Abstract
Introduction: Isolated limb perfusion (ILP) is a treatment option for patients with in-transit metastases of malignant melanoma in the extremities, as well as locally advanced sarcoma. ILP allows for a delivery of high-dose chemotherapy to an isolated extremity with minimal systemic toxicity. However, local toxicity like oedema, blistering, nerve damage and compartment syndrome can occur. Myoglobin measurements have been used as a screening method to predict the most severe cases of local toxicity. The aim was to investigate if myoglobin is a predictive factor for local toxicity after ILP in patients with melanoma in-transit metastases.
Methods: One hundred and ninety-three patients were treated for the first time with ILP for in-transit metastases between 2001 and 2015. Myoglobin was measured once the first hours after the perfusion (POD0), and for the first five post-operative days (POD1-5). Local toxicity was graded according to Wieberdink, and grouped in mild (I and II), moderate (III), and severe (IV and V). Wieberdink-groups were compared with myoglobin measurements, and myoglobin measurements were compared between gender, perfusion time, perfusion temperature and cannulated vessels.
Results: There is no statistically significant difference in myoglobin serum levels during the first five days post perfusion between patients suffering from mild, moderate or severe local toxicity. There is no difference between toxicity groups when it comes to distribution of sex, tumour size, or tumour numbers.
Conclusion: Levels of myoglobin do not predict local toxicity for patients with melanoma in-transit metastases treated with ILP when measured during the first five post-operative days.
Introduction
About 5–10% of the patients with recurrent malignant melanoma develop in-transit metastases. If the tumours are few, surgical resection is an option, but if they are many or are rapidly recurring, several therapeutic options exist. These include surgical resection, intra-lesional and topical treatments, local radiation, regional perfusion based therapies, systemic therapy and amputation [Citation1]. Another treatment option to gain local control is isolated limb perfusion (ILP), originally described by Creech and Krementz [Citation2] in 1958, where they proposed a technique for regional perfusion using an oxygenated perfusion circuit.
ILP allows for the delivery of a high dose of chemotherapy to a surgically isolated extremity with minimal systemic toxicity. However, local toxicity is often evident with signs of erythema and oedema developing within 48 hours of the procedure in most patients. The maximal reaction can be seen about two weeks after the treatment, most often with increased erythema and oedema but also pain. In some, more severe cases, other side effects of the treatment can occur, such as temporary loss of nails and hair, blistering, temporary neuralgia, rhabdomyolysis with renal failure, and compartment syndrome [Citation3].
The Wieberdink grading system of local toxicity [Citation4] is most often used when grading local toxicity. Several factors have been shown to be associated with local toxicity; the dosage of the chemotherapeutic drug (more important in female and obese patients), the temperature of the perfusate (lower temperature gives less toxicity), and blood gas levels (pCO2 and pH) [Citation5].
Myoglobin is a heme protein that is increases in serum in response to damaged muscle tissue and has been used as a marker of rhabdomyolysis since the 1960 s [Citation6]. In the case of an event leading to rhabdomyolysis, myoglobin is promptly released into the blood, where it can be measured within minutes [Citation7,Citation8]. Myoglobin was shown by Hohenberger et al. to be useful in monitoring toxicity after ILP, even though there was a great individual difference regarding the amount of myoglobin released into the blood [Citation9]. Measurements of myoglobin serum levels have been used as a screening method to predict the most severe cases of local toxicity at Sahlgrenska University Hospital since 2001, but also in other hospitals [Citation10,Citation11]. The aim of this study is to investigate if serum levels of myoglobin the first five days after ILP could predict local toxicity and its severity in patients with melanoma in-transit metastases.
Methods
Patients
A consecutive series of 207 patients with in-transit metastases of malignant melanoma were treated with first-time ILP with melphalan (no patients with TNF-alpha were included) at Sahlgrenska University Hospital between 2001 and 2015, which included all patients treated in Sweden during this time period. Excluded are 1 patient with missing myoglobin measurements and 13 patients that were excluded due to inability to retrieve data regarding local toxicity, resulting in 193 patients remaining in this study. The median age was 70 years (range 23–93 years) and 104 of these were female (54%) and 89 were male (46%), patient and treatment characteristics are summarised in . The study was approved by the Regional Ethical Review Board at the University of Gothenburg (reference number 721-08).
Table 1. Patient and treatment characteristics.
Local toxicity
Local toxicity was measured according to Wieberdink and classified from I to V, where I is no reaction, II slight erythema and/or oedema, III is considerable erythema and/or oedema with some blistering, IV is extensive epidermolysis and/or obvious damage to deep tissues, and V induces a reaction that may necessitate amputation. For this study, group I and II were pooled, representing mild toxicity, III medium toxicity, and IV and V severe toxicity. Data on local toxicity was retrieved from patient medical records.
