Abstract
Background: Isolated hepatic perfusion (IHP) is a treatment option for patients with liver metastases. Previous studies have found that liver toxicity is one of the limiting factors, and in an attempt to reduce the toxicity a buffering agent was added to the perfusate. The aim was to retrospectively analyse if this buffering reduced toxicity and complication rates.
Methods: A retrospective review of 52 consecutive patients with uveal melanoma liver metastases treated with IHP between 2005 and 2013. Patients were followed by daily liver function tests (LFT). Toxicity was graded according to Common Terminology Criteria for Adverse Events version 4.0 (CTCAE; United States Department of Health & Human Services, Washington, D.C), complications according to Clavien-Dindo and response according to RECIST-criteria.
Results: Thirty-six patients were treated with a buffered perfusate and 16 patients without buffer. There was no difference in age, gender, largest tumour size or number of tumours between the groups. There was a significantly lower mean in peak ALT, AST, PK (INR) and bilirubin when comparing buffer with no-buffer. There were five major complications without a significant difference between the groups (8.3 vs. 12.5%, p = 0.33). There was a lower complete response (CR) rate (11 vs. 44%, p = 0.023) and a trend for shorter time to local progression (9.2 vs. 17.6 months, p = 0.096); however, not significant in multivariate analysis. There was no difference in survival (24.2 vs. 26.0 months, p = 0.43) between the two groups.
Conclusions: Adding buffer to the perfusate during IHP significantly reduces postoperative LFTs; however, without a reduced complication rate. Interestingly, buffering also seems to reduce the response rate; however, this did not translate into a survival difference. To address if buffering adds any clinical benefit to the patients concerning toxicity, a larger prospective trial is necessary.
Introduction
In adults, uveal melanoma is the most common intraocular malignancy, with a higher incidence in fair-skinned populations [Citation1]. Approximately, 35–50% of the patients ultimately develop systemic metastases [Citation2], with about 90% of patients having isolated liver metastases [Citation3]. For patients with metastatic disease, the median overall survival is 4–12 months [Citation3–5].
There is no systemic treatment that has been able to show a prolonged survival in a phase III trial, and limited response rates have been reported [Citation6–9]. More promising data has been shown for loco-regional treatment strategies. The median OS for patients treated with liver resection has been reported to 14 months [Citation10], intra-arterial fotemustine showed an OS of 15 months [Citation11] and trans-arterial chemoembolisation have shown an OS of 5–29 months [Citation12,Citation13].
Isolated hepatic perfusion (IHP) is another loco-regional treatment option, where the outcome for the first five patients was reported in 1960 by Ausman [Citation14]. The liver is surgically isolated from the body and treated with high doses of chemotherapeutics. Since then, IHP has been clinically evaluated in several studies, mainly for liver metastases derived from colorectal cancer, melanoma and neuroendocrine tumours, but also for primary hepatic malignancies [Citation15–17]. Previous reports for patients with uveal melanoma have shown response rates between 33 and 100%, and a median overall survival of 9–22 months [Citation18–26].
In the early stages of this treatment, doses of chemotherapeutics were high and patients with tumour burden >50% were treated, which resulted in high post-procedural mortality related to liver failure [Citation22]. Since then, doses have been reduced and patients with large tumour burden are usually excluded from treatment. Despite this, procedure related liver toxicity is still common. In a report by Alexander et al., where only 1 out of 29 patients had >50% tumour burden, 65% of patients suffered from grade 3–4 hepatotoxicity according to the Common Terminology Criteria for Adverse Events (CTCAE) [Citation19].
Also in our own institutional experience, the most common complication after IHP is liver toxicity [Citation26]. With the hypothesis that the acidic environment in the perfusate was responsible for some of the liver toxicity after IHP, a buffering agent was added to the perfusate in 2009 [Citation27]. The rationale for this was that we for many years have used a buffered perfusate in isolated limb perfusion (ILP) without a negative effect on outcome [Citation28].
The aim of this study is to retrospectively analyse if this buffering of the perfusate actually reduced liver toxicity and complication rates. Primary outcome was hepatic toxicity measured as LFTs and graded according to CTCAE version 4.0 (United States Department of Health & Human Services, Washington, D.C). Secondary outcomes were response, complications, time to local progression and survival.
