Abstract
Purpose: To evaluate lyso-thermosensitive liposomal doxorubicin (LTLD, ThermoDox®) in combination with loco-regional mild hyperthermia (HT) for targeted drug delivery to the bladder wall and potential treatment of bladder cancer.
Material and methods: Porcine in vivo studies were performed with the following groups: (i) intravenous (IV) LTLD with hyperthermia (LTLD + HT); (ii) IV doxorubicin (DOX) with hyperthermia (IV DOX + HT) and (iii) IV LTLD without hyperthermia (LTLD – HT). Drug formulations were delivered via 30 min IV infusion coinciding with 1-h bladder irrigation (45 °C water for HT groups, 37 °C for non-HT group), followed by immediate bladder resection. DOX concentrations were measured in consecutive sections parallel to the bladder lumen by liquid chromatography following drug extraction. Computer models were developed to simulate tissue heating and drug release from LTLD.
Results: Comparing mean DOX concentrations at increasing depths from the lumen to outer surface of the bladder wall, the ranges for LTLD + HT, IV DOX + HT and LTLD – HT, respectively, were 20.32–3.52 μg/g, 2.34–0.61 μg/g and 2.18–0.51 μg/g. The average DOX concentrations in the urothelium/lamina and muscularis, respectively, were 9.7 ± 0.67 and 4.09 ± 0.81 μg/g for IV LTLD + HT, 1.2 ± 0.39 and 0.86 ± 0.24 μg/g for IV DOX + HT, and 1.15 ± 0.38 and 0.62 ± 0.15 μg/g for LTLD – HT. Computational model results were similar to measured DOX levels and suggest adequate temperatures were reached within the bladder wall for drug release from LTLD.
Conclusions: Doxorubicin accumulation and distribution within the bladder wall was achieved at concentrations higher than with free IV doxorubicin by mild bladder hyperthermia combined with systemic delivery of LTLD.
Introduction
Urinary bladder cancer is the fourth most common cancer in men and accounts for ∼5% of new cancer diagnoses in USA [Citation1]. The majority of patients (∼70%) present with non-muscle invasive bladder cancer (NMIBC) confined to the mucosa [Citation2]. Patients with NMIBC are generally treated with transurethral resection of the bladder tumour (TURBT) and adjuvant intravesical chemotherapy or bacillus Calmette–Guerin (BCG) [Citation2]. However, as many as 78% of NMIBC patients experience a recurrence and 45% experience disease progression within 5 years despite locoregional therapy [Citation3]. Radical cystectomy is indicated for high-risk patients who have failed first-line treatment, however alternate therapies are needed since some patients are not candidates for surgery or elect for more conservative treatments.
The incomplete response of bladder tumours to intravesical drugs has been attributed in part to inadequate drug delivery resulting in subtherapeutic drug concentrations in the bladder wall [Citation4–8]. High drug concentrations are needed at depth in the bladder wall to treat invasive tumours that reside there [Citation9]. The urothelium, which lines the bladder lumen, acts as a transport barrier that restricts passage of molecules such as urea and ammonia from the urine (as well as intravesical drugs) into systemic circulation [Citation10–12]. In addition, glycosaminoglycans comprising the mucin layer overlying the urothelium may further inhibit transport of intravesical substances. Indeed, studies have shown that drugs such as mitomycin C and doxorubicin (DOX) penetrate poorly across the urothelium limiting dosing of tumours [Citation5,Citation6,Citation9,Citation11]. As such, methods to enhance the permeability of the bladder wall or alternative routes for drug delivery are necessary.
One promising strategy to improve the efficacy of intravesical chemotherapy is the use of hyperthermia as an adjunct to therapy. Synergistic cytotoxic effects of several chemotherapeutic agents administered in combination with hyperthermia have been demonstrated in bladder cancer cell lines grown in vitro [Citation13]. Several mechanisms have been proposed for the chemosensitisation effects of hyperthermia including changes in intracellular metabolism, impairment of cellular proliferation and increased intrinsic sensitivity of tumour cells to cytotoxic drugs [Citation14,Citation15]. Hyperthermia may also serve to increase the penetration of drugs across the urothelium by increasing tissue permeability [Citation16,Citation17]. In a systematic review of chemohyperthermia trials, the combination of intravesical mitomycin C and hyperthermia appeared to be more effective than mitomycin C alone in reducing recurrence and possibly improving bladder preservation rate [Citation18]. When compared to BCG in a randomised controlled, open-label, multicenter trial, chemohyperthermia with mitomycin C demonstrated greater recurrence free survival, although recurrence rates for both groups remained relatively high [Citation19].