Myoglobin
Myoglobin was measured as S-Myoglobin (μg/L), once approximately 6 h after the perfusion (called Post-Operative Day 0, POD0), and for the first five post-operative days (POD1–5) at 06.00 in the morning.
ILP
The extremity containing the tumour was separated from the systemic circulation by clamping and cannulation of the major artery and vein. In the case of femoral perfusion, either an inflatable tourniquet or an Esmarch bandage was placed to compress collateral vessels and thereby preventing systemic leakage. In the case of a brachial or external iliac approach an Esmarch bandage was used. Leakage monitoring was used using technetium-99 m-labeled human serum albumin (Vasculosis, Cis-Bio International, Gif-sur-Yvette, France) injected into the extracorporeal membrane oxygenation circuit, and using a precordial scintillation probe (MedicView, Sweden) to detect and measure leakage if present. The temperature in the perfused tissue was kept between 41.0 and 41.5 °C during the years 2001 and 2003, and between 39.0 and 40.0 °C during 2004 and 2015. Melphalan was administrated in doses of 13 mg/L for the upper limb and 10 mg/L for the lower limb. The perfusion time between 2001 and 2005 was 120 min. This was changed in 2005 to 90 min, and again in 2012 to 60 min. After completed perfusion, the limb was irrigated with Ringer’s solution (Ringer Acetat, Baxter Medical, Kista, Sweden), 1000–2000 mL for the upper limb, and 3000–4000 mL for the lower limb.
Statistical analysis
Nominal data was analysed using Fisher's exact test. Myoglobin data was analysed by the non-parametric Kruskal–Wallis test or Mann–Whitney U test. A p values less than 0.05 was considered statistically significant. All data was analysed using SPSS version 22 (SPSS, Chicago, IL).
Results
Of the included 193 patients, the distribution of local toxicity according to Wieberdink was grade I 6%, grade II 61%, grade III 22%, grade IV 6% and grade V 1%. There was no significant difference between patient characteristics and toxicity ().
Myoglobin levels increased at POD 0 to a median of 80 μg/L (IQR 139) and decreased the following five days (POD1 median 66 μg/L [IQR 84], POD2 median 50 μg/L [IQR 41], POD3 median 41 μg/L [IQR 31], POD4 median 39 μg/L [IQR 23] and POD5 median 39 μg/L [IQR 22]). There was no statistically significant difference in myoglobin levels during POD0-POD5 when comparing the groups of patients suffering mild, moderate or severe local toxicity (POD0, p = 0.13, POD1 p = 0.13, POD2 p = 0.40, POD3 p = 0.55, POD4 p = 0.18, POD5 p = 0.37) ().
Figure 1. Logarithmized values of myoglobin taken on the day of surgery (POD0) and the following five days (POD1–5) for groups with mild (I–II) moderate (III) and serve (IV–V) local toxicity.
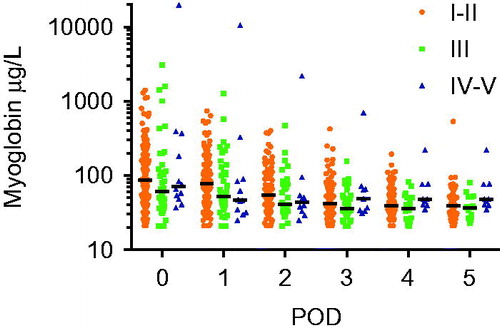
Eleven patients had a Wieberdink IV reaction, and one patient a Wieberdink V reaction. All these patients showed early symptoms for compartment syndrome (pain out of proportion and loss of sensibility) within the first 36 h. There was one outlier with a myoglobin of 19680 μg/L already within two hours after ILP, however, this patient had severe symptoms for compartment syndrome and also kidney failure due to a massive rhabdomyolysis. For all the other patients the median myoglobin at POD0 was 58 μg/L (range 37–392 μg/L), where only two patients had levels more than 90 μg/L (392 and 183 μg/L, respectively). The median myoglobin level at POD1 was 46 μg/L (range 25–90 μg/L).
There is a statistically significant difference in myoglobin levels between the sexes on the day of perfusion and during the first four days post perfusion, with males having higher levels (POD0 female median 63 μg/L (IQR 87) vs. male median 114 μg/L (IQR 170) p = <0.001, ).
Figure 2. Median of myoglobin taken on the day of surgery (POD0) and the following five days (POD1–5) for female and male patients, different perfusion vessels, different perfusion time and different temperatures.