Patients and methods
Patients
Medical records of 52 consecutive patients treated between 2005 and 2013 with IHP for uveal melanoma liver metastases were retrospectively reviewed. Patients were eligible for IHP if they had no signs of extra hepatic disease on PET-CT, or CT of the thorax and abdomen and less than 50% of the liver parenchyma replaced by tumour. The patients had not received any prior systemic therapy. The study was approved by the Regional Ethical Review Board at the University of Gothenburg.
Isolated hepatic perfusion
The IHP procedure has been described in detail previously [Citation27]. In summary, a veno-venous bypass is placed between the external iliac vein and the internal jugular vein to allow for shunting of the caval vein. A venous return catheter is placed in the caval vein, which is clamped supra- and infrahepatically. An arterial inflow catheter is placed in the proper hepatic artery. The portal vein and the hepatoduodenal ligament are clamped. The inflow and outflow catheters are connected to an oxygenated perfusion system with a flow rate of 500–1200 mL/min. Thermistor probes are placed into the liver, the perfusate is heated with a target temperature in the liver of 40 °C. Systemic leakage was measured by injecting 100 MBq Technetium labelled albumin into the perfusion system. A scintillation probe is placed over the veno-venous bypass pump to detect any eventual leakage. Melphalan at a dose of 1 mg/kg bodyweight is administered as two bolus doses given 30 min apart, and the liver is perfused for 60 min. In 2009, the clinical practice was altered and 100 mL of the buffering agent Tribonat (disodium phosphate, sodium bicarbonate, trometamol; Fresenius Kabi) was added to the perfusate as a routine.
Liver toxicity and complications
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin and prothrombin complex (PK-INR) were recorded daily during the first postoperative week and classified according to CTCAE 4.0. When analysed, CTCAE grade 3–4 was considered as severe toxicity. Complications were classified according to Clavien-Dindo [Citation29], where grade 3–4 was considered as severe complications.
Response evaluation
Response was assessed with CT or MRI 8–12 weeks after the perfusion and graded according to the RECIST-criteria [Citation30]. Complete response (CR) was defined as a complete disappearance of all visible lesions and partial response (PR) was a reduction by at least 30%. Progressive disease (PD) was when new lesions appeared or previously known lesions increased with more than 25%. Stable disease (SD) was when none of the other criteria was reached.
Statistical analyses
Data are presented with mean and SD or median and IQR when appropriate. Peak values of LFTs were compared using Mann–Whitney U test. Groups were compared by Fisher’s exact test and multivariate analysis by logistic regression using the Enter method. Survival was calculated from the time of the procedure and analysed by the Kaplan–Meier method. For multivariate analysis, Cox-regression was used using the Enter method. A two-sided alpha of 0.05 was considered significant. All statistical analyses were made using SPSS version 0.23 (SPSS, Chicago, IL).
Results
Patients
Fifty-two patients were included, thirty-six (69%) patients underwent treatment with a buffered perfusate (buffer) and 16 (31%) without (no-buffer). Twenty patients were male (38%) and 32 were female (62%) and the median age was 61.9 (range 17.8–77.2) years. There was no significant difference in baseline patient characteristics between the buffer and the no-buffer group, except for a higher bilirubin value in the buffer group (6.2 vs.10.9 μmol/L, p < 0.001); however, all patients had normal bilirubin values below 20 μmol/L ().
Table 1. Patient and treatment characteristics.
IHP
All patients completed the IHP and received their planned dose of melphalan (1 mg/kg bodyweight). IHP was performed following the standard procedures without any leakage during the perfusion. There was no significant temperature difference in the liver parenchyma between the buffer and no-buffer group; however, it was a difference in the perfusion circuit temperature (39.8 vs. 39.5 °C, p = 0.045) ().
Liver toxicity
There was a significantly lower mean in the peak values of ALT (7.3 vs. 29.6, p = 0.001), AST (7.7 vs. 21.8, p < 0.001), ALP (1.7 vs. 3.4, p = 0.001) and bilirubin (17.0 vs. 43.7, p = <0.001) the first week after treatment, when comparing the group that received buffer with no-buffer. There was no difference in mean peak PK-INR (1.3 vs. 1.3, p = 0.656) (.
Figure 1. Kinetics of liver functions tests 7 d after IHP with or without buffering of the perfusate. Legend: *p < 0.05, **p < 0.01 and ***p < 0.001.