In addition to its chemosensitisation effects, hyperthermia can also stimulate the release of chemotherapeutic agents from thermosensitive nanocarriers at the therapeutic target. Pre-clinical studies have demonstrated high levels of doxorubicin in tumours when LTLD is administered intravenously in combination with tumour-localised mild hyperthermia [Citation20]. A commercial LTLD formulation (ThermoDox®, Celsion Corp., Lawrenceville, NJ) is currently undergoing phase III clinical evaluation in combination with radiofrequency ablation for treatment of hepatocellular carcinoma [Citation21]. Doxorubicin is also known to be active against bladder cancer and is part of a standard perioperative chemotherapy regimen for muscle invasive disease. We hypothesised that the use of LTLD in combination with mild hyperthermia would achieve high accumulation and distribution of DOX in the bladder wall that could be beneficial in the future treatment of bladder tumours. The objective of this study was to evaluate the feasibility of loco-regional application of mild hyperthermia to the bladder combined with systemic delivery of LTLD as a strategy for targeted delivery of DOX to the bladder.
Material and methods
Animal model
Female Yorkshire swine (16–18 weeks old) weighing between 53 and 63 kg were purchased from Oak Hill Genetics (Ewing, IL). Due to a known potential anaphylactoid reaction to liposomal formulations mediated by histamine, swine received the following premedication regimen: dexamethasone (0.12 mg/kg, IM) was administered twice daily for 48 h prior to day of study. On the day of study, dexamethasone (0.12 mg/kg, IM), famotidine (0.5 mg/kg, IM) and diphenhydramine (2 mg/kg, IM) were given 1.5–3 h before infusion of study formulations. Finally, meloxicam (0.3 mg/kg, IV) was given 10 min before infusion of study formulations. Anaesthetic induction was achieved with IV propofol (1 mg/kg) following sedation with intramuscular ketamine (25 mg/kg), midazolam (0.5 mg/kg) and glycopyrrolate (0.01 mg/kg). Surgical plane of anaesthesia was maintained with 100% oxygen and isoflurane (1–3%) administered via intubation. Euthanasia was performed under general anaesthesia by intravenous administration of Beuthanasia-D (pentobarbital sodium 390mg/mL and phenytoin sodium 50 mg/mL). All animal procedures were approved by the Animal Care and Use Committee of the NIH Clinical Center and performed in accordance with applicable federal regulations.
Experimental groups
The study consisted of six swine divided into three treatment groups (with one additional swine excluded due to thermal injury to the bladder wall following faulty temperature measurements) as follows: (i) Intravenous (IV) LTLD with hyperthermia (IV LTLD + HT, N = 2); (ii) IV DOX with hyperthermia (IV DOX + HT, N = 2) and (iii) IV LTLD without hyperthermia (IV LTLD – HT, N = 2). The target intravesical water temperature during irrigation was 45 °C for Groups 1 and 2, and 37 °C for Group 3. Drug formulations were administered via a 30-min IV infusion at a dose of 0.7 mg/kg.
Bladder hyperthermia
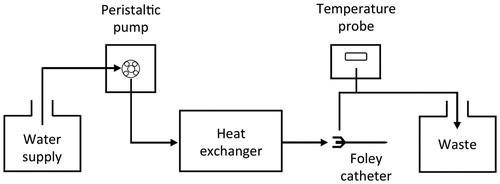
Mild hyperthermia of the bladder was performed by irrigation with warm water (45 °C). The irrigation system consisted of a custom in-line heat exchanger connected to a peristaltic pump, a water supply tank and an 18 F triple lumen Foley catheter inserted into the bladder via the urethra (). The heat exchanger consisted of coiled copper tubing submerged in a water bath. The temperature inside the bladder was monitored using an optical temperature probe inserted in the outflow of the Foley catheter and fine temperature control was achieved by slight manual adjustment of the flow rate of water and/or the water bath temperature. Hyperthermia was maintained for 1 h from the start of drug infusion.
Computational modelling of tissue heating and drug delivery in the bladder wall
We created computer models that simulate both tissue heating and drug delivery, as described in detail in prior studies [Citation22,Citation23]. An axisymmetric 2D model was created where a 4.2-mm thick bladder section was modelled, including 0.71 mm mucosa (urothelium + lamina propria). As prior studies demonstrated vast differences in vascular density between mucosa and the underlying muscle [Citation24,Citation25], we included this heterogeneity in the model. One prior study in swine measured ∼10 times higher vascular density in mucosa compared to bladder muscle [Citation25]. Since vascular density scales approximately linearly with perfusion [Citation26], we assumed a 10 times higher perfusion at the endothelial surface that dropped down throughout the mucosal layer to the lower muscle perfusion. Since together with vascular density, microvascular surface area is also increased 10×, we assumed a 10 times higher permeability-surface area product in the drug delivery model that decreased throughout the mucosal layer. Intravesicular hyperthermia by circulating water at 45 °C was simulated by applying a convective heat transfer coefficient to the internal bladder surface as boundary condition. Due to lack of sufficient data to calculate this coefficient, we assumed h = 100 W/(Km2) corresponding to forced convection at moderate intensity [Citation27]. lists the tissue properties used in the heat transfer model.
Table 1. Heat transfer model tissue properties.
