found between the two groups (). The patients all had a menstrual cycle in the following month.
Table 3. Complications after percutaneous microwave ablation.
Short-term treatment effectiveness
Patient pain scores for dysmenorrhoea showed a statistically significant reduction from the baseline of 6.71 ± 0.96 to 2.92 ± 0.79 at three months after the procedure (p< .01). The SSS significantly decreased from 21.8 ± 5.5 to 16.4 ± 4.8 at the three-month follow-up (p< .01). Twenty-two patients (88%) reported improvement of dysmenorrhoea, and 14 patients (82.3%) reported improvement of menorrhagia. In the control group, the VAS score declined from a baseline of 7.02 ± 0.71 to 2.75 ± 1.13 and the SSS significantly decreased from 22.0 ± 4.9 to 17.4 ± 5.0, which were not significantly different from the study group (). Both groups at 3 months after ablation showed increased haemoglobin levels at 11.4 ± 1.3 g/dL in the study group versus 11.5 ± 2.0 g/dL in the control group compared with 10.1 ± 1.6 g/dL at the baseline in the study group and 10.7 ± 1.9 g/dL at the baseline in the control group, respectively (p< .05).
Discussion
The complication rate of thermal ablation is low according to previous studies. However, thermal ablation can cause severe complications such as penetration and thermal injuries of pelvic organs. Several studies have reported bowel perforation after hepatic or renal RFA/MWA without artificial ascites [Citation21–23]. Hyo et al. reported a case of rectouterine fistula after laparoscopic ultrasound-guided RFA of an intramural uterine fibroid with pelvic endometriosis [Citation24]. Meanwhile, clinical outcomes significantly differ between patients who do or do not achieve a sufficient ablation rate [Citation8,Citation12]. Therefore, it should be the ultimate goal of the thermal ablation to ensure a sufficient ablation rate to achieve a good clinical outcome. It is also essential that the operator performs the procedure without any complications. Adenomyosis mostly affects the posterior wall of the uterus and usually diffusely invades the whole uterus. To assure sufficient ablation, sometimes the likelihood of the ablation zone abutting a pelvic organ is increased. However, to our best knowledge, no research has been conducted on thermal protective measures during thermal ablation of uterine adenomyosis or fibroids. Our study showed that percutaneous introduction of artificial ascites is technically feasible and has the distinct advantage of separating the uterus from the adjacent organs. The fluid between the uterus and the adjacent structures may reduce the temperature around the uterus, hence protecting the adjacent structures from thermal injury. Eight patients who had an ablation zone on the anterior uterine wall in the control group had transient macroscopic haematuria. Zhou previously reported this complication [Citation8]. We believe that this phenomenon represents mild thermal bladder injury. Although this adverse effect was minor, it indicates the hidden thermal injury of MWA to the surrounding tissues even when the lesions were considered that they could be safely attempted without thermoprotective measures after pre-ablation evaluation. The difference between the groups reflected the protective effect of artificial ascites during MWA. Although complete separation was not achieved in two patients with distinct pelvic adhesions in the present study, the artificial ascites improved the visualisation and identification of high-risk locations with distinct pelvic adhesions, thus facilitating operator caution (). Furthermore, our study demonstrated that artificial ascites could achieve a better MW antenna path in some cases with a poor antenna path (). No remarkable difficulties are associated with percutaneous puncture assisted by transabdominal ultrasonic guidance in most cases. Nevertheless, this procedure may sometimes be difficult due to bowel interference, especially in cases with pelvic adhesions. Unfortunately, pelvic surgery (especially caesarean section) and pelvic endometriosis are commonly observed in association with adenomyosis [Citation25–28]. Both pelvic surgery and pelvic endometriosis can cause pelvic adhesions. In our study, hydrodissection was helpful for archiving a sonic window and widening the distance between the uterus and interfering structures, thus improving the poor antenna path (). We believe that the artificial ascites technique is worth employing in the case of uterine adenomyosis. However, thorough assessment through a pre-procedural ultrasound examination is essential during the process of patient selection. If the introduction of artificial ascites is difficult due to severe pelvic adhesions, an alternative treatment should be considered.