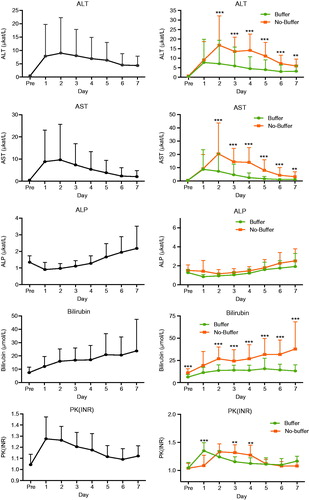
Seventy-five percent of patients suffered from severe hepatotoxicity (grade 3–4) when classified according to CTCAE 4.0. When comparing severe toxicity between the groups, there was a weak trend in favour of the group that received buffering (69 vs. 80%, p = 0.11).
Complications
There was no post-operative mortality. There were five patients (9.6%) with severe complications (Clavien-Dindo III and IV), without a significant difference between the buffer and no-buffer group (8.3 vs. 12.5%, p = 0.64). Four of the patients had severe hepatotoxicity (5.5 vs. 12.5%, p = 0.58). The fifth patient was operated at the ninth post-operative day for a perforated duodenal ulcer without signs of hepatic toxicity. Neither age, sex, largest tumour diameter or tumour number could predict severe complications. There was no significant difference in length of hospital stay between the buffer and no-buffer group (median 7.0 vs. 9.0 d, p = 0.898).
Response
Eleven patients had a CR (21%), 24 patients a PR (46%), nine patients SD (17%) and eight patients PD (16%) (). There was a trend towards a difference in ORR between the buffer and the no-buffer group (58.3 vs. 87.5%, p = 0.055). However, in multivariate analysis the only significant predictor for overall response was age (OR 0.86, 95% CI 0.77–0.96, p = 0.007).
Table 2. Distribution of response according to treatment with or without buffering.
The CR rate was 11% for the buffer group and 44% for the no-buffer group (p = 0.023), but there was only a trend for higher response rate in the no-buffer group in multivariate analysis (OR 6.6, 95% CI 0.88–50, p = 0.067) (.
Table 3. Univariate and multivariate analyses for time to local progression.
Time to local progression
Median time to local progression was 10 months. There was a trend for shorter time to local progression in the buffer group compared to no-buffer (9.2 vs. 17.6 months, p = 0.096). However, in multivariate analysis only the tumour size and the number of tumours were significant prognostic factors ().
Table 4. The univariate and multivariate analyses performed for survival.
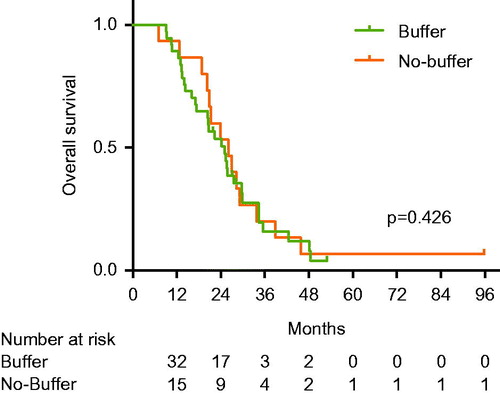
Survival
Median overall survival was 25 months and there was no difference between the buffer and no-buffer group (24.2 vs. 26.0 months, p = 0.426) (). In multivariate analysis, tumour size, tumours number and overall response were the only significant prognostic factors for overall survival ().
Discussion
This study suggests that buffering of the perfusate during IHP treatment significantly reduces the peak value of ALT, AST, ALP and bilirubin. However, when grading hepatic toxicity according to CTCAE 4.0, there was only a weak trend towards lower hepatotoxicity and this did not translate into a less severe complication rate. Interestingly, patients treated with a buffered perfusate showed a trend towards lower overall response rate in multivariate analyses; however, did this not translate into a difference in overall survival.
The major limitation in this study is the retrospective design and the limited number of patients included in the analyses. However, what strengthens the data is that it is a consecutive series with no difference in surgical technique or eligibility criteria before or after the introduction of a buffered perfusate.