The local bladder tissue temperature determined local rate of DOX release from LTLD within the vasculature; this release rate was based on in vitro measurements of DOX release from LTLD [Citation22]. The drug delivery model simulated intravascular drug release from LTLD, transvascular transport of released drug, and cell uptake, as described in prior studies [Citation22,Citation23]. Liposomal extravasation was not considered, as prior studies demonstrate intravascular release to be the dominating delivery mechanism [Citation31,Citation32]. As in the in vivo studies described above, we simulated infusion of LTLD for 30 min, while heating is simulated for 60 min starting concurrently with the infusion. Tissue drug concentrations are reported based on a weighted average of drug concentrations inside cells, in the interstitial space and in plasma; the volume fractions of cells, interstitial space and vascular space served as weighing factors to calculate total tissue concentration [Citation31]. Simulations were performed for LTLD + HT, IV DOX + HT and LTLD – HT groups.
Sectioning of the bladder wall
The concentration of DOX was measured in consecutive bladder wall sections parallel to the bladder lumen by adapting a method previously reported by Wientjes et al. [Citation33]. Briefly, immediately following euthanasia, the bladder was exposed via a midline abdominal incision. Superior and inferior aspects were marked using sutures and major blood vessels and the urethra were clamped and cut in order to excise the bladder. The dome and trigone were trimmed and the bladder wall was subsequently dissected into ∼2 × 2 cm regions. Tissue samples were rapidly frozen by submersion in liquid nitrogen with the urothelial side placed flat on a stainless steel surface. Samples from the anterior (ventral) and posterior (dorsal) bladder wall were analysed.
Frozen tissue sections were attached to a microtome sample holder using Tissue-Tek cryoadhesive (Sakura Finetek, Torrance, CA) leaving the urothelial side exposed towards the cutting blade. A 10 μm thick section was cut and stained with haematoxylin and eosin for histological analysis. Tissue totalling 100 μm in thickness was then sectioned and placed in pre-weighed homogenisation vials. This process was repeated across the entire thickness of the tissue sample. At 400 μm intervals, a 10 μm thick section was cut and mounted on a microscope slide, stained with H&E, and evaluated by a pathologist to correlate tissue histology with depth/position in the bladder wall. Transverse sections of the bladder wall (cross sections) were also cut and either stained with H&E or mounted on slides unstained for fluorescence microscopy.
Qualitative analysis of drug distribution in the bladder wall
Qualitative assessments of DOX distribution in the bladder wall were made by visualisation of DOX fluorescence in transverse bladder wall sections using an epifluorescent microscope (Axio Imager M1; Zeiss, Thornwood, NY) equipped with a monochrome CCD camera, motorised scanning stage and stitching software (Zen; Zeiss, Thornwood, NY). Images of DOX fluorescence were captured using a custom filter cube (excitation, 480/40 nm; emission, 600/60 nm) corresponding to doxorubicin’s excitation and emission spectra and 10× objective lens. Cell nuclei were stained using TO-PRO-3 iodide (1 μM) and imaged using a Cy5 filter set. Images were converted to 8-bit, overlayed, and pseudo-coloured (DOX in red; nuclei in blue) using ImageJ software (National Institutes of Health, Bethesda, MD). All images were captured using the same exposure and consistent window/level settings were maintained during image processing.
Quantitative analysis of drug distribution in the bladder wall
Doxorubicin was extracted from tissue samples using a previously reported method with slight modification [Citation20,Citation34]. Briefly, a solution of acetonitrile, water and potassium chloride (3:10:1) was added to each vial containing 100 μm tissue sections and homogenised. Following homogenisation, the samples were dried in a water bath at 50 °C under flowing air. A solution (0.9 mL) of potassium phosphate (KH2PO4) with pH 3.8 was added to vials containing dried homogenised bladder tissue. Daunarubicin (100 μL, 2.5 mg/mL in water) was added as an internal standard. The vials were vortexed and incubated at 37 °C for 15 min. Acetone [(CH3)2CO, 250 μL] and a saturated solution of zinc sulphate (ZnSO4, 100 μL) were added to each vial, vortexed and incubated at 37 °C for 15 min. Vials were then centrifuged and the supernatant transferred to 2 mL screw cap vials, and dried as previously described. The dried residue was dissolved completely in 200 μL of the HPLC mobile phase, thoroughly vortexed, and centrifuged. The concentration of doxorubicin in the supernatant was evaluated by HPLC using a Zobrax Eclipse Plus C18 reversed-phase column (Agilent, Santa Clara, CA) connected to a fluorescence detector. The mobile phase consisted of 0.1% trifluoroacetic acid in acetonitrile and deionised water (34:66) at a flow rate of 1.5 mL/min at 30 °C.
A two-sample t-test was used to determine statistical significance between sample groups. Differences were considered statistically significant if p < 0.05 (two- tailed).