Additionally, our study showed that the cooling effect of the artificial ascites did not reduce the efficacy of MWA. The uterus is bathed in room temperature ascites fluid during MWA. Consequently, this may play a role in depriving the ablation zone of the heat produced by MW energy. Data on the heat-sink effect of artificial ascites during thermal ablation is scarce. Kim et al. [Citation29] found that the cooling effect of artificial ascites used during RF ablation was not likely to cause a significant heat-sink phenomenon for hepatic RFA, which could affect the volume of the ablation zone in an in vivo experimental study using a rabbit model. Sang Yu Nam et al. [Citation30] found that artificial ascites did not exhibit a heat-sink effect on the volume of the ablation zone after percutaneous RFA for the treatment of a human hepatic tumour abutting the diaphragm. However, this study was conducted on only a single, localised and small lesion (<3 cm), and no comparison of the ablation energy was conducted. In our study including medium (>3 cm and <5 cm), large (>5 cm) and diffuse lesions, no significant difference was found in the NPV ratio or EPV between the groups with or without artificial ascites. Our results suggest that no more energy was required to achieve the same volume of necrotic tissue during MWA when artificial ascites was applied.
The results of our study demonstrate that MWA with artificial ascites is feasible and effective for the improvement of access to enable safe treatment in patients with adenomyosis. The median NPV ratio was 76%, which was similar to our previous experience without artificial ascites for adenomyosis [Citation13]. However, our value was greater than the value reported by Ling (61%) with HIFU treatments for adenomyosis [Citation9]. An NPV ratio >50% was achieved in all the patients in our study. This contrasts with the report from Zhou [Citation8], which states that NPV ratios >50% were achieved in 68.1% of patients after HIFU treatment for adenomyosis. The primary reason for the difference in the technological efficacy between our study and the HIFU studies is likely related to the principal mechanism of action of the different therapeutic methods [Citation31]. The short-term treatment efficacy was encouraging. However, it is difficult to compare the present results with previous studies because the severity of the adenomyosis, follow-up period and criteria for defining the degrees of improvement are different. Depending on the series, 84.7–96.4% [Citation7–11] of patients reportedly had overall improvements, including full, moderate or slight improvement of dysmenorrhoea and 79.8–93% improvement of menorrhagia at three months after the thermal ablation as described in the literature [Citation7–14]. Our results are within the ranges reported.
This study presents several inherent limitations. First, we could not evaluate the true benefit of artificial ascites on the thermal protection of the neighbouring structures during percutaneous MWA for adenomyosis, because we could not perform a prospective randomised controlled study. The value of the comparison between the two groups might be affected by case selection bias because all lesions in the artificial ascites group were problematic, and thus, more difficulties were encountered in the artificial ascites group than in the control group. Second, we concentrated on only the feasibility, safety and technical efficacy of this technique. The long-term therapeutic effect is still under evaluation.
Conclusions
Percutaneous MWA with artificial ascites is feasible, safe and can be effective in improving access for treatment of adenomyosis.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Struble J, Reid S, Bedaiwy MA. (2016). Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol 23:164–85.
- Sheng J, Zhang WY, Zhang JP, Lu D. (2009). The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 79:189–93.
- Bragheto AM, Caserta N, Bahamondes L. (2007). Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception 76:195–9.
- Siskin GP, Tublin ME, Stainken BF, et al. (2001). Uterine artery embolization for the treatment of adenomyosis: clinical response and evaluation with MR imaging. AJR Am J Roentgenol 177:297–302.
- Holub Z, Mara M, Kuzel D, et al. (2008). Pregnancy outcomes after uterine artery occlusion: prospective multicentric study. Fertil Steril 90:1886–91.
- Homer H, Saridogan E. (2010). Uterine artery embolization for fibroids is associated with an increased risk of miscarriage. Fertil Steril 94:324–30.
- Sang-Wook Y, Ah KK, Hee CS. (2008). Successful use of magnetic resonance–guided focused ultrasound surgery to relieve symptoms in a patient with symptomatic focal adenomyosis. Fertil Steril 90:13–15.
- Zhou M, Chen JY, Tang LD, et al. (2011). Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril 95:900–5.
