Abstract
Background: Hyperthermic isolated limb perfusion (HILP) represents a limb-sparing treatment for unresectable soft tissue sarcoma (STS) of the extremities with substantial complete response rates. HILP often provides good functional limb preservation, hence a significant improvement also in terms of quality of life of the patient. Notwithstanding these clear advantages, the traditional technique is still hindered by relatively high post-operative morbidity.
Method: We treated a 78-year-old female with unresectable angiosarcoma of the left leg using a new surgical approach: an entirely laparoscopic HILP.
Results: No conversion from laparoscopic to “open” surgery was necessary. Since no abdominal muscle section was performed, post-operative pain was low and easily manageable; early mobilisation and early discharge were achieved. Patient developed moderate toxicity, which resolved spontaneously within 3–4 weeks, with complete return to normal daily activities after 30 d. Complete clinical response with preservation of leg function was obtained.
Conclusions: We describe for the first time an entirely laparoscopic HILP. Demonstration of this technique’s efficacy and safety on a large series of patients is clearly necessary but its therapeutic efficacy appears to be comparable to the standard technique. Furthermore, laparoscopic HILP has shown low post-operative morbidity: no wound complications, mild and easily manageable post-operative pain and early discharge from the hospital and early resuming of daily activities.
Introduction
We propose a novel laparoscopic hyperthermic isolated limb perfusion (HILP) technique that could represent an innovative compromise between the efficacy of HILP and the low morbidity rates of other less invasive approaches.
HILP has gone through multiple reshuffles, modifications and revisions. In 1950, Klopp et al. were the pioneers of direct injection of nitrogen mustard into the arterial supply of tumours while venous drainage temporary occlusion was performed [Citation1]. In 1958, Creech and Krementz introduced isolated limb perfusion (ILP) as a novel method of drug administration for patients with advanced cancer of a limb [Citation2]. ILP, as firstly described, consisted of: exposition and temporary isolation of the limb’s major blood vessels and perfusion of the extremity with high dosage of chemotherapeutic drugs via a heart–lung machine [Citation3]. The authors goal was to obtain high tissue drug concentrations with minimal systemic exposure and hence few complications. Capitalizing on the observation that heat had its own antineoplastic properties, in 1975, Stehlin modified the technique to include hyperthermia [Citation4,Citation5]. Hyperthermia causes thermal chemosensitization, defined as an increased drug uptake and chemical activity, and induces severe oxygen deprivation in soft tissue sarcoma (STS) [Citation6]. Mild hyperthermia (38–39, 0 °C) is considered the best compromise between efficacy and acceptable levels of toxicity [Citation7].
Recent studies confirmed that HILP, regardless of the chosen drug combination, represents a safe and efficient limb-sparing treatment that provides substantial and durable local control of the disease in patients with either primary, recurrent or metastatic melanoma or soft-tissue sarcoma of a limb [Citation8–18]. In particular, Seyedi et al. showed a complete response after HILP of all five patients with angiosarcoma [Citation19]. In another case report, complete regression of a 10 × 25 cm upper extremity angiosarcoma was achieved through HILP [Citation4].
Currently, in most international melanoma and STS centres, HILP in the lower limb is performed using the traditional technique which requires wide surgical incisions: a single ilio-inguinal incision or two separate incisions, one to access inguinal vessels and nodes and one to reach iliac-obturators vessels and nodes. It also demands the preparation of skin flaps, the interruption of fasciae and muscles of the lower abdominal wall and often the section of the inguinal ligament, resulting in high morbidity for the patient.
Despite a considerable evolution, traditional HILP is still a long (3–4 h) and complex multidisciplinary procedure. Surgeons are required to possess a thorough knowledge of vascular surgery, a high degree of coordination and a range of non-surgical skills [Citation3]. In addition, the available literature on ILP showed great variability in surgical technique, drug choice, temperature setting, indications and response evaluation [Citation3,Citation20–24].
