Abstract
Pseudomyxoma peritonei (PMP) is an uncommon disease characterised by mucinous ascites, classically originating from a ruptured low grade mucinous neoplasm of the appendix. The natural history of PMP revolves around the “redistribution phenomenon”, whereby mucinous tumour cells accumulate at specific sites with relative sparing of the motile small bowel and to a lesser extent other parts of the gastrointestinal tract. Peritoneal tumour accumulates due to gravity and at the sites of peritoneal fluid absorption, namely, the greater and lesser omentum and the under-surface of the diaphragm, particularly on the right. The optimal treatment is complete macroscopic tumour excision termed cytoreductive surgery (CRS) combined with Hyperthermic Intra-Peritoneal Chemotherapy (HIPEC). Total operating time for complete CRS and HIPEC for extensive PMP is around 10 h and generally involves bilateral parietal and diaphragmatic peritonectomies, right hemicolectomy, radical greater omentectomy with splenectomy, cholecystectomy and liver capsulectomy, a pelvic peritonectomy with, or without, rectosigmoid resection and bilateral salpingo-oophorectomy with hysterectomy in females. A unique feature of low grade PMP, which differs from other peritoneal malignancies, includes slow disease progression, which may be asymptomatic until advanced stages. Additionally, very extensive disease with a high “PCI” (Peritoneal Carcinomatosis Index) may still be amenable to complete excision and cure. In cases where complete tumour removal is not feasible, maximum tumour debulking can still result in long-term survival in PMP. PMP is challenging, complex but nevertheless the most rewarding peritoneal malignancy amenable to cure by CRS and HIPEC.
Introduction
Pseudomyxoma peritonei (PMP) is an uncommon clinical condition, characterised by mucinous ascites, and is generally associated with a perforated epithelial neoplasm of the appendix [Citation1,Citation2]. Carl Rokitansky was the first to describe an appendiceal mucocele in 1842 [3]. Subsequently, in 1884, Werth coined the term PMP [Citation4] in relation to an ovarian neoplasm. In 1937, Robert Michaelis Von Olshausen, a German gynaecologist, proposed the hypothesis that epithelial cells from the lining of a ruptured appendiceal cyst took root in the peritoneal cavity and continued to secrete gelatinous material, leading to PMP [Citation3].
While epithelial neoplasms of the appendix remains the most common cause of PMP, similar pathological features may originate from mucinous neoplasms of the colorectum, ovaries or indeed almost any abdominal organ. PMP of non-appendiceal origin was thought to have a worse prognosis, as the underlying pathology was more likely to be a mucinous adenocarcinoma. However, a recent series of 225 PMP patients has failed to demonstrate any survival and prognostic differences based on the site of origin [Citation5]. It is likely that the biology of the disease is the main determinant of outcome.
PMP has traditionally been considered as a benign process. However, the disease has a wide spectrum, from slow growing benign lesions to rapidly progressive infiltrative disease Therefore, at best, PMP should always be considered as a “borderline malignant” process [Citation2].
Incidence
The true incidence of PMP in the population is not known, with little available data. Historical data from autopsy studies estimated the incidence of a mucocele of the appendix to be about 0.2% [Citation6]. The incidence of PMP was initially proposed to be around 1 per million population per year [Citation7], but this was not based on robust evidence [Citation2]. Recent data by Smeenk et al. from the Netherlands has estimated the incidence of mucinous epithelial neoplasms of the appendix to be around 0.3% and progression to PMP in about 20% of these patients [Citation8]. Extrapolating the data, this puts the incidence of PMP at approximately 2 per million annually. However, experience from high volume centres suggests that the actual incidence may be higher at 3–4 operable cases per million per year [Citation9].
Pathophysiology
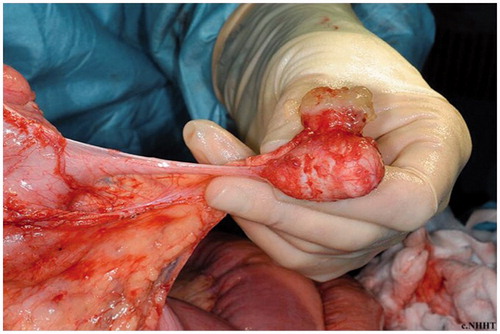
PMP is a clinico-pathological entity resulting from mucin producing peritoneal and omental tumour implants from a perforated mucinous neoplasm. Rupture of the primary tumour results in free-floating mucin and epithelial cells (), which implant in various parts of the peritoneal cavity. These then produce mucin and are responsible for the development of the typical “jelly belly” [Citation10].
The classical distribution of PMP deposits is via what has been termed the “redistribution phenomenon” [Citation11]. This phenomenon results from movement of free-floating epithelial cells with little, or no, adhesional properties. Cells move with the peritoneal fluid and by gravity. Proliferation of tumour deposits in predetermined sites of the peritoneal cavity is therefore dictated by gravity and concentration at the sites of peritoneal fluid absorption [Citation2,Citation11].
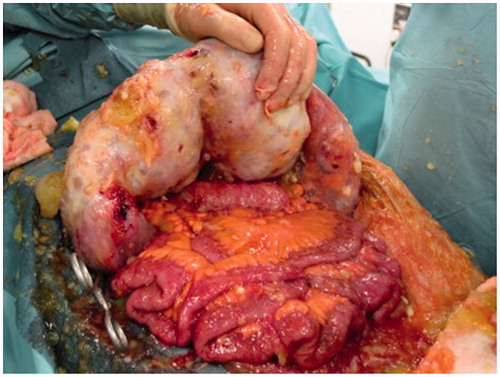
The major sites of peritoneal fluid absorption are the greater and lesser omentum, and the undersurface of the diaphragm, particularly the right hemi diaphragm with resultant omental cake (), and sub-diaphragmatic disease conglomeration.
