Abstract
Background: High intensity focused ultrasound (HIFU) is a promising ablation technique for benign thyroid nodules. However, its effect on underlying thyroid function remains unknown. We aimed to evaluate the 6 months changes in serum thyroid stimulating hormone (TSH) and free thyroxine (FT4) after HIFU treatment.
Methods: Eighty-three consecutive patients who underwent single HIFU ablation for symptomatic benign thyroid nodule were analysed. Eligible patients had serum TSH and FT4 checked before treatment (baseline), 1 week, 3 and 6 months following HIFU treatment. Primary endpoints were hypothyroidism (FT4 < 12 pmol/L) and hyperthyroidism (FT4 > 23 pmol/L) in the 6 months following treatment. To express extent of nodule ablation relative to the total gland volume, an ablation volume ratio was calculated by [(Ablated nodule volume/total thyroid volume)/(total thyroid volume)] × 100.
Results: Relative to baseline, 1-week serum TSH significantly dropped (from 1.16 to 0.76 mIU/L, p < 0.001) while 1-week serum FT4 significantly rose (from 16.0 to 17.8 pmol/L, p < 0.001). However, 3- and 6-months TSH and FT4 did not changed significantly from baseline (p > 0.05). No patients developed hyperthyroidism while one (1.4%) developed hypothyroidism (FT4 = 11 pmol/L) at 3 months and 6 months. Interestingly, this patient had a previous lobectomy and an ablation volume ratio of 64.00%.
Conclusions: Hypothyroidism following single HIFU ablation occurred rarely (1.4%) and resulted in little clinical relevance. Given that only one patient developed hypothyroidism following single HIFU ablation, it remains unclear how patients with different amount of parenchyma and relative extent of ablation may affect subsequent thyroid function.
Introduction
Thyroid nodules are common and although most are benign and remain relatively unchanged, some do become large and cause local pressure symptoms [Citation1–3]. Under such circumstance, surgical resection is usually recommended [Citation1,Citation2]. However, surgery is associated with complication risks, permanent loss of function, high cost and need for general anaesthesia. As a result, there has been a growing interest in developing less invasive, non-surgical treatment alternative for benign thyroid nodules [Citation4–6]. High intensity focused ultrasound (HIFU) is one of these non-surgical alternatives that utilises focused ultrasound energy to induce thermal ablation within the nodule. It has been shown to be not only effective in inducing nodule necrosis and shrinkage but also relieving symptoms in patients with nodule-related complaints [Citation7–9].
However, thermal ablation of benign thyroid nodules is not without side-effects and thyroid dysfunction is one potential treatment side-effect [Citation10–12]. To our knowledge, only two studies specifically assessed the thyroid function following HIFU ablation of benign thyroid nodules [Citation7,Citation13]. Although they both reported no significant changes in thyroid function at 24 h and 3 months afterwards [Citation7,Citation13], only a small number of patients was evaluated. Furthermore, these studies did not account for patients’ residual thyroid parenchyma and extent of ablation which may play a role in post-treatment thyroid dysfunction. For example, it is known that patients with part of their thyroid gland surgically resected are more prone of developing hypothyroidism over time than those with an intact whole gland [Citation14–16]. Also despite being a focused ablation treatment, the energy in the HIFU beam may damage the surrounding parenchyma on its way to the target area. When given at a higher intensity, off-focus high energy destruction as well as non-linear propagation and cavitation effects have been shown to occur [Citation17]. We hypothesised that the amount of underlying thyroid parenchyma and ablation size may influence the risk of thyroid dysfunction following single HIFU ablation. Given these issues, our study aimed to evaluate changes in thyroid function in the first 6 months following HIFU ablation with a particular focus on patients with different amount of thyroid parenchyma and extent of ablation.