In the past, several attempts have been made to develop a less invasive and simplified procedure. Isolated limb infusion (ILI) is a short (30 min), low-flow ILP, performed under hypoxic conditions via percutaneously placed catheters. Just like ILP, it achieves high-dose regional therapy with cytotoxic agents and substantially avoids systemic side effects, but with reduced invasiveness [Citation25]. Drug concentrations in the limb can be up to 10-fold of the maximum tolerated systemic concentration [Citation26]. Hypoxia produces marked acidosis of the limb and they act synergistically, enhancing cytotoxic effects of several drugs (e.g. melphalan) (Stratford, Adams, & Godden, 1988) [Citation27,Citation28]. ILI technique is usually uncomplicated even in case lymph node dissection because it exploits contralateral groin percutaneous accesses. It has been successfully used also for STS [Citation29] and it is considered well tolerated even in elderly, infirm patients [Citation30]. Nonetheless, Beasley et al. analysed the result of 162 ILIs performed in eight different institutions and found that ILI was indeed a safe alternative for HILP but with consistently lower rates of complete responses [Citation31].
In order to reduce post-operative morbidity and maintain notable rates of complete tumour response, we propose a new surgical approach: HILP completely performed with minimally invasive approach. This is the first case described in literature of an entirely laparoscopic HILP.
Methods
A 78-year-old female with an unresectable angiosarcoma of the left leg was treated at the Surgery Unit 1 of IRCCS “San Martino-IST” of Genoa, Italy, in August 2016.
During the previous five months, the patient had undergone two consecutive wide excisions of the cutaneous angiosarcoma associated with skin grafts reconstruction. Afterwards, she developed multiple new metastatic lesions on the outer margins of the grafts. Positron emission tomography–computed tomography (PET-CT) confirmed a locally advanced tumour with no evidence of secondary localizations. We debated the case with our institutional STS disease management team (DMT) and considering our experience with laparoscopy, especially in radical laparoscopic lymph nodes dissection [Citation32], and our experience with traditional HILP and HIPEC [Citation33,Citation34], we obtained approval and agreed upon an accurate planning of the novel procedure. Therefore, entirely laparoscopic HILP with melphalan was performed to achieve local control of the metastatic angiosarcoma with limb salvage.
Technique: In our institute, we usually prefer to use iliac approach over femoral approach because we registered a lower rate of wound complications and lower post-operative morbidity. Furthermore, we favour its first line use in order to maintain intact femoral vessels if additional perfusion is required.
We recommend to perform laparoscopic HILP via four ports [Citation35]: a 10 mm umbilical port, for the camera, a second 10 mm suprapubic operative port, a third 5 mm operative port on the ipsilateral pararectus line and a fourth 5 mm port on the contralateral pararectus line.
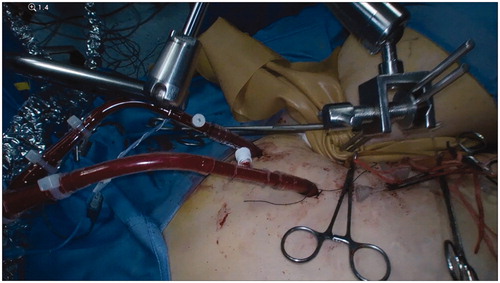
The patient should be placed in supine position, with 30° right tilt and 30° Trendelenburg tilt. General anaesthesia is necessary. We suggest to administer prophylactic antibiotics and to place a Foley catheter to empty the bladder. A rubber Esmarch tourniquet is applied at the proximal limit of the limb and fixed by Steinman orthopaedic pins inserted into the iliac crest and held in place by a Martin’s arm retractor ().
Figure 1. Esmarch tourniquet applied at the proximal limit of the limb, fixed with Steinman orthopaedic pins inserted into the iliac crest and held in place by a Martin’s arm retractor.
The anatomical boundaries of the surgical field include: the genitofemoral nerve, laterally, the obliterated umbilical artery, medially, the pubic bone, distally and the bifurcation of the common iliac artery, proximally. We usually perform a lymph node dissection with the sole goal of obtaining a proper exposition of the external iliac vessels and their branches; hence no radical surgical approach is required. Gradually, the lymphatic tissue anterior to the external iliac vessels is carefully divided longitudinally, obtaining a circumferential skeletonisation of both iliac vessels. During this phase, we use both bipolar and ultrasound devices. The dissection along the medial aspect of the lymphatic iliac tissue allows the identification of the obturator nerve. The entire specimen is therefore placed in a laparoscopic bag to avoid contact with adjacent tissues.