Figure 2. Intra-operative photograph with a massive omental cake. Note that the small bowel is free of disease.
The other main redistribution mechanism is by gravity, with tumour deposits aggregating in dependent sites. This accounts for tumour commonly being found in the rectovesical pouch or pouch of Douglas, the paracolic gutters and the retro hepatic space.
Mobile organs such as the small bowel are usually spared early on in the disease process. However fixed parts of the intestines, such as the pylorus, the duodeno-jejunal flexure and ligament of Treitz, the ascending and sigmoid colon may be heavily involved by tumour, sometimes warranting resection. The small bowel may be involved early in the disease if there are adhesions from previous surgery or previous attempts at removal. The relative sparing of the small bowel () and its mesentery allow for complete resection of tumour, without the need for major small bowel resection. In aggressive tumours, small bowel involvement may occur early, and precludes complete tumour removal. Tumour on the serosa of the small bowel, or at the junction of the small bowel and its mesentery, is difficult to excise surgically and precludes complete tumour removal.
Extra abdominal spread of PMP is rare, but well reported in the literature. The most common site of extra abdominal spread is the pleural cavity, which can sometimes be amenable to treatment with intra-pleural chemotherapy after surgical excision [Citation12].
PMP appears to be more common in women, who often present with rapidly enlarging ovarian masses, secondary to trans-celomic spread [Citation8,Citation13]. Women also tend to present at an earlier stage than men. This may again be secondary to the rapidly enlarging ovarian masses, which become symptomatic or are obvious clinically. Earlier presentation in women may also results from more liberal use of cross sectional imaging in women on suspicion of ovarian cancer. Males often present at an advanced stage, as the disease is asymptomatic initially. Early diagnosis in men and women commonly results when detected incidentally at appendicectomy.
While classical PMP originates from an appendiceal tumour, similar clinical, radiological and even pathological features may originate from any other true adenocarcinoma of the appendix, colon or rectum [Citation2]. PMP in women was considered to commonly originate in the ovary but this has now been refuted, and there is evidence that the underlying aetiology in most cases is the appendix, with secondary involvement of the ovaries [Citation2]. Immunohistochemistry and immunological markers have proven that the disease is of intestinal origin with secondary ovarian involvement [Citation14–17]. An ovarian primary may account for some cases and it has also been noted that a dermoid tumour of the ovary may produce classical PMP with intestinal immune-histochemical features due to the pleuro-potential features of these ovarian neoplasms.
Primary peritoneal PMP has been described and indeed there are case reports of PMP originating from practically every organ in the abdomen, including stomach, gallbladder, pancreas [Citation18], urinary bladder and urachus [Citation19].
Pathology
Histopathology and classification of PMP has always been confusing and challenging. There are many classification systems, often with confusing and overlapping terminology.
One of the first widely recognised classification systems was reported by Ronnett et al. in 1995 [Citation20]. They divided PMP into three categories: disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA) and an intermediate category containing tumours with inconsistent or discordant features (PMCA-I/D). DPAM was characterised by peritoneal lesions composed of abundant extracellular mucin containing scanty simple to focally proliferative mucinous epithelium, with little cytological atypia or mitotic activity, with, or without, an associated appendiceal mucinous adenoma. PMCA was characterised by peritoneal lesions composed of more abundant mucinous epithelium with the architectural and cytologic features of carcinoma, with, or without, an associated primary mucinous adenocarcinoma. Although the term DPAM suggested a benign process, this was not in keeping with clinical behaviour, with disease progression even at the lower end of the spectrum.
Bradley et al. [Citation21] attempted to reclassify PMP in 2006. After reviewing patient outcomes, they classified PMP into two distinct categories: mucinous carcinoma peritonei low grade (MCP-L) and mucinous carcinoma peritonei high grade (MCP-H). MCP-L incorporated Ronnett’s cases with DPAM and PMCA-I. Cases that were moderately to poorly differentiated were classified as MCP-H, including patients with signet ring cells.
In 2010, WHO published a classification, which divided PMP into low grade and high grade [Citation22].
A consensus statement on the classification and pathological reporting of PMP and associated appendicular neoplasia has recently been published by Carr et al. on behalf of the Peritoneal Surface Oncology Group International (PSOGI) [Citation23]. Appendicular neoplasms have been classified as low grade and high grade appendiceal mucinous neoplasms, synonymous with DPAM and PMCA, respectively. The term mucinous adenocarcinoma is to be reserved for lesions with infiltrative invasion. A checklist for pathological reporting of PMP has also been developed to ensure uniform reporting and meaningful comparison of outcomes [Citation23].
Clinical features
PMP is often asymptomatic in the initial stages, and classically presents with vague abdominal symptoms when the disease burden is marked. Many patients may have been investigated by gastro-intestinal tract endoscopies, which inevitably are normal, and have often been labelled as having irritable bowel syndrome.