Results
Bladder wall histology
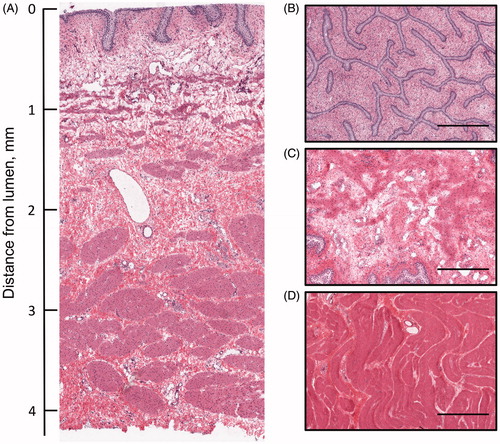
Transverse and parallel plane histologic sections of the bladder wall demonstrated distinct tissue layers comprising the urothelium, lamina propria and muscle layers which measured 0.041 ± 0.014, 0.667 ± 0.084 and 3.467 ± 0.053 mm in thickness, respectfully. The mean bladder wall thickness was 4.175 ± 0.512 mm (). One animal in the IV DOX + HT group had redness on the luminal surface that was presumably in close proximity to the heated inlet jet of the Foley catheter; however this region of tissue was not within the area sampled for analysis. For all animals, no differences were observed in histologic sections of the bladder wall between animals that received hyperthermia of the bladder compared to those that did not.
Figure 2. Orthogonal sections of the bladder wall stained with H&E. (A) Cross section (transverse) of the bladder wall with luminal surface at top. Sections parallel to the bladder lumen taken from the (B) urothelium, (C) lamina propria and (D) muscularis. Scale bar for parallel sections represents 0.5 mm.
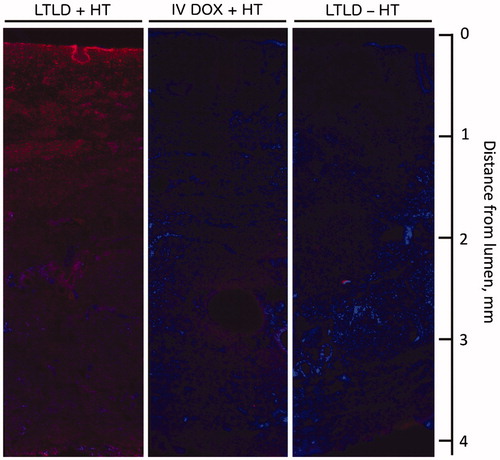
DOX distribution was visualised in bladder wall cross sections by fluorescence microscopy (). High-fluorescence intensity of DOX was observed in the bladder wall for animals in the LTLD + HT group relative to IV DOX + HT and LTLD – HT groups. DOX fluorescence in sections from the LTLD + HT group was most intense in the mucosa with lower intensity in the muscle.
Figure 3. Cross sections of bladder wall showing doxorubicin (red) and cell nuclei (blue) in animals treated with LTLD + HT, IV DOX + HT and LTLD – HT arranged with luminal surface at the top and serosal surface at the bottom. The highest fluorescence intensity of DOX was observed for LTLD + HT compared to IV DOX + HT and LTLD − HT groups in both the mucosa and muscle. (Refer the online version for color figure.)
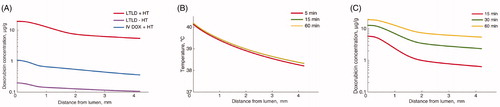
The computer models demonstrated adequate temperature for heat-activated drug release from LTLD liposomes in the bladder wall (). Drug uptake by tissues increased during bladder hyperthermia, suggesting importance of adequate heating duration (). Temperature was below what is needed for maximum rate of drug release from LTLD in parts of the muscle layer, suggesting drug uptake may be enhanced by more efficient hyperthermia (e.g. more rapid water circulation). Similar to in vivo results, there was a concentration gradient present in the computer models. Tissue drug concentrations were in the range of 19.31–5.43 μg/g for LTLD + HT, 1.07–0.36 μg/g for IV DOX + HT and 0.20–0.11 μg/g for LTLD – HT. In all cases, the higher concentration was in the mucosa and the lower concentration in the distal muscle (i.e. outer bladder surface).
Figure 4. Results from computer models simulating tissue heating and drug delivery in the bladder wall. (A) Final doxorubicin tissue concentration profile in the bladder wall for different treatment groups. (B) Temperature profile following 5, 15 and 60 min of bladder hyperthermia. Steady-state temperature is reached after ∼15 min. (C) Doxorubicin tissue concentration profile for LTLD + HT group after 15, 30 and 60 min demonstrating importance of hyperthermia duration.
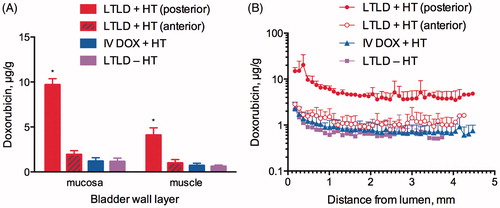
Quantitative assessment of DOX concentration in the bladder wall confirmed that the greatest accumulation of DOX occurred in the LTLD + HT group across all tissue layers compared to IV DOX + HT and LTLD – HT (p < 0.05). For all treatment groups, the highest DOX concentrations were found in the urothelium/lamina propria (mucosa). Comparing the mean DOX concentrations at all depths in the bladder wall, the ranges for LTLD + HT, IV DOX + HT and LTLD – HT, respectively, were 20.32–3.52 μg/g, 2.34–0.61 μg/g and 2.18–0.51 μg/g. The average DOX concentrations in the urothelium/lamina and muscle, respectively, were 9.7 ± 0.67 and 4.09 ± 0.81 μg/g for IV LTLD + HT, 1.2 ± 0.39 and 0.86 ± 0.24 μg/g for IV DOX + HT, and 1.15 ± 0.38 and 0.62 ± 0.15 μg/g for LTLD–HT. Lower DOX concentrations were found in the anterior bladder wall of animals receiving LTLD + HT, 1.93 ± 0.44 μg/g (N = 2) and 1.00 ± 0.23 μg/g (N = 2) in the mucosa and muscle layers, respectively, compared to the posterior bladder wall, with a range in mean DOX concentrations at all depths in the bladder wall of 2.85–0.79 μg/g. There was no significant difference in DOX concentration between the anterior and posterior bladder wall for the other two groups.