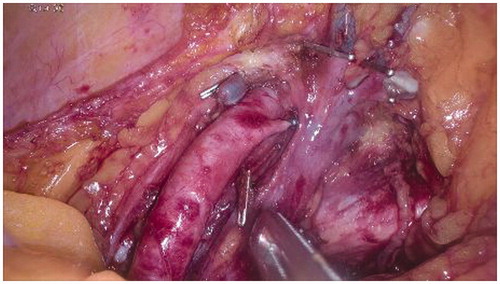
All side branches of external iliac vessels are clipped with particular attention to collateral ones beyond the inguinal ligament (i.e. inferior deep epigastric artery and vein, deep internal and external circumflex vessels) (). These clips are carefully removed at the end of the perfusion. Obturator vein is ligated and sectioned. These deep collaterals are not affected by external Esmarch banding, and their control is crucial for minimising leakage during perfusion.
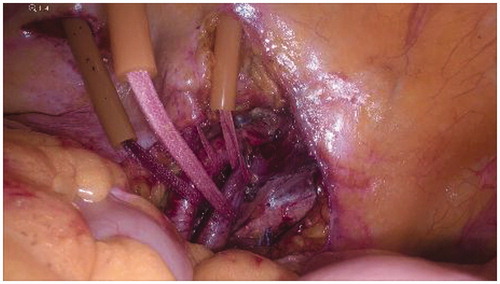
Four tourniquets, introduced through four 5 mm skin incisions, need to be applied proximally and distally on each external iliac vessel (). Two cannulae should be introduced through two additional 5 mm skin incisions on the ipsilateral side of the abdominal wall: a 16 F cannula for the vein and a 14 F cannula for the artery ().
Two thermistors shall now be inserted into the subcutaneous tissue, proximally to the tumour sites, with the purpose of measuring limb temperature during perfusion [Citation4].
Systemic heparinization (5000 UI heparin) is indispensable. Deep vein thrombosis prophylaxis measures such as preoperative low molecular weight heparin (LMWH) and contralateral elastic compression stockings are indicated. Anticoagulation is monitored throughout the procedure making sure that the activated clotting time (ACT) is always >350 ss [Citation36].
The external iliac vessels are therefore clamped and cannulated (firstly the vein and then the artery). While artery cannula positioning is almost effortless, the insertion of the vein cannula is typically quite laborious since the cannula has to be pushed distally to reach at least the inguinal ligament and since the venous valves produce a certain resistance. We advise to attempt to best align the longitudinal axes of the cannula and the vein and proceed with extremely delicate retraction-jiggling-advancement movements of the cannula when a stop is perceived. A useful tip might be using the tourniquets in order to exploit venous filling and resulting turgor.
The extracorporeal circulation system (ECS) consists of a roller-pump similar to the one used for cardiopulmonary bypass surgery (Rand device). A single-head pump is sufficient. Priming is composed of 500 ml of balanced electrolyte solution, 200 ml of polygeline (Emagel®) and 5000 U of heparin.
After setting the ECS, the limb ought to be perfused in conditions of mild hyperthermia for 60 min using melphalan (100 mg to 10 mg/l) with a flow rate of 300–400 ml/min.
No leakage monitoring system is required since no TNF-α was used and therefore limited systemic toxicity has been shown. We chose to avoid TNF-α in consideration of its systemic toxicity [Citation37], patient comorbidity, absence of “high tumor burden” (lesions larger than 3–5 cm or more than 10–15 lesions) and the evaluation of DMT of our Institution.
At the completion of the infusion, the circuit is interrupted and the perfused solution is washed out from the limb with 2.5 l of saline solution, 0.9% and 1.5 l of polygeline solution.
The pump is then turned off and the cannulae can be removed [Citation3].