The initial lesion in the appendix is usually slow growing and therefore asymptomatic. Patients often do not recall any acute abdominal pain associated with tumour rupture. The tumour seals the luminal communication to the caecum, therefore preventing gross bacterial contamination. The increasing mucous accumulation from tumour implantation slowly leads to abdominal distension, discomfort and pain, with palpable abdominal masses (omental cake and ovarian masses in women). This eventually progresses to malnutrition, bowel obstruction and respiratory compromise, which set off a cascade of terminal events.
In 2000, Esquivel and Sugarbaker described common presenting features of PMP [Citation24]. They reported on 217 patients and found the most common presentation to be acute appendicitis (27%). The next most common presentation was abdominal distension (23%), while 14% were picked up when being investigated for a new onset hernia, mostly inguinal. Other presentations included ascites, abdominal pain and vague abdominal symptoms, accounting for 17% of cases. In women, the diagnosis was made most commonly during investigation for a suspected ovarian mass (39%). In our series of 222 patients over a two-year period (2011–2012) [Citation25], we have reported that CT is the most common mode of diagnosis, with about half of the patients being diagnosed as a result of CT abnormalities in patients with vague symptoms and often abdominal distension as a presenting symptom. Overall, 36.5% of the patients were diagnosed at CT alone (with or without image guided biopsy) and a further 14.4% following a suspicious CT scan. Additionally, 20.7% were diagnosed at laparoscopy, or laparotomy, for an acute abdomen or on subsequent histopathology. In this experience only 5% of patients were diagnosed while being investigated for a new onset hernia, and 5.4% at diagnostic laparoscopy.
It is increasingly noted that more patients are currently being diagnosed on imaging for unrelated pathology or as an unexpected finding at laparoscopy/laparotomy for an acute abdomen [Citation13,Citation26].
Physical examination may reveal a distended abdomen with a palpable omental cake. Ovarian masses may be palpable in females. A rectal examination may reveal deposits in the pouch of Douglas or the rectovesical pouch.
Investigations
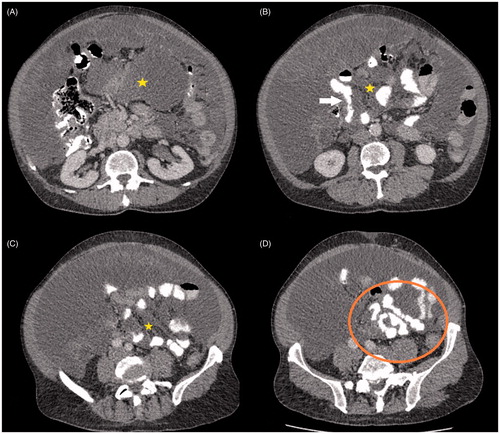
CT scan of the chest, abdomen and pelvis with intra-venous and oral contrast is the imaging modality of choice for PMP [Citation27,Citation28]. CT can often reveal the primary in the appendix, which may be calcified or ruptured, in addition to omental caking and mucinous ascites. The classical feature of PMP on imaging is scalloping of the liver surface, which is indentation of the liver capsule by tumour deposits, which differentiates PMP from serous ascites (). The key feature determining feasibility of complete cytoreduction is involvement of the small bowel mesentery and serosa (). Involvement of the porta hepatis is also key when considering the feasibility of complete cytoreduction. Oral and intra-venous contrast CT is recommended to optimally assess the small bowel.
Figure 3. CT coronal (A) and axial (B) sections with pseudomyxoma peritonei showing scalloping of the liver surface (arrows).
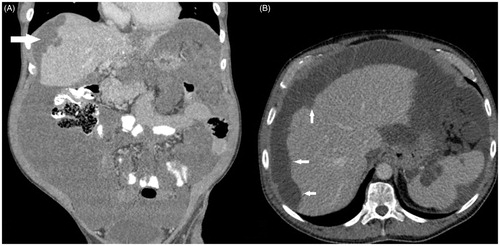
Figure 4. CT axial sections of same patient showing extensive small bowel and mesenteric involvement. There is low density mucin in the mesentery (*). Small bowel involvement is seen as distortion and narrowing of bowel lumen (arrow) and scalloping of bowel lumen seen as changing calibre of bowel (circle).
Magnetic resonance imaging (MRI) of the abdomen and pelvis may be helpful in assessing the small bowel as well as the hepatoduodenal ligament [Citation29–31]. Some have reported that additional diffusion weighted MRI imaging may help to improve the sensitivity and specificity in detecting peritoneal metastases [Citation32]. The sensitivity and specificity in detection, and characterisation, of peritoneal metastasis also increases with increasing experience of the radiologist [Citation33].
Despite optimal CT imaging and MRI, small bowel involvement by low volume diffuse disease may sometimes be difficult to detect in patients with peritoneal malignancy, and is often best assessed by visual inspection at laparoscopy or laparotomy. However, diagnostic laparoscopy to accurately assess small bowel involvement in PMP is often disappointing due to the presence of a large omental cake and mucinous ascites. Laparoscopy is more useful in patients with other peritoneal malignancies, such as colorectal peritoneal metastasis or mesothelioma [Citation34], with the aim of detecting low volume serosal, or mesenteric disease, which may preclude complete tumour resection.
Functional imaging with PET CT scanning does not contribute much to the diagnosis of PMP and is more useful in identifying extra abdominal disease in addition to hepatic metastases in patients with adenocarcinoma [Citation35].