The median peak value of the liver enzymes AST, ALT, PK (INR) and bilirubin was lower in the group receiving buffering. A possible explanation for this could be that acidosis causes a directly toxic effect on the liver parenchyma. Therefore, an increase in pH would lead to less cellular destruction and lower LFTs. Another explanation is that acidosis increases the cytotoxic effect of melphalan on tumour cells and/or liver parenchyma. It has earlier been shown in vitro that acidosis enhances cell death by itself but also through an increased cytotoxicity from melphalan [Citation31]. This has also been demonstrated in a xenograft mice model on cutaneous melanoma. The combination of melphalan and acidosis in ILP of cutaneous melanoma was more effective on response and time to local progression, compared to a control group and the two entities alone [Citation32]. This could support the theory that the anti-tumour and cytotoxic activity of melphalan is increased by acidosis.
Even though hepatic toxicity was reduced in the buffer group, the amount of patients having severe hepatotoxicity did not differ from earlier reports regarding IHP [Citation19]. Approximately, 10% of patients suffered from severe complications after the treatment. All of them recovered from the treatment and no treatment related mortality was recorded.
There was no statistically significant difference in complications between patients whom had severe hepatic toxicity compared to minor, but four out of five patients with severe complications had a severe hepatotoxicity. Since patients with >50% tumour burden routinely were excluded, the rate of complications and post-operative mortality has drastically decreased [Citation22]. No analysed factors in this study could predict severe complications.
Patients treated in the buffer group had a lower overall response rate as well as a shorter time to local progression raising severe concerns about a diminished effect of the treatment. It could be hypothesised that the same mechanisms that cause damage to tumour cells will also damage the liver parenchyma, and, therefore, an attempt to reduce toxicity could also reduce response. However, when analysing both response and time to local progression using multivariate analysis, there were no significant differences between the two groups. Most importantly, there was also no difference at all between the two groups concerning overall survival.
There might be a beneficial effect of perfusate buffering; however, the number of severe complications was few, so there was no statistically significant difference in morbidity. Worth noting is that the median overall survival in the patient group is 25 months, which is significantly longer than most other series of systemic treatments [Citation7–9,Citation33]. To further investigate this, a randomised multicentre trial (the SCANDIUM trial, NCT01785316) was initiated in 2013 where patients with liver metastases from uveal melanoma are randomised to either IHP or best alternative care (BAC) [Citation34]. This protocol still uses a buffered perfusate; however, further research is needed to address if buffering should be used routinely or not.
Acknowledgements
This study was supported by grants from Signe and Olof Wallenius Foundation, The Göteborg Medical Society, Assar Gabrielsson Foundation, Wilhelm and Marina Lundgren’s Foundation.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
References
- Bergman L, Seregard S, Nilsson B, et al. (2002). Incidence of uveal melanoma in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci 43:2579–83.
- Kujala E, Makitie T, Kivela T. (2003). Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci 44:4651–9.
- Diener-West M, Reynolds SM, Agugliaro DJ, et al. (2005). Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol 123:1639–43.
- Rietschel P, Panageas KS, Hanlon C, et al. (2005). Variates of survival in metastatic uveal melanoma. J Clin Oncol 23:8076–80.
- Gragoudas ES, Egan KM, Seddon JM, et al. (1991). Survival of patients with metastases from uveal melanoma. Ophthalmology 98:383–9. discussion 390.
- Bedikian AY, Legha SS, Mavligit G, et al. (1995). Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer 76:1665–70.
- Bedikian AY, Papadopoulos N, Plager C, et al. (2003). Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Res 13:303–6.
- Zimmer L, Vaubel J, Mohr P, et al. (2015). Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS One 10:e0118564.
- Kottschade LA, McWilliams RR, Markovic SN, et al. (2016). The use of pembrolizumab for the treatment of metastatic uveal melanoma. Melanoma Res 26:300–3.
- Mariani P, Piperno-Neumann S, Servois V, et al. (2009). Surgical management of liver metastases from uveal melanoma: 16 years’ experience at the Institut Curie. Eur J Surg Oncol 35:1192–7.
- Peters S, Voelter V, Zografos L, et al. (2006). Intra-arterial hepatic fotemustine for the treatment of liver metastases from uveal melanoma: experience in 101 patients. Ann Oncol 17:578–83.
- Gonsalves CF, Eschelman DJ, Thornburg B, et al. (2015). Uveal melanoma metastatic to the liver: chemoembolization with 1,3-bis-(2-chloroethyl)-1-nitrosourea. AJR Am J Roentgenol 205:1–5.