The distribution of DOX for all treatment groups was characterised by a drop in concentration across the mucosa and superficial muscle layers before reaching constant concentrations in deep muscle layers ().
Figure 5. (A) DOX concentration in the mucosa and muscle layers from samples of the bladder wall. “*” indicates significant differences compared to unmarked groups within the same bladder wall layer (p < 0.05). (B) DOX concentration measured in parallel sections of the bladder wall as a function of distance from the luminal surface. Error bars represent standard deviation of the mean. DOX concentrations in samples taken from the posterior and anterior aspects of the bladder are only displayed for the LTLD + HT group since regional differences between samples in the other groups were insignificant.
Discussion
This study evaluated the feasibility of targeted drug delivery to the bladder wall consisting of intravenous administration of LTLD in combination with locoregional application of mild hyperthermia. We found high accumulation and spatial distribution of DOX in the bladder wall of swine that received LTLD with hyperthermia compared to IV DOX with hyperthermia or LTLD alone. This drug delivery paradigm is a promising alternative to intravesical therapies as it provides selective targeting of chemotherapy to the bladder wall while avoiding the drug penetration barrier imposed by the urothelium.
Targeted drug delivery to the bladder using thermosensitive liposomes relies on two key components: heating of the bladder wall to temperatures sufficient to achieve drug release from the liposomes, and drug transport to and within the bladder wall via systemic circulation. A variety of methods exist for heating the bladder including use of external regional electromagnetic (EM) systems, intravesical microwave applicators, and intravesical thermal conduction systems (i.e. circulation of warm fluids) [Citation35]. In this work, swine bladders were irrigated with warm water to achieve hyperthermia of the bladder wall. The degree of bladder wall heating depends primarily on the net effect of heat conduction from the warm water inside the bladder (and to a lesser degree on convection of the circulating water) and convective heat loss due to blood perfusion [Citation36,Citation37].
Computational modelling of tissue heating and drug delivery was consistent with DOX concentrations measured in the bladder wall that decreased from mucosa to muscle for all treatment groups. For targeted drug delivery, the thermal gradient across the bladder wall should ideally result in heating of the tissue layers to temperatures >39 °C in order to maximise drug release from the thermosensitive liposomes [Citation38]. Using a model of heat transport in perfused tissues based on a conservative estimate for convective heat transport at the intraluminal surface, we calculated that an intravesical temperature of 45 °C, previously reported for bladder chemohyperthermia [Citation18,Citation39,Citation40], resulted in bladder wall temperatures >39 °C up to a distance of ∼2 mm from the bladder lumen (). The modelled thermal gradient across the bladder wall revealed an estimated temperature drop of ∼2 °C between the luminal surface and deepest muscle layers and a drop of ∼7 °C relative to the temperature of the circulating water. Deposition of DOX from LTLD based on the computational model in regions of the bladder wall heated below 39 °C is due to drug release that occurs below this temperature at a reduced rate [Citation38]. Greater heating of deep muscle may be achieved by increasing the flow rate of warm water in the bladder in order to reduce dwell time and increase convective heat transfer.
Intravesical drug delivery for treatment of bladder tumours is inherently limited by dilution of drug in the bladder due to perpetual urine production during treatment, poor drug penetration of the bladder wall, and the effect of urine pH on the stability of some drugs [Citation8]. In contrast, systemic administration of drug encapsulated in thermosensitive liposomes circumvents these limitations since the drug is distributed via blood vessels and released from the carrier within the bladder wall. This approach allows for targeted drug delivery to tumours localised in the bladder wall while reducing systemic drug exposure and toxicity to other organs. In this study, a 30-min drug infusion was initiated during bladder heating and heating was maintained for another 30 min after completion of the infusion in order to increase the overlap between tissue hyperthermia and elevated plasma drug levels [i.e. the area under the pharmacokinetic curve (AUC)] based on the plasma pharmacokinetics reported for LTLD delivered via IV infusion [Citation41,Citation42]. Swine received a premedication regimen consisting of a corticosteroid, antihistamines and a non-steroidal anti-inflammatory prior to the administration of treatments in order to mitigate known hypersensitivity reactions to liposomal drug formulations that can result in anaphylaxis and adverse cardiopulmonary events [Citation43,Citation44]. One swine experienced significant flushing of the skin that subsided upon completion of the liposomal doxorubicin infusion. No significant adverse events were observed.