Percutaneous image guided biopsies (USG or CT) are often unhelpful as the resultant material may be acellular mucin, even in patients with adenocarcinoma and thus percutaneous biopsy is selectively utilised. However, in cases where a percutaneous biopsy has been performed, finding acellular mucin is highly suggestive of PMP.
Serum tumour markers are helpful in predicting aggressiveness of disease. In patients who are secretors, elevated tumour markers help in follow-up and early identification of recurrence [Citation36]. The three tumour markers commonly used in PMP are the carcino-embryonic antigen (CEA), carbohydrate antigen 125 (CA 125) and carbohydrate antigen 19.9 (CA 19.9) [Citation37].
CEA is expressed by tumours of the gastrointestinal tract, particularly colorectal cancers. CA 125 is used mainly as a marker for ovarian tumours and CA 19.9, while classically associated with pancreatic and upper gastrointestinal tract tumours, is also expressed in peritoneal malignancy. CA125 and CA19.9 can be elevated in patients with any source of peritoneal irritation.
Although published series are few, have small numbers and short follow-up, all reports suggest that patients with elevated tumour markers are at higher risk of recurrence [Citation38–41]. Tumours with elevated tumour markers, irrespective of histology (high or low grade), can behave aggressively, and abnormal markers is an independent prognostic feature [Citation42,Citation43]. Unsurprisingly, elevated tumour markers have also been shown to be predictors of incomplete cytoreduction [Citation44]. Pre-operative high tumour markers could also be used to guide the interval and frequency of follow-up.
The largest reported series by Taflampas et al. [Citation37] reported on 519 patients undergoing complete tumour removal by cytoreductive surgery (CRS) and hyperthermic intra-peritoneal chemotherapy (HIPEC) for perforated appendiceal tumours. Patients with normal tumour markers pre-operatively had significantly higher disease free (DFS) and overall (OS) survival when compared with patients with elevated tumour markers. DFS and OS correlated with the number of elevated markers. This finding was independent of underlying histopathological classification of low grade and high grade tumours. There has been a recent pilot study reporting that CEA levels reduce, or normalise, at 7 days after complete CRS [Citation45]. It was postulated that this may have prognostic value and further work in this area is awaited.
Various other inflammation based indices, such as the neutrophil lymphocyte ration and the Onodera nutritional index, have been shown to be independent prognostic markers for overall survival in PMP patients undergoing CRS and HIPEC [Citation43].
Treatment
The recommended treatment for PMP is a combined strategy of complete macroscopic tumour removal (entitled complete cytoreductive surgery, CCRS), combined with HIPEC [Citation46–48].
Completeness of cytoredution (CC) is assessed at the end of surgery by measuring the diameter of the largest remaining tumour deposits [Citation49]. Complete removal of all visible disease is scored as CC0 cytoreduction and residual disease less than 0.25 cm is scored as CC1. Any residual tumour deposit between 0.25 and 2.5 cm is scored as a CC2 cytoreduction while residual tumour deposits >2.5 cm are scored as CC3 cytoreduction. CC0 and CC1 are considered CCRS in patients with PMP. CCRS is one of the most important prognostic factors in PMP [Citation50,Citation51]. Other methods used to assess completeness of resection include the post-operative residual tumour (R) classification [Citation52].
CCRS with HIPEC is a major undertaking with an average operating time of 9 h (range: 2–24 h) with significant associated morbidity and occasional post-operative mortality [Citation50].
A careful assessment of the patient and their fitness for surgery is paramount. All patients receive thromboembolic prophylaxis and perioperative antibiotics. Full mechanical bowel preparation is generally recommended. Patients receive prophylactic vaccination, a useful precautionary measure in the event that a splenectomy is necessary.
Patient positioning is vital, to allow access to all parts of the abdomen and perineum, while reducing the risks of compartment syndrome and neurological complications associated with prolonged surgery. Our preferred strategy is using “Allen” stirrups and a modified lithotomy position.
A long midline incision from the Xiphisternum to the pubis is usually required, with excision of the umbilicus and any old scars, if involved by tumour. The extent of disease is assessed by the peritoneal carcinomatosis index (PCI) with the understanding that this system was never designed for PMP but rather for other peritoneal malignancies, such as colorectal or gastric cancer [Citation49]. This scoring system divides the abdomen into nine anatomical areas with four further areas of the small bowel (upper and lower jejunum, upper and lower ileum). Tumour is assessed in each area and a score of 0–3 is given for each of the 13 areas (0 for no tumour, 1 for nodules <0.5 cm, 2 for nodules between 0.5 and 5 cm and 3 for nodules >5 cm). The total score is then calculated by adding all the scores, and ranges from 0 to 39. Similarly a radiological PCI can be estimated from CT and MRI scans. PMP patients with a high PCI may still be amenable to a curative resection, if the small bowel is not extensively involved.
While PCI is the most widely used scoring system, other systems like the Dutch simplified PCI [Citation53], and the Japanese carcinomatosis staging system [Citation54], are also used by individual authors.
Our favoured approach is to start with a right parietal peritonectomy and medial mobilisation of the caecum to expose the gonadal vessels and the right ureter [Citation55]. This dissection is then carried cephalad to perform a right diaphragmatic peritonectomy with full mobilisation of the liver. A liver capsulectomy is performed by a “roly ball” diathermy set at the highest level. A high power smoke extraction system is used to reduce smoke contamination.
Similar steps are followed on the left side beginning with a left parietal peritonectomy to mobilise the left and sigmoid colon, exposing the left ureter and gonadal vessels. This is continued cephalad to perform a left diaphragmatic peritonectomy if needed.