Methods
This retrospective analysis was approved by local institutional review board. Eighty-three consecutive patients who underwent a HIFU ablation for a symptomatic benign thyroid nodule from 2015 to 2016 were analysed. The inclusions were: (1) the nodule was benign (i.e. Bethesda class II on cytology) [Citation18]; (2) the nodule was causing local symptoms or cosmetic concern; (3) the nodule was predominantly solid (≥70% solidity on USG); (4) the nodule was within the treatable depth for HIFU ablation (i.e. 5–30 mm from the skin); (5) the patient had a normal serum free T4 (FT4) (12–23 pmol/L) at baseline; (6) the ablation to the nodule was considered successful and complete. Complete ablation meant having the entire middle layer of the nodule ablated by HIFU within a single session; (7) at least 6 months follow-up after ablation. The exclusions were (1) patients aged ≤18 years old or >70 years old; (2) patients with elevated anti-thyroid (i.e. anti-thyroglobulin or anti-microsomal) autoantibodies or a thyroid condition likely to alter underlying function like Graves’ disease or thyroiditis; (3) patients taking anti-thyroid medications or thyroxine supplement before treatment or (4) patients who had received two or more HIFU ablations either initially or previously. All patients had their thyroid function (serum TSH and FT4) checked on the day of treatment (baseline), 1 week, 3 and 6 months following HIFU treatment. The primary study outcome was the incidence of thyroid dysfunction (i.e. hypothyroidism and hyperthyroidism) in the first 6 months following a single HIFU treatment. Hypothyroidism was defined as a biochemical state of low serum FT4 (<12 pmol/L) while hyperthyroidism was defined as a biochemical state of high serum FT4 (>23 pmol/L).
Pre-treatment evaluation
In addition to thyroid function tests, a detailed history on previous thyroid diseases and surgery was conducted. The volume of the right and left lobe was estimated by measuring the three orthogonal dimensions of each lobe. Dimensions were carried out by USG using the LOGIQ e (GE Healthcare) scanner equipped with a 10–14 MHz linear matrix transducer by an independent experienced sonographer. The three orthogonal measurements were the cranio-caudal dimension (length) of the lobe and the other two perpendicular diameters (i.e. the medio-lateral (width) and antero-posterior (thickness) dimensions). All measurements were made to the nearest 0.1 mm. To estimate volume of the lobe, the formula for an ellipsoid was used: volume (mL) = (width (in cm) × thickness (in cm) × length (in cm)) × (π/6) where π was taken as 3.1416. To estimate volume of the ablated nodule within the lobe, the same formula was used.
Ablation volume ratio
To express the relative extent of nodule ablation to the entire gland volume, we used ablation volume ratio which was defined as: [(Ablated nodule volume/total thyroid volume)/(total thyroid volume)] × 100. For simplicity, total thyroid volume was the sum of the right and left lobes only. The volume of the isthmus which is usually proportionally small was not included in our calculation. For those with one lobe already surgically removed (i.e. history of lobectomy), only the volume of the remaining lobe was used.
HIFU treatment
All treatments were performed by one person (B.H.L.) with >2 years of experience using the USG-guided HIFU device (EchoPulse; Theraclion, Paris, France). This device comprised an energy generator, a treatment head, a skin cooling device and a touch-screen interface for planning. The treatment head incorporated an image transducer (7.5 MHz, 128 elements, linear array) and HIFU transducer (3 MHz, single element, 60 mm in diameter). After positioning, patients were sedated with diazepam (10–15 mg) and pethidine (50–100 mg). Under USG guidance, the treatment head was adjusted until the entire index nodule was within the treatable depth from 5 to 30 mm from the skin. The device computer (Beamotion version TUS 3.2.2, Theraclion, Paris, France) automatically divided the nodule into multiple ablation subunits. Each subunit measured approximately 7.3 mm in thickness and 5 mm in width. Each subunit received a continuous 8-s pulse of HIFU energy followed by 40 s of cooling time before the beam moved to the adjacent subunit. This cycle continued until all subunits were ablated. To ensure safety, the device automatically selected the following safety margins: (1) 0.5 cm from the skin, (2) at least 0.3 cm from the trachea and (3