The exposure of tumour cells to therapeutic drug concentrations depends on the extent of drug accumulation and distribution in the bladder wall. We found that LTLD delivered in combination with bladder hyperthermia resulted in approximately 8-fold and 5-fold greater mean DOX accumulation in the mucosa and muscle layers, respectively, than IV DOX delivered with bladder hyperthermia and about 8-fold and 7-fold greater mean DOX accumulation in the mucosa and muscle layers, respectively, than LTLD delivered without bladder hyperthermia (). This finding supports the understanding that drug delivery using LTLD is mediated by the temperature-responsive nature of the liposomes and adequate heating of the target tissues. DOX concentrations throughout the bladder wall were greater for LTLD + HT compared to those reported following intravesical administration of DOX in humans [Citation6].
Penetration of intravesical drugs is characterised by a steep drop in concentration across the bladder wall due to limited tissue permeability that leads to low drug concentrations in deep tissue layers where high drug concentrations are needed to combat tumours with limited chemosensitivity [Citation5,Citation6,Citation11,Citation33]. Wientjes et al. [Citation6] reported an exponential decline in DOX concentration in the perfused tissues below the urothelium following intravesical administration of DOX in patients and a high degree of inter-patient variability. The concentration of DOX in the deep muscle layer was just 4% of the concentration at the interface between the urothelium and lamina propria. We found that drug concentration in the bladder wall for all treatment groups declined with increasing distance from the lumen until reaching stable concentrations in the muscle layers. The concentration of DOX was highest for the LTLD + HT group in all tissue layers including the deep muscle compared to controls. Moreover, <3-fold drop in mean concentration was observed between the mucosa and muscle layers in all treatment groups.
The higher proportion of drug located near or within the urothelium/lamina is likely due to higher blood vessel density in these layers relative to muscle layers, since this trend was observed in all treatment groups. Swine bladder walls were comprised primarily of urothelium, lamina propria and muscularis and were similar in proportion and composition to human bladders [Citation45,Citation46]. Greater blood vessel density in proximity to the lumen was observed by qualitative assessment of histologic bladder wall specimens () and is consistent with the high density of subepithelial capillaries observed in swine and human bladders [Citation24,Citation25,Citation47]. The mean concentrations of DOX for the LTLD + HT group in the mucosal and muscle layers, respectively, were ∼9- and 4-fold greater than the IC50 of doxorubicin reported by Gan et al. [Citation9] in histocultures of Grade II bladder tumours. Therefore, this treatment strategy may have the potential to deliver therapeutic quantities of drug to regions of the bladder wall that are typically underdosed during intravesical drug delivery.
This was a feasibility study with several limitations. First, the computational modelling of tissue heating and drug delivery in the bladder wall required several assumptions and approximations. Convective heating at the inner wall surface is based on a conservative estimate, as exact calculation was not possible. Further, convective heating likely varies depending on location within the bladder, depending on local water flow conditions (flow velocity, laminar vs. turbulent flow, etc.). Also, the cell uptake model is based on in vitro studies of a lung cancer cell line. The cell uptake kinetics for bladder cancer or normal bladder parenchyma may differ from our assumptions. Similarly, our assumption of changes in vascular density across the bladder wall is an approximation, as no exact data were available. Finally, differences in the rate of tissue perfusion may affect temperature and drug concentrations across the bladder wall.
Despite efforts to maintain constant bladder distention during treatment and between subjects, variability in bladder volume could not be entirely eliminated and the influence of bladder distension on drug penetration is not known. We tested samples from two anatomic locations in the bladder and found that samples from the anterior midline had lower drug concentrations than samples taken from the posterior aspect of the bladder for swine treated with LTLD + HT. Since the swine were in the supine position during the procedure, we speculate that this may be a result of air collection anteriorly inside the bladder, or perhaps less likely, anatomic variations in blood flow, that could have resulted in heterogeneous heating of the anterior bladder wall. Finally, this study was performed in a limited number of animals. These animals were non-tumour bearing and it is possible that drug delivery to tumours may be affected by differences in vascular perfusion, efficiency of angiogenic vasculature, and heating.
DOX delivery to the bladder could be further optimised by adjusting several treatment parameters. Increasing the heating time or increasing the dose of DOX may provide a larger window during which DOX can be released from LTLD by maximising the overlap of hyperthermia and DOX plasma AUC. In addition, increasing the flow rate of water and using a closed loop recirculation system may lessen entrapment of air inside the bladder and provide greater temperature uniformity. Other standardised heating modalities may be explored in order to evaluate differences in thermal gradients across the bladder wall and potential effects on targeted drug delivery using temperature-sensitive liposomes. Several such systems are commercially available including the Combat BRS and Synergo® systems that make use of warm fluid for conductive heating and radiofrequency radiation, respectively. Overall, the efficacy of intravenous LTLD in combination with locally applied hyperthermia may be promising as a stand-alone therapy or in combination with intravesical therapies.