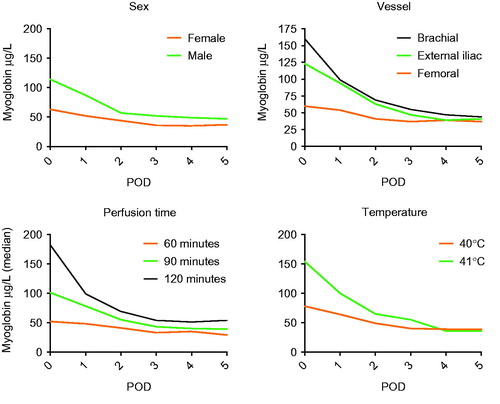
For patients with different perfusion times, a statistically significant difference in myoglobin measurements on the day of perfusion and the following three days post perfusion, as well as day five, can be found. The measurements show that 60 min perfusion gives the lowest levels of myoglobin (60 min median value 52 μg/L, POD0, n = 69, 90 min median value 101 μg/L, POD0, n = 83, 120 min median value 182 μg/L, POD0, n = 41, p = <0.001–0.002, ). Regarding the perfusion temperature, one finds a statistically significant difference in myoglobin measurements on the day of perfusion, as well as the following five days post perfusion. A perfusate with lower temperature gives lower myoglobin measurements (40 °C median value 78 μg/L, POD0, n = 165, 41 °C median value 154 μg/L, POD0, n = 24, p = <0.001, ).
When comparing different perfusion vessels there is a significant difference during the first three post-operative days. The highest levels of myoglobin can be found in patients perfused using the brachial vessels (median value 160 μg/L, POD0, n = 31), followed by the external iliac vessels (median value 123 μg/L, POD0, n = 46). The patients perfused via the femoral vessels had the lowest myoglobin medians (median value 60 μg/L, POD0, n = 116) (p = <0.001–0.015, ).
Discussion
The aim of this study was to investigate whether myoglobin is a predictive factor for local toxicity after ILP in patients with melanoma in-transit metastasis. This study demonstrates that when three groups, suffering from mild, moderate, and severe toxicity, were compared, no statistically significant difference could be found using S-myoglobin.
The most interesting of the three groups is the one suffering severe local toxicity (Wieberdink IV and V); these are the patients most likely to have complications developing into grave or even life-threatening forms at worst, and thus this is where you can have the most impact with a predicting factor. The median for all 12 patients on POD0 is 71 μg/L (the cut-off point at the local laboratory is <90 μg/L). As is evidently clear, one patient is an outlier with a myoglobin level at POD0 of 19 680 μg/L, when the closest measurement being 392 μg/L. The outlier patient had clinical signs of compartment syndrome just a few hours after surgery, with disabled sensory, as well as motoric, function in the extremity. He got treatment (forced alkaline diuresis), an acute fasciotomy, and the acute situation was managed. The signs of compartment syndrome were noticed before the POD0 measurement of myoglobin was known, so the myoglobin itself was not a predictor for the outcome.
To our knowledge, there are no other studies comparing local toxicity and myoglobin. One study, by Hohenberger et al. [Citation7], demonstrates that myoglobin levels are elevated 90 min after the start of the perfusion. They concluded that myoglobin levels were significantly higher after a perfusion than before it, but could also conclude that there are great individual differences when it comes to the measured levels. This individual difference was speculated upon and one theory is that there is a large individual difference in myoglobin levels found inside the muscle fibres, as well as the individual difference when it comes to muscle volume. Bodié et al. [Citation12] could show that myoglobin was of limited value as a biomarker for muscle tissue damage in rats. Better markers were fatty acid binding protein 3, myosin light chain 3, and skeletal troponin I. They also concluded that there was a big individual variance regarding the measured levels.
Myoglobin is measured on the day of the perfusion and for the coming 3–7 days, and has been measured since the late 1990 s by many centres performing the procedure [7,10,11]. This has been partly due to a belief that a high level of serum myoglobin the first days could predict the most severe complications, like compartment syndrome, and renal failure [Citation7]. Myoglobin can be used to predict renal failure, following thoraco-abdominal aortic surgery, according to Miller et al. [Citation13] and following suspected rhabdomyolysis according to Premru et al. [Citation14]. Miller et al. found that patients suffering from renal failure had a significant raise in serum myoglobin compared to patients that was unaffected. The levels of myoglobin measured for those affected were much higher, they found that a clinically important value is one >1000 μg/L. In this study, the median of the toxicity group suffering from severe toxicity (Wieberdink IV and V) was 71 μg/L. Perhaps that rhabdomyolysis is not the actual trigger for severe toxicity following ILP?