A radical greater omentectomy is performed inside the gastro epiploic vessels. The spleen is carefully assessed for disease and if involved, the splenic vessels are ligated and splenectomy performed. Care must be taken to avoid damage to the tail of the pancreas, as this can lead to pancreatitis and a pancreatic fistula.
Pelvic dissection is performed by complete mobilisation of the rectum in the mesorectal plane. This is then continued anteriorly towards the bladder and the peritoneum carefully stripped off the bladder. Often the rectum and sigmoid can be preserved, but in case of tumour infiltration or prior pelvic surgery, an anterior resection may be required.
The ovaries are routinely removed, as there is a high chance of ovarian metastasis. The removal of the uterus may, or may not, be required depending on the extent of disease.
The appendiceal tumour may just require an appendicectomy if the base is free and there is no tumour on the right colon. In these cases, the base of the appendix is stapled off. In cases where the base is involved, or there is extensive right colon involvement, or in the presence of ileocolic nodes, a formal radical right hemicolectomy may be required. Nodal involvement is uncommon and, if suspected, a node may be sent for frozen section.
Disease in the lesser omentum, porta hepatis and aortocaval groove is difficult to safely remove. Lesser omental dissection is usually carried out starting from the lesser curve of the stomach, taking care to preserve the left gastric vessels. This dissection is then carried to the porta hepatis, anterior to the portal triad structures.
The gallbladder is often removed, usually in a retrograde fashion to facilitate identification of the portal anatomy. The gallbladder should be removed if there is evidence of disease on the gall bladder of if there are stones in the gallbladder. The aortocaval groove is entered after complete mobilisation of the left liver and retraction of the liver to the right side. The caudate lobe is identified and the diseased peritoneum between the caudate lobe, the right crus of the diaphragm and the inferior vena cava is removed.
In about 12% of cases, a distal gastrectomy is needed to completely remove the disease on the stomach [Citation56]. The stomach and the duodenum are stapled off, and after HIPEC, an end-to-end anastomosis is performed. The gastric remnant is anastomosed to the duodenum.
Once cytoreduction is complete, intra-operative hyperthermic chemotherapy is delivered. We use the open “colosseum” technique, while some centres may use the closed technique whereby the abdomen is closed prior to commencement of HIPEC. We use Mitomycin C at a dose of 10 mg/m2, heated to 43° and continuously infused using a HIPEC machine for 1 h. Once again, the dose and timing differs between centres, with some centres using doses up to 20 mg/m2 of Mitomycin C and 90 min of HIPEC. The dose is reduced in renal failure, morbid obesity, old age and the presence of major co-morbidities. Some of the small bowel serosal disease may be rubbed off during the chemotherapy and anastomoses are performed after HIPEC. Hand-sewn anastomosis is preferred, except for a colorectal anastomosis, where a stapled anastomosis is optimal. A low rectal anastomosis is usually protected by a defunctioning ileostomy, which is usually closed at about three months provided a water-soluble contrast study shows no evidence of a leak.
We routinely insert chest drains if the diaphragm has been stripped. After major PMP surgery, particularly if the diaphragms have been stripped, patients are cared for in the intensive care unit post-operatively, and many require elective overnight ventilation.
Early post-operative intra-peritoneal chemotherapy (EPIC), while not standard practice in all centres, is used selectively at our institute, by administering 15 mg/kg of 5 fluorouracil daily for 4 days post-operatively via a Tenckhoff catheter. Some of the factors determining the use of post-operative chemotherapy include completeness of cytoreduction, age and performance status of the patient, grade of tumour and duration of surgery.
While we have described our preferred surgical technique above, many variations in order of surgery, specific technology and techniques are reported from different centres. However, the general principals of attaining CCRS remain the same.
When complete CCRS is not possible, a maximum tumour debulking (MTD) can still achieve good survival and significant improvements in quality of life. This generally involves a greater omentectomy with subtotal colectomy and an end ileostomy. In a recent study by Dayal et al. [Citation9], 205 patients undergoing MTD had an overall survival of 47% at 3 years, 30% at 5 years and 22% at 10 years after surgery, with a median survival of 32.8 months.
The use of perioperative systemic chemotherapy is not standardised and based on individual preferences. It has been shown that while post-operative systemic chemotherapy may not be helpful in low-grade appendiceal neoplasms, there are benefits in high-grade neoplasms [Citation57]. Preoperative systemic chemotherapy is generally not helpful, expect in borderline resectable or unresectable cases [Citation57].
Management of unexpected appendiceal neoplasms
An appendiceal neoplasm may sometimes be encountered at surgery for appendicitis or incidentally noted at laparotomy, or laparoscopy, for an unrelated procedure. In these cases, note should be made of the size of the tumour, involvement of the base of the appendix, involvement of the mesoappendix and associated perforation. Careful examination of the rest of the peritoneal cavity should be undertaken to look for obvious metastatic disease or presence of mucin.
Tumours smaller than 2 cm, without involvement of the base or the mesoappendix, can be safely treated with a simple appendicectomy [Citation26,Citation58]. Care should be taken to resect the mesoappendix adequately and prevent perforation of the specimen. If performed laparoscopically, midline ports should be used for specimen extraction.
Non-mucinous neoplasms greater than 2 cm, involving the appendix base or the mesoappendix, should be treated with a right hemicolectomy. In cases of uncertainty, it may be preferable to perform an appendicectomy and wait for definitive histopathology [Citation26].