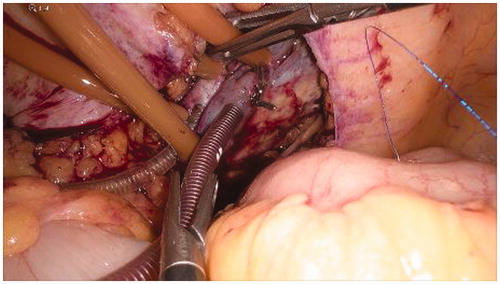
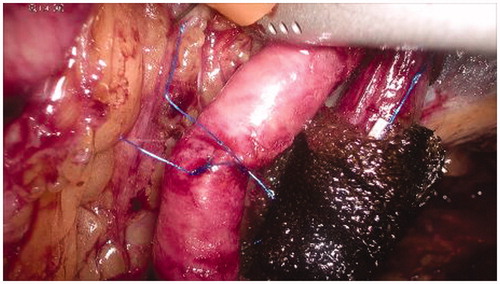
The vein and the arterial breaches are repaired manually through simple interrupted stitches with prolene 5/0 () in order to restore the normal blood flow of the limb ().
Figure 5. Manual repair of the vascular breaches through simple interrupted stitches with prolene 5/0.
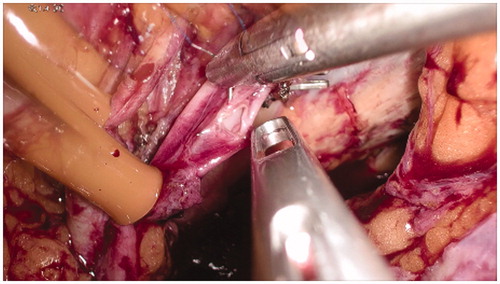
Figure 6. Completion of the vascular sutures and restoring of the normal blood flow in the external iliac vessels.
Finally, the procedure is completed by peritoneal repair with running suture, the placement of an 18 F fluted drain through the lateral 5 mm port site, and the extraction of the specimen through the 10 mm trocar site, using the laparoscopic bag.
Results
The procedure was completed in 300 min. No conversion from laparoscopic to “open” surgery was necessary. There was no need for a nasogastric tube. No abdominal muscle or fascia significant transection was performed (apart from the 10 mm port incision) therefore post-operative pain was moderate and managed with minor analgesic (paracetamol 1500 mg/d). Patient was mobilised early and was able to walk on the very first post-operative day. Bowel movements were observed within 2 d from surgery and patient was discharged on the fifth day after surgery.
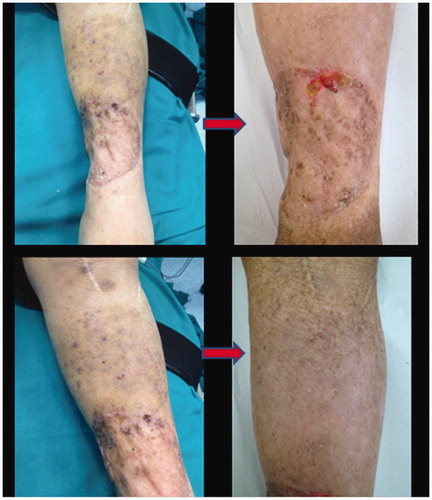
Patient developed moderate toxicity (Wieberdink grade III [Citation38]): mild oedema, mild calor, erythema, discomfort and few skin blisters, mostly located on the soles of her feet. No full-thickness tissue loss was observed. Pain and significant discomfort were probably related to muscle swelling, and neurotoxicity. Evaluation of the venous and arterial system by ultrasound Doppler was performed after 21 d and no major complications were observed. Ten days later the patient developed a mild, self-limited neutropenia. The patient’s angiosarcoma displayed complete clinical response to the treatment while the leg function was preserved ().
Most of the complications following laparoscopic HILP resolved spontaneously within 3–4 weeks and the patients resumed normal daily activities after 30 d.