- Valpione S, Aliberti C, Parrozzani R, et al. (2015). A retrospective analysis of 141 patients with liver metastases from uveal melanoma: a two-cohort study comparing transarterial chemoembolization with CPT-11 charged microbeads and historical treatments. Melanoma Res 25:164–8.
- Aust JB, Ausman RK. (1960). The technique of liver perfusion. Cancer Chemother Rep 10:23–33.
- Bartlett DL, Libutti SK, Figg WD, et al. (2001). Isolated hepatic perfusion for unresectable hepatic metastases from colorectal cancer. Surgery 129:176–87.
- Grover A, Alexander HR, Jr. (2004). The past decade of experience with isolated hepatic perfusion. Oncologist 9:653–64.
- Feldman ED, Wu PC, Beresneva T, et al. (2004). Treatment of patients with unresectable primary hepatic malignancies using hyperthermic isolated hepatic perfusion. J Gastrointest Surg 8:200–7.
- Alexander HR, Libutti SK, Bartlett DL, et al. (2000). A phase I-II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin Cancer Res 6:3062–70.
- Alexander HR, Jr., Libutti SK, Pingpank JF, et al. (2003). Hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to liver. Clin Cancer Res 9:6343–9.
- Noter SL, Rothbarth J, Pijl ME, et al. (2004). Isolated hepatic perfusion with high-dose melphalan for the treatment of uveal melanoma metastases confined to the liver. Melanoma Res 14:67–72.
- Pingpank JF, Libutti SK, Chang R, et al. (2005). Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J Clin Oncol 23:3465–74.
- Rizell M, Mattson J, Cahlin C, et al. (2008). Isolated hepatic perfusion for liver metastases of malignant melanoma. Melanoma Res 18:120–6.
- Verhoef C, de Wilt JH, Brunstein F, et al. (2008). Isolated hypoxic hepatic perfusion with retrograde outflow in patients with irresectable liver metastases; a new simplified technique in isolated hepatic perfusion. Ann Surg Oncol 15:1367–74.
- van Iersel LB, Hoekman EJ, Gelderblom H, et al. (2008). Isolated hepatic perfusion with 200 mg melphalan for advanced noncolorectal liver metastases. Ann Surg Oncol 15:1891–8.
- van Etten B, de Wilt JH, Brunstein F, et al. (2009). Isolated hypoxic hepatic perfusion with melphalan in patients with irresectable ocular melanoma metastases. Eur J Surg Oncol 35:539–45.
- Ben-Shabat I, Belgrano V, Ny L, et al. (2016). Long-term follow-up evaluation of 68 patients with uveal melanoma liver metastases treated with isolated hepatic perfusion. Ann Surg Oncol 23:1327–34.
- Ben-Shabat I, Hansson C, Sternby Eilard M, et al. (2015). Isolated hepatic perfusion as a treatment for liver metastases of uveal melanoma. J Vis Exp 95:52490.
- Olofsson R, Mattsson J, Lindner P. (2013). Long-term follow-up of 163 consecutive patients treated with isolated limb perfusion for in-transit metastases of malignant melanoma. Int J Hyperthermia 29:551–7.
- Dindo D, Demartines N, Clavien PA. (2004). Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–13.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. (2009). New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–47.
- Skarsgard LD, Skwarchuk MW, Vinczan A, et al. (1995). The cytotoxicity of melphalan and its relationship to pH, hypoxia and drug uptake. Anticancer Res 15:219–23.
- Kelley ST, Menon C, Buerk DG, et al. (2002). Acidosis plus melphalan induces nitric oxide-mediated tumor regression in an isolated limb perfusion human melanoma xenograft model. Surgery 132:252–8.
- Carvajal RD, Sosman JA, Quevedo F. (2013). Phase II study of selumetinib (sel) versus temozolomide (TMZ) in gnaq/Gna11 (Gq/11) mutant (mut) uveal melanoma (UM). J Clin Oncol 31:CRA9003.
- Olofsson R, Ny L, Eilard MS, et al. (2014). Isolated hepatic perfusion as a treatment for uveal melanoma liver metastases (the SCANDIUM trial): study protocol for a randomized controlled trial. Trials 15:317.