Conclusions
Targeted delivery of doxorubicin to the bladder can be achieved by combining loco-regional application of mild hyperthermia using warm water irrigation and systemic administration of lyso-thermosensitive liposomal doxorubicin (LTLD, ThermoDox®). This drug delivery paradigm applies heat at the target to selectively deliver chemotherapy to the bladder wall where it is needed using low temperature-sensitive liposomes. This drug + device combination appears promising for delivery of chemotherapy to the bladder wall and may provide a viable alternative for patients who have failed standard first line therapy for bladder cancer.
Acknowledgements
The authors would like to thank Combat Medical and Medical Enterprises Group for discussions and technical advice. Thanks also to Nick Borys, MD for guidance on ThermoDox administration. Many respectful thanks to Mark Dewhirst, DVM, PhD for so many thoughtful and engaging discussions over 15 years on the deployment of liposomal doxorubicin locally via thermal interventions for novel cancer indications.
Disclosure statement
This work was supported by the intramural research programme of the National Institutes of Health (NIH), and the Center for Interventional Oncology NIH grant CL040015-08 (B.W.); NIH grant R01CA181664 (D.H.); and NIH C06 RR018823 from the Extramural Research Facilities Program of the National Center for Research Resources (MUSC). NIH and Celsion Corp. have a Cooperative Research and Development Agreement (CRADA).
Additional information
Funding
References
- American Cancer Society. (2016). Cancer facts & figures 2016. Atlanta: American Cancer Society.
- Clark PE, Agarwal N, Biagioli MC, et al. (2013). Bladder cancer. Bladder Cancer 11:446–75.
- Sylvester RJ, Van Der Meijden APM, Oosterlinck W, et al. (2006). Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49:466–75.
- Wientjes MG, Dalton JT, Badalament RA, et al. (1991). Bladder wall penetration of intravesical mitomycin C in dogs. Cancer Res 51:4347–54.
- Wientjes MG, Badalament RA, Wang RC, et al. (1993). Penetration of mitomycin C in human bladder. Cancer Res 53:3314–20.
- Wientjes MG, Badalament RA, Au JL. (1996). Penetration of intravesical doxorubicin in human bladders. Cancer Chemother Pharmacol Pharm 37:539–46.
- Knemeyer I, Wientjes MG, Au JL-S. (1999). Cremophor reduces paclitaxel penetration into bladder wall during intravesical treatment. Cancer Chemoth Pharm 44:241–8.
- Au JL-S, Badalament RA, Wientjes MG, et al. (2001). Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. J Natl Cancer Inst 93:597–604.
- Gan Y, Wientjes MG, Badalament RA. (1996). Pharmacodynamics of doxorubicin in human bladder tumors. Clin Cancer Res 2:1275–83.
- Dalton JT, Wientjes MG, Badalament RA, et al. (1991). Pharmacokinetics of intravesical mitomycin C in superficial bladder cancer patients. Cancer Res 51:5144–52.
- Hu L, Wientjes MG, Li J, Au JL-S. (2010). Bladder tissue pharmacokinetics of intravesical mitomycin C and suramin in dogs. AAPS J 12:586–91.
- Tammela T, Wein AJ, Monson FC, Levin RM. (1993). Urothelial permeability of the isolated whole bladder. Neurourol Urodyn 12:39–47.
- Van Der Heijden AG, Verhaegh G, Jansen CFJ, et al. (2005). Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: an in vitro study. J Urol 173:1375–80.
- Urano M, Kuroda M, Nishimura Y. (1999). For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperther 15:79–107.
- Owusu RA, Abern MR, Inman BA. (2013). Hyperthermia as adjunct to intravesical chemotherapy for bladder cancer. Hyperthermia as adjunct to intravesical chemotherapy for bladder cancer. Biomed Res Int 2013:262313.
- Paroni R, Salonia A, Lev A, et al. (2001). Effect of local hyperthermia of the bladder on mitomycin C pharmacokinetics during intravesical chemotherapy for the treatment of superficial transitional cell carcinoma. Brit J Clin Pharmacol 52:273–8.
- Milla P, Fiorito C, Soria F, et al. (2014). Intravesical thermo-chemotherapy based on conductive heat: a first pharmacokinetic study with Mitomycin C in superficial transitional cell carcinoma patients. Cancer Chemoth Pharm 73:503–9.
- Lammers RJM, Witjes JA, Inman BA, et al. (2011). The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. Eur Urol 60:81–93.
- Arends TJH, Nativ O, Maffezzini M, et al. (2016). Results of a randomised controlled trial comparing intravesical chemohyperthermia with Mitomycin C versus bacillus Calmette-Guérin for adjuvant treatment of patients with intermediate- and high-risk non-muscle-invasive bladder cancer. Eur Urol 69:1046–52.
- Ranjan A, Jacobs GC, Woods DL, et al. (2012). Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Control Release 158:487–94.
- Celsion. (2016). Study of ThermoDox with standardized radiofrequency ablation (RFA) for treatment of hepatocellular carcinoma (HCC) (OPTIMA): NLM identifier NCT02112656; [Dec 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02112656.
- Gasselhuber A, Dreher MR, Negussie A, et al. (2010). Mathematical spatio-temporal model of drug delivery from low temperature sensitive liposomes during radiofrequency tumour ablation. Int J Hyperther 26:499–513.