Patients with perforated mucinous neoplasms and suspected PMP are best treated with CRS and HIPEC [Citation26,Citation48]. In these cases, a simple appendicectomy should be performed, if feasible, along with an omental biopsy, with onward referral to a specialist centre.
Outcomes
Prior to the strategy of CRS and HIPEC, the outcome of many patients with PMP was poor with repeated laparotomy and debulking which becomes increasingly ineffective over time. Two publications from the Mayo clinic in 1990 reported a 10 year survival of 32% for low grade PMP and a 5 year survival of 6% for adenocarcinoma of the appendix [Citation59,Citation60].
With the introduction of CRS and HIPEC, the survival of patients with PMP has improved dramatically [Citation61,Citation62]. Disease free survival at 1, 5 and 10 years have been reported as 75%, 56–70% and 67%, with overall 5-year survival rate of 69–75% and overall 10-year survival rates of 57% [Citation46,Citation50,Citation51,Citation63,Citation64].
In 2006, an expert consensus concluded that there was significant survival benefits with CRS and HIPEC when compared to historical controls [Citation47]. A pooled analysis, published by Chua et al. [Citation50] in 2012, included 2298 patients from 16 centres, undergoing CRS and HIPEC. In this multicentre study the overall 3-, 5-, 10- and 15-year survival rates were 80%, 74%, 63% and 59%, respectively. Post-operative mortality was 2% and major complication rate was 24%. Patients who had complete cytoreduction (CC0 and CC1) had an 85% 5-year survival when compared to patients who had a CC2 or CC3 cytoreduction. This again confirms that completeness of cytoreduction is one of the most important prognostic factors. Complete cytoredution also depends on the extent of previous surgery as tumour entrapment and deposition in scar tissue decreases the chances of a complete cytoreduction [Citation65].
A systemic review in 2013 put the median 3-, 5- and 10-year survival rates at 77.8%, 79.5% and 55.9% [Citation66]. A recent report on 1000 appendiceal tumours treated with a strategy of CRS and HIPEC reported a median 5- and 10-year overall survival of 87.4% and 70.3% for patients undergoing CCRS compared with only 39.2% and 8.1% for patients undergoing MTD. 30-day mortality was 0.8% and 1.7% in the CCRS and MTD groups with post-operative morbidity being 15.2% and 14.5% [Citation67].
Other prognostic factors in this largest single centre report were grade of tumour and tumour marker levels [Citation67].
It is pertinent to note that CRS and HIPEC are major undertakings associated with major morbidity and mortality. Operative mortality ranges between 0.6% and 4.4% and major morbidity between 7% and 49% [Citation46,Citation50,Citation51,Citation63,Citation64]. An experienced multidisciplinary team comprising of surgeons, anaesthetists, intensivists, radiologists, pathologists, specialist nurses, stoma therapists, dieticians, physiotherapists and pharmacists improves outcomes and reduces complications.
Follow-up
A definitive follow-up strategy is important to detect recurrences early and plan subsequent management. Our standard practice has been to perform a follow-up CT scan and serum tumour markers at 1 year after surgery and then annually thereafter for 10 years. Earlier imaging may be warranted if patients are symptomatic or recurrence is suspected.
If recurrence is detected on follow-up, further management has to be individualised, with no definitive guidelines available. Options include a repeat cytoreduction with HIPEC, systemic chemotherapy or a watch and wait approach, with intervention only if the disease progresses or symptoms develop. The nature of the primary tumour and primary surgery, location of the recurrence, disease burden, fitness for surgery as well as symptoms and patient wishes, all play a part in deciding further management.
Conclusion
PMP is a rare, borderline malignant, clinico-pathological entity originating from a perforated mucinous neoplasm of the appendix, with secondary spread by the “redistribution” phenomenon. The optimal management includes CCRS followed by HIPEC. This is a complex surgical procedure associated with significant morbidity and mortality, and is best performed in experienced centres. Even advanced disease may be amenable to surgical resection, and in cases where complete excision is not possible, a maximal tumour debulking offers significant survival advantage. The principles and techniques developed from management of PMP have been translated to treating other more common peritoneal malignancies, particularly colorectal peritoneal metastases and primary peritoneal malignancies. Control and cure of peritoneal malignancy is increasingly achievable in selected cases and work is ongoing in this complex field.
References
- Sugarbaker PH. (1996). Pseudomyxoma peritonei. Cancer Treat Res 81:105–19.
- Moran BJ, Cecil TD. (2003). The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am 12:585–603.
- Weaver CH. (1937). Mucocele of appendix with pseudomucinous degeneration. Am J Surg 36:523–6.
- Werth R. (1884). Klinische und anatomische Untersuchungen zur Lehre von den Bauchgeschwülsten und der Laparatomie. Arch Für Gynäkol 24:100–18.
- Baratti D, Kusamura S, Milione M, et al. (2016). Pseudomyxoma peritonei of extra-appendiceal origin: a comparative study. Ann Surg Oncol 23:4222–30.
- Castle OL. (1915). Cystic dilation of the vermiform appendix. Ann Surg 61:582–8.
- Complete cytoreduction for pseudomyxoma peritonei (Sugarbaker technique). Guidance and guidelines. NICE [Internet]. Available from: https://www.nice.org.uk/guidance/ipg56 [last accessed 24 Nov 2016].
- Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. (2008). Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol 34:196–201.
- Dayal S, Taflampas P, Riss S, et al. (2013). Complete cytoreduction for pseudomyxoma peritonei is optimal but maximal tumor debulking may be beneficial in patients in whom complete tumor removal cannot be achieved. Dis Colon Rectum 56:1366–72.
- Behling H. (1967). Mucocele of the appendix and jelly-belly. Minn Med 50:1109–12.
- Sugarbaker PH. (1994). Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg 219:109–11.
- Pestieau SR, Esquivel J, Sugarbaker PH. (2000). Pleural extension of mucinous tumor in patients with pseudomyxoma peritonei syndrome. Ann Surg Oncol 7:199–203.
- Järvinen P, Lepistö A. (2010). Clinical presentation of pseudomyxoma peritonei. Scand J Surg 99:213–16.
- Chuaqui RF, Zhuang Z, Emmert-Buck MR, et al. (1996). Genetic analysis of synchronous mucinous tumors of the ovary and appendix. Hum Pathol 27:165–71.
- Guerrieri C, Frånlund B, Fristedt S, et al. (1997). Mucinous tumors of the vermiform appendix and ovary, and pseudomyxoma peritonei: histogenetic implications of cytokeratin 7 expression. Hum Pathol 28:1039–45.
- Szych C, Staebler A, Connolly DC, et al. (1999). Molecular genetic evidence supporting the clonality and appendiceal origin of pseudomyxoma peritonei in women. Am J Pathol 154:1849–55.
- Ronnett BM, Shmookler BM, Diener-West M, et al. (1997). Immunohistochemical evidence supporting the appendiceal origin of pseudomyxoma peritonei in women. Int J Gynecol Pathol 16:1–9.
- Lee SE, Jang J-Y, Yang SH, Kim S-W. (2007). Intraductal papillary mucinous carcinoma with atypical manifestations: report of two cases. World J Gastroenterol 13:1622–5.
- Smeenk RM, Bex A, Verwaal VJ, et al. (2006). Pseudomyxoma peritonei and the urinary tract: involvement and treatment related complications. J Surg Oncol 93:20–3.
- Ronnett BM, Zahn CM, Kurman RJ, et al. (1995). Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol 19:1390–408.
- Bradley RF, Stewart JH, Russell GB, et al. (2006). Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol 30:551–9.
- Carr NJ, Sobin LH. (2010). Adenocarcinoma of the appendix. In: Bosman FT, Carneiro F, Hruban RH, et al., eds. WHO classification of tumors of the digestive system. Lyon: IARC, 12212–15.
- Carr NJ, Cecil TD, Mohamed F, et al. (2016). A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) modified Delphi process. Am J Surg Pathol 40:14–26.
- Esquivel J, Sugarbaker PH. (2000). Clinical presentation of the pseudomyxoma peritonei syndrome. Br J Surg 87:1414–18.
- Glaysher M, Gordon-Dixon A, Chandrakumaran K, et al. (2014). Pseudomyxoma peritonei of appendiceal origin: mode of presentation in the modern era. Colorectal Dis 16:53.
- Murphy EMA, Farquharson SM, Moran BJ. (2006). Management of an unexpected appendiceal neoplasm. Br J Surg 93:783–92.
- Sulkin TVC, O’Neill H, Amin AI, Moran B. (2002). CT in pseudomyxoma peritonei: a review of 17 cases. Clin Radiol 57:608–13.
- Jacquet P, Jelinek JS, Chang D, et al. (1995). Abdominal computed tomographic scan in the selection of patients with mucinous peritoneal carcinomatosis for cytoreductive surgery. J Am Coll Surg 181:530–8.
- Cotton F, Pellet O, Gilly F-N, et al. (2006). MRI evaluation of bulky tumor masses in the mesentery and bladder involvement in peritoneal carcinomatosis. Eur J Surg Oncol 32:1212–16.
- Tirumani SH, Fraser-Hill M, Auer R, et al. (2013). Mucinous neoplasms of the appendix: a current comprehensive clinicopathologic and imaging review. Cancer Imaging 13:14–25.
- Low RN, Barone RM, Gurney JM, Muller WD. (2008). Mucinous appendiceal neoplasms: preoperative MR staging and classification compared with surgical and histopathologic findings. Am J Roentgenol 190:656–65.
- Low RN, Sebrechts CP, Barone RM, Muller W. (2009). Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings-a feasibility study. Am J Roentgenol 193:461–70.
- Menassel B, Duclos A, Passot G, et al. (2016). Preoperative CT and MRI prediction of non-resectability in patients treated for pseudomyxoma peritonei from mucinous appendiceal neoplasms. Eur J Surg Oncol 42:558–66.
- Moran BJ, Cecil TD. (2014). Treatment of surgically resectable colorectal peritoneal metastases. Br J Surg 101:5–7.
- Passot G, Glehen O, Pellet O, et al. (2010). Pseudomyxoma peritonei: role of 18F-FDG PET in preoperative evaluation of pathological grade and potential for complete cytoreduction. Eur J Surg Oncol 36:315–23.
- Wagner PL, Austin F, Sathaiah M, et al. (2013). Significance of serum tumor marker levels in peritoneal carcinomatosis of appendiceal origin. Ann Surg Oncol 20:506–14.
- Taflampas P, Dayal S, Chandrakumaran K, et al. (2014). Pre-operative tumour marker status predicts recurrence and survival after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for appendiceal pseudomyxoma peritonei: analysis of 519 patients. Eur J Surg Oncol 40:515–20.