Conclusions
We describe for the first time HILP completely perform with minimally invasive approach. Our aim was only to test the feasibility of a HILP by a totally laparoscopic approach: we reproduced the same steps of the standard technique with the additional difficulties deriving from the minimally invasive approach. The therapeutic efficacy appeared comparable to the one achieved with the standard technique, with no need for conversion to an “open” procedure, and no intraoperative complications. Furthermore, laparoscopic HILP showed the possibility of an excellent exposure of the anatomic structures even in a patient with small pelvis and excessive fat tissue. Moreover, registered post-operative morbidity was impressively low: no wound complications, very low post-operative pain, early discharge from the hospital and recovery of daily activities were all readily reached. This technique is convincing, feasible and safe if performed by a surgeon with high laparoscopic experience and accurate knowledge of vascular and general surgery. Femoral video-assisted approach might be feasible soon thanks to our institution’s experience with video-assisted inguinal lymph node dissection, even if cavity narrowness could represent a significant obstacle [Citation32]. Clearly, demonstration of minimally invasive HILP efficacy and safety on a large series of patients is necessary but we are confident that laparoscopic HILP will provide a powerful option in the future.
Patient consent
The patient described in this article has given written consent to the non-standard procedure and to the publication of material pertaining to herself. She acknowledges that full anonymisation has been attained.
Disclosure statement
The Authors, Nicola Solari, MD, Francesco Sucameli, MD, Marco Gipponi, MD, Franco De Cian, MD, and Ferdinando Cafiero, MD, have no financial interest in any of the products, devices, or drugs mentioned in this article.
The paper is not based on a previous communication to a society or meeting.
References
- Klopp C, Alford T, Bateman J, et al. (1950). Fractionated intra-arterial cancer chemotherapy with methyl bis amine hydrochloride: a preliminary report. Ann Surg 132:811–32.
- Creech OJ, Krementz E, Ryan R, Winblad J. (1958). Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg 148:616–32.
- Klauser J, Lev-Chelouche D, Meller I, et al. (2001). Isolated limb perfusion in the treatment of advanced soft-tissue sarcomas. Musculoskeletan Cancer Surg 4:75–83.
- Stehlin JJ. (1969). Hyperthermic perfusion with chemotherapy for cancers of the extremities. Surg Gynecol Obstet 129:305–8.
- Stehlin J, Giovanella B, de Ipolyi P, et al. (1975). Results of hyperthermic perfusion for melanoma of the extremities. Surg Gynecol Obstet 140:339–48.
- Jakob J, von Rege I, Weiss C, Hohenberger P. (2012). Impact of hyperthermic isolated limb perfusion on tumour oxygenation in soft tissue sarcoma. Int J Hyperthermia 28:591–6.
- de Wilt J, Manusama E, van Tiel S, et al. (1999). Prerequisites for effective isolated limb perfusion using tumour necrosis factor alpha and melphalan in rats. Br J Cancer 80:161–6.
- Beasly G, Ross M, Tyler D. (2008). Future directions in regional treatment strategies for melanoma and sarcoma. Int J Hyperthermia 24:301–9.
- Di Filippo F, Calabro A, Giannarelli D, et al. (1989). Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer 63:2551–61.
- Kim C, Puleo C, Letson G, Reintgen D. (2001). Hyperthermic isolated limb perfusion for extremity sarcomas. Cancer Control 8:269–73.
- Taeger G, Grabellus F, Podleska L, et al. (2008). Effectiveness of regional chemotherapy with TNF-alpha/Melphalan in advanced soft tissue sarcoma of the extremities. Int J Hyperthermia 24:193–203.
- Rastrelli M, Campana L, Valpione S, et al. (2016). Hyperthermic isolated limb perfusion in locally advanced limb soft tissue sarcoma: a 24-year single-centre experience. Int J Hyperthermia 32:165–72.
- Grünhagen D, de Wilt J, van Geel A, Eggermont A. (2006). Isolated limb perfusion for melanoma patients-a review of its indications and the role of tumour necrosis factor-alpha. Eur J Surg Oncol 32:371–80.
- Rossi C, Comandone A, Veltri A. (2010). Locoregional therapies and surgical oncology. In: Antonio M, ed. New technologies in surgical oncology. Milan: Springer Milan, 113–28.