- Long L, Chen J, Xiong Y. (2015). Efficacy of high-intensity focused ultrasound ablation for adenomyosis therapy and sexual life quality. Int J Clin Exp Med 8:11701–7.
- Xiong Y, Yue Y, Shui L, et al. (2015). Ultrasound guided high intensity focused ultrasound (USgHIFU) ablation for the treatment of patients with adenomyosis and prior abdominal surgical scars: a retrospective study. Int J Hyperthermia 31:777–83.
- Shui L, Mao S, Wu Q, et al. (2015). High-intensity focused ultrasound (HIFU) for adenomyosis: Two-year follow-up results. Ultrasonics Sonochemistry 27:677–681.
- Gong C, Yang B, Shi Y, et al. (2016). Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int J Hyperthermia 32:496–503.
- Yu Y, Jing Z, Zhi-Yu H, et al. (2015). Ultrasound-guided percutaneous microwave ablation for adenomyosis: efficacy of treatment and effect on ovarian function. Sci Rep 5:10034.
- Xu RF, Zhang J, Han ZY, et al. (2016). Variables associated with vaginal discharge after ultrasound-guided percutaneous microwave ablation for adenomyosis. Int J Hyperthermia 32:504–10.
- Ma X, Zhang J, Han ZY, et al. (2014). Research of dose–effect relationship parameters of percutaneous microwave ablation for uterine leiomyomas – a quantitative study. Sci Rep 4:6469.
- Song I, Rhim H, Lim HK, et al. (2009). Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol 19:2630–40.
- Zhang M, Liang P, Cheng ZG, et al. (2014). Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia 30:134–41.
- Uehara T, Hirooka M, Ishida K, et al. (2007). Percutaneous ultrasound-guided radiofrequency ablation ofhepatocellular carcinoma with artificially induced pleural effusion and ascites. J Gastroenterol 42:306–11.
- Kondo Y, Yoshida H, Shiina S, et al. (2006). Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg 93:1277–82.
- Kroencke TJ, Scheurig C, Kluner C, et al. (2006). Uterine fibroids: contrast-enhanced MR angiography to predict ovarian artery supply-initial experience. Radiology 241:181–9.
- Liang P, Wang Y, Yu X, Dong B. (2009). Malignant liver tumors: treatment with percutaneous microwave ablation-complications among cohort of 1136 patients. Radiology 251:933–40.
- Iannuccilli JD, Dupuy DE, Beland MD. (2016). Effectiveness and safety of computed tomography-guided radiofrequency ablation of renal cancer: a 14-year single institution experience in 203 patients. Eur Radiol 26:1656–64.
- Livraghi T, Solbiati L, Meloni MF, et al. (2003). Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226:441–51.
- Jeong HJ, Kwon BS, Choi YJ. (2014). Rectouterine fistula after laparoscopic ultrasound-guided radiofrequency ablation of a uterine fibroid. Obstet Gynecol Sci 57:553–6.
- Panganamamula UR, Harmanli OH, Isik-Akbay EF. (2004). Is prior uterine surgery a risk factor for adenomyosis? Obstet Gynecol 104:1034–8.
- Bergholt T, Eriksen L, Berendt N. (2001). Prevalence and risk factors of adenomyosis at hysterectomy. Hum Reprod 16:2418–21.
- Bazot M, Fiori O, Darai E. (2006). Adenomyosis in endometriosis-prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod 21:1101–2.
- Gonzales M, de Matos LA, Gonçalves MODC. (2012). Patients with adenomyosis are more likely to have deep endometriosis. Gynecol Surg 9:259–64.
- Kim YS, Rhim H, Choi D, Lim HK. (2009). Does artificial ascites induce the heat-sink phenomenon during percutaneous radiofrequency ablation of the hepatic subcapsular area?: an in vivo experimental study using a rabbit model. Korean J Radiol 10:43–50.
- Kang TW, Rhim H, Lee MW. (2011). Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy. AJR Am J Roentgenol 196:907–13.
- Zhao WP, Han ZY, Zhang J. (2015). A retrospective comparison of microwave ablation and high intensity focused ultrasound for treating symptomatic uterine fibroids. Eur J Radiol 84:413–17.