- Gasselhuber A, Dreher MR, Partanen A, et al. (2012). Targeted drug delivery by high intensity focused ultrasound mediated hyperthermia combined with temperature-sensitive liposomes: computational modelling and preliminary in vivo validation. Int J Hyperther 28:337–48.
- Prozorowska E, Jackowiak H. (2014). The vascular corrosion casting (VCC) and scanning electron microscopy study on changes of vascular networks arrangement in the organs undergoing cyclic volume changes. In: Méndez-Vilas A, ed. Microscopy: advances in scientific research education. 6th ed. Badajoz, Spain: Formatex Research Center, 112–18.
- Nielsen KK. (1995). Blood flow rate and total blood flow related to length density and total length of blood vessels in mini-pig urinary bladder after chronic outflow obstruction and after recovery from obstruction. Neurourol Urodyn 14:177–86.
- Qin H-y, Sun H, Wang X, et al. (2013). Correlation between CT perfusion parameters and microvessel density and vascular endothelial growth factor in adrenal tumors. PLoS One 8:e79911.
- Incropera FP, DeWitt DP. (2006). Introduction to convection. Fundamentals of heat and mass transfer. 6th ed. New York: John Wiley & Sons, 347–400.
- Mcintosh RL, Anderson V. (2010). A comprehensive tissue properties database provided for the thermal assessment of a human at rest. Biophys Rev Lett 5:129–51.
- Duck FA. (1990). Thermal properties of tissue. Physical properties of tissue: a comprehensive reference book. London: Academic Press, 167–223.
- Nielsen KK, Nielsen SL, Nordling J, Kromann-Andersen B. (1991). Rate of urinary bladder blood flow evaluated by 133Xe washout and radioactive microspheres in pigs. Urol Res 19:387–91.
- Gasselhuber A, Dreher MR, Rattay F, et al. (2012). Comparison of conventional chemotherapy, stealth liposomes and temperature-sensitive liposomes in a mathematical model. PLoS One 7:e47453.
- Manzoor A, Lindner LH, Landon CD, et al. (2012). Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res 72:5566–75.
- Wientjes MG, Dalton JT, Badalament RA, et al. (1991). A method to study drug concentration-depth profiles in tissues: mitomycin C in dog bladder wall. Pharm Res 8:168–73.
- Al-Abd AM, Kim NH, Song SC, et al. (2009). A simple HPLC method for doxorubicin in plasma and tissues of nude mice. Arch Pharm Res 32:605–11.
- Stauffer PR, van Rhoon GC. (2016). Overview of bladder heating technology: matching capabilities with clinical requirements. Int J Hyperther 32:407–16.
- Yuan Y, Cheng KS, Craciunescu OI, et al. (2012). Utility of treatment planning for thermochemotherapy treatment of nonmuscle invasive bladder carcinoma. Med Phys 39:1170–81.
- Schooneveldt G, Bakker A, Balidemaj E, et al. (2016). Thermal dosimetry for bladder hyperthermia treatment. An overview. Int J Hyperther 32:417–33.
- Needham D, Dewhirst MW. (2001). The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev 53:285–305.
- Colombo R, Da Pozzo LF, Lev A, et al. (1996). Neoadjuvant combined microwave induced local hyperthermia and topical chemotherapy versus chemotherapy alone for superficial bladder cancer. J Urol 155:1227–32.
- Colombo R, Da Pozzo LF, Lev A, et al. (1998). Local microwave hyperthermia and intravesical chemotherapy as bladder sparing treatment for select multifocal and unresectable superficial bladder tumors. J Urol 159:783–7.
- Wood BJ, Poon RT-P, Locklin JK, et al. (2012). Phase I study of heat-deployed liposomal doxorubicin during radiofrequency ablation for hepatic malignancies. J Vasc Interv Radiol 23:248–55.
- Swenson CE, Haemmerich D, Maul DH, et al. (2015). Increased duration of heating boosts local drug deposition during radiofrequency ablation in combination with thermally sensitive liposomes (ThermoDox) in a porcine model. PLoS One 10:e0139752.
- Sercombe L, Veerati T, Moheimani F, et al. (2015). Advances and challenges of liposome assisted drug delivery. Front Pharmacol 6:286.
- Szebeni J, Bedocs P, Csukas D, et al. (2012). A porcine model of complement-mediated infusion reactions to drug carrier nanosystems and other medicines. Adv Drug Deliv Rev 64:1706–16.
- Hakenberg OW, Linne C, Manseck A, Wirth MP. (2000). Bladder wall thickness in normal adults and men with mild lower urinary tract symptoms and benign prostatic enlargement. Neurourol Urodynam 19:585–93.
- Manieri C, Carter SS, Romano G, et al. (1998). The diagnosis of bladder outlet obstruction in men by ultrasound measurement of bladder wall thickness. J Urol 159:761–5.
- Miodonski AJ, Litwin JA. (1999). Microvascular architecture of the human urinary bladder wall: a corrosion casting study. Anat Rec 254:375–81.