- Deneve J, Zager J. (2012). Isolated regional therapy for advanced extremity soft tissue sarcomas. Surg Oncol Clin N Am 21:287–99.
- Eggermont A, de Wilt J, ten Hagen T. (2003). Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol 4:429–37.
- Klaase J, Kroon B, van Geel A, et al. (1994). Prognostic factors for tumor response and limb recurrence-free interval in patients with advanced melanoma of the limbs treated with regional isolated perfusion with melphalan. Surgery 115:39–45.
- Vrouenraets B, Nieweg O, Kroon B. (1996). Thirty-five years of isolated limb perfusion for melanoma: indications and results. Br J Surg 83:1319–28.
- Seyedi J, Lienard D, Bourgeois P, Lejeune F. (1990). Treatment of advanced angiosarcomas of the limbs by isolation perfusion. Reg Cancer Treatment 3:98–103.
- Padussis J, Steerman S, Tyler D, Mosca P. (2008). Pharmacokinetics & drug resistance of melphalan in regional chemotherapy: ILP versus ILI. Int J Hyperthermia 24:239–49.
- Ariyan CEBM. (2008). History of regional chemotherapy for cancer of the extremities. Int J Hyperthermia 24:185–92.
- Haveman J, Van Der Zee J, Wondergem J, et al. (2004). Effects of hyperthermia on the peripheral nervous system: a review. Int J Hyperthermia 20:371–91.
- Möller M, Lewis J, Dessureault S, Zager J. (2008). Toxicities associated with hyperthermic isolated limb perfusion and isolated limb infusion in the treatment of melanoma and sarcoma. Int J Hyperthermia 24:275–89.
- Seinen J, Hoekstra H. (2013). Isolated limb perfusion of soft tissue sarcomas: a comprehensive review of literature. Cancer Treat Rev 39:569–77.
- Thompson J, Kam P, Waugh R. (1998). Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol 14:238–47.
- Minor D, Allen R, Alberts D. (1985). A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer 55:2638–44.
- Stratford I, Adams G, Godden J. (1988). Potentiation of the anti-tumour effect of melphalan by the vasoactive agent, hydralazine. Br J Cancer 58:122–7.
- Siemann D, Chapman M, Beikirch A. (1991). Effects of oxygenation and pH on tumor cell response to alkylating chemotherapy. Int J Radiat Oncol Biol Phys 30:287–9.
- Moncrieff M, Kroon H, Kam P. (2008). Isolated limb infusion for advanced soft tissue sarcoma of the extremity. Ann Surg Oncol 15:2749–56.
- Kroon H, Lin D, Kam P. (2009). Safety and efficacy of isolated limb infusion with cytotoxic drugs in elderly patients with advanced locoregional melanoma. Ann Surg 249:1008–13.
- Beasly G, Caudle A, Petersen R, et al. (2009). A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. Am Coll Surg 208:706–15.
- Solari N, Gipponi M, Di Somma C, et al. (2016). Videoscopic inguinal-iliac-obturator lymph-node dissection: new videoscopic technique for regional lymphadenectomy in patients with melanoma. Anticancer Res 36:6579–83.
- Papadia FBV, Patuzzo R, Maurichi A, et al. (2013). Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19-TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. J Surg Oncol 2:173–9.
- De Cian F, Mondini G, Demarchi R, et al. (1996). Conventional isolated hyperthermic antiblastic perfusion in the treatment of recurrent limb melanoma. Anticancer Res 16:2017–24.
- Picciotto F, Volpi E, Zaccagna A, Siatis D. (2003). Transperitoneal laparoscopical iliac lymphadenectomy for treatment of malignant melanoma. Surg Endosc 17:1536–40.
- Lander H, Zammert M, FitzGerald D. (2016). Anticoagulation management during cross-clamping and bypass. Best Pract Res Clin Anaesthesiol 30:359–70.
- Cornett WR, McCall LM, Petersen RP. (2006). Z0020, American college of surgeons oncology group trial. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor. J Clin Oncol 24:4196–201.
- Wieberdink J, Benckhuysen C, Braat R, et al. (1982). Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol 18:905–10.