- Alexander-Sefre F, Chandrakumaran K, Banerjee S, et al. (2005). Elevated tumour markers prior to complete tumour removal in patients with pseudomyxoma peritonei predict early recurrence. Colorectal Dis 7:382–6.
- Baratti D, Kusamura S, Martinetti A, et al. (2007). Prognostic value of circulating tumor markers in patients with pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 14:2300–8.
- Carmignani CP, Hampton R, Sugarbaker CE, et al. (2004). Utility of CEA and CA 19-9 tumor markers in diagnosis and prognostic assessment of mucinous epithelial cancers of the appendix. J Surg Oncol 87:162–6.
- van Ruth S, Hart AAM, Bonfrer JMG, et al. (2002). Prognostic value of baseline and serial carcinoembryonic antigen and carbohydrate antigen 19.9 measurements in patients with pseudomyxoma peritonei treated with cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 9:961–7.
- Koh J-L, Liauw W, Chua T, Morris DL. (2013). Carbohydrate antigen 19-9 (CA 19-9) is an independent prognostic indicator in pseudomyxoma peritonei post cytoreductive surgery and perioperative intraperitoneal chemotherapy. J Gastrointest Oncol 4:173–81.
- Kusamura S, Baratti D, Hutanu I, et al.. The role of baseline inflammatory-based scores and serum tumor markers to risk stratify pseudomyxoma peritonei patients treated with cytoreduction [Internet]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/?term=Eur+J+Surg+Oncol.+2015+Aug%3B41(8)%3A1097-105 [last accessed 06 Feb 2017].
- Kusamura S, Hutanu I, Baratti D, Deraco M. (2013). Circulating tumor markers: predictors of incomplete cytoreduction and powerful determinants of outcome in pseudomyxoma peritonei. J Surg Oncol 108:1–8.
- Di Fabio F, Aston W, Mohamed F, et al. (2015). Elevated tumour markers are normalized in most patients with pseudomyxoma peritonei 7 days after complete tumour removal. Colorectal Dis 17:698–703.
- Youssef H, Newman C, Chandrakumaran K, et al. (2011). Operative findings, early complications, and long-term survival in 456 patients with pseudomyxoma peritonei syndrome of appendiceal origin. Dis Colon Rectum 54:293–9.
- Moran B, Baratti D, Yan TD, et al. (2008). Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei). J Surg Oncol 98:277–82.
- Sugarbaker PH, Chang D. (1999). Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol 6:727–31.
- Jacquet P, Sugarbaker PH. (1996). Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359–74.
- Chua TC, Moran BJ, Sugarbaker PH, et al. (2012). Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 30:2449–56.
- Yan TD, Bijelic L, Sugarbaker PH. (2007). Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann Surg Oncol 14:2289–99.
- Hermanek P, Wittekind C. (1994). The pathologist and the residual tumor (R) classification. Pathol Res Pract 190:115–23.
- Witkamp AJ, de Bree E, Kaag MM, et al. (2001). Extensive cytoreductive surgery followed by intra-operative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur J Cancer 37:979–84.
- Kajitani T. (1981). The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg 11:127–39.
- Taflampas P, Moran BJ. (2013). Extraperitoneal resection of the right colon for locally advanced colon cancer. Colorectal Dis 15:e56–9.
- Di Fabio F, Mehta A, Chandrakumaran K, et al. (2016). Advanced pseudomyxoma peritonei requiring gastrectomy to achieve complete cytoreduction results in good long-term oncologic outcomes. Ann Surg Oncol 23:4316–21.
- Blackham AU, Swett K, Eng C, et al. (2014). Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol 109:740–5.
- Stocchi L, Wolff BG, Larson DR, Harrington JR. (2003). Surgical treatment of appendiceal mucocele. Arch Surg 138:585–90.
- Gough DB, Donohue JH, Schutt AJ, et al. (1994). Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg 219:112–19.
- Nitecki SS, Wolff BG, Schlinkert R, Sarr MG. (1994). The natural history of surgically treated primary adenocarcinoma of the appendix. Ann Surg 219:51–7.
- Sugarbaker PH. (1989). Surgical treatment of peritoneal carcinomatosis: 1988 Du Pont lecture. Can J Surg 32:164–70.
- Sugarbaker PH. (1995). Peritonectomy procedures. Ann Surg 221:29–42.
- Omohwo C, Nieroda CA, Studeman KD, et al. (2009). Complete cytoreduction offers longterm survival in patients with peritoneal carcinomatosis from appendiceal tumors of unfavorable histology. J Am Coll Surg 209:308–12.
- Elias D, Gilly F, Quenet F, et al. (2010). Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol 36:456–62.
- Spiliotis J, Efstathiou E, Halkia E, et al. (2012). The influence of tumor cell entrapment phenomenon on the natural history of pseudomyxoma peritonei syndrome. Hepatogastroenterology 59:705–8.
- McBride K, McFadden D, Osler T. (2013). Improved survival of patients with pseudomyxoma peritonei receiving intraperitoneal chemotherapy with cytoreductive surgery: a systematic review and meta-analysis. J Surg Res 183:246–52.
- Ansari N, Chandrakumaran K, Dayal S, et al. (2016). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1000 patients with perforated appendiceal epithelial tumours. Eur J Surg Oncol 42:1035–41.