It has been shown on several occasions that perfusion time affects the measured toxicity [Citation15]. Our results show that there is statistical significant difference in myoglobin levels between the 60 min, 90 min, and 120 min of perfusion time. The temperature of the perfusate was also found to have an impact on the myoglobin levels, where lower temperatures gave lower measurements of myoglobin. Since the higher temperature also was combined with a longer perfusion time, which has been shown to correlate to tissue damage as well, any conclusion cannot be made concerning if it is the temperature or the perfusion time that affects the myoglobin levels the most.
The significant difference in myoglobin measurements between the sexes, with males having significantly higher values than females, is something that could be expected, considering the higher muscle mass of the male part of the population [Citation16].
When it comes to the difference in myoglobin levels between the patients being perfused using different vessels it is not as intuitive as the difference between the sexes. The highest levels of myoglobin can be found in patients perfused using the brachial vessels. One explanation is that there is an overuse of melphalan when calculated using a formula made for the lower body, and the arm having less muscle volume in comparison. Another explanation could be that this is because of a difference in sex ratio. In the brachial group, only 35% are female, whereas the femoral group is dominated by female patients (60%).
The Wieberdink grading [Citation4] is the standard way of grading toxicity after an ILP(7). One thing that has to be taken into consideration regarding this grading system, is the fact that the grading is dependent on when the patient is examined, since the maximum reaction often occur after the patient has left the hospital. The major limitation of this study is that there are relatively few patients with Wieberdink IV and V toxicity.
According to this study, serum levels of myoglobin do not predict the severity of local toxicity for patients undergoing ILP as a palliative treatment for in-transit metastases of malignant melanoma, when measured during the first five post-operative days. The implications of the findings in this study could be that myoglobin no longer will be measured routinely as a screening method to predict local toxicity.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Abbott AM, Zager JS. (2014). Locoregional therapies in melanoma. Surg Clin North Am 94:1003–15, viii.
- Creech O, Krementz ET, Ryan RF, Winblad JN. (1958). Chemotherapy of Cancer: Regional Perfusion Utilizing an Extracorporeal Circuit. Ann Surg 148:616–32.
- Perry MC, Doll DC, Freter CE. Perry's the chemotherapy source book. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012.
- Wieberdink J, Benckhuysen C, Braat RP, et al. (1982). Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol 18:905–10.
- Neto JPD, Oliveira F, Bertolli E, et al. (2012). Isolated limb perfusion with hyperthermia and chemotherapy: predictive factors for regional toxicity. Clinics 67:237–41.
- Perkoff GT. (1965). Myoglobin. Am J Med 39:527–32.
- Hohenberger P, Haier J, Schlag PM. (1997). Rhabdomyolysis and renal function impairment after isolated limb perfusion: comparison between the effects of perfusion with rhTNFα and a ‘triple-drug’ regimen. Eur J Cancer 33:596–601.
- Sorichter S, Puschendorf B, Mair J. (1999). Skeletal muscle injury induced by eccentric muscle action: muscle proteins as markers of muscle fiber injury. Exercise Immunol Rev 5:5–21.
- Hohenberger P, Haier J, Kettelhack C, et al. (1997). Erfassung regionaler und systemischer Toxizität der isolierten, hyperthermen Extremitätenperfusion mit Tumor-Nekrose-Faktor α und Melphalan. Der Chirurg 68:914–20.
- Möller MG, Lewis JM, Dessureault S, Zager JS. (2008). Toxicities associated with hyperthermic isolated limb perfusion and isolated limb infusion in the treatment of melanoma and sarcoma. Int J Hypertherm 24:275–89.
- Bonifati DM, Ori C, Rossi CR, et al. (2000). Neuromuscular damage after hyperthermic isolated limb perfusion in patients with melanoma or sarcoma treated with chemotherapeutic agents. Cancer Chemother Pharmacol 46:517–22.
- Bodié K, Buck WR, Pieh J, et al. (2016). Biomarker evaluation of skeletal muscle toxicity following clofibrate administration in rats. Exp Toxicol Pathol 68:289–99.
- Miller Iii CC, Villa MA, Sutton J, et al. (2009). Serum myoglobin and renal morbidity and mortality following thoracic and thoraco-abdominal aortic repair: does rhabdomyolysis play a role? Eur J Vasc Endovasc Surg 37:388–94.
- Premru V, Kovač J, Ponikvar R. (2013). Use of myoglobin as a marker and predictor in myoglobinuric acute kidney injury. Ther Apher Dial 17:391–5.
- Olofsson R, Mattsson J, Lindnér P. (2013). Long-term follow-up of 163 consecutive patients treated with isolated limb perfusion for in-transit metastases of malignant melanoma. Int J Hypertherm 29:551–7.
- Chen IW, David R, Maxon HR, et al. (1980). Age-, sex-, and race-related differences in myoglobin concentrations in the serum of healthy persons. Clin Chem 26:1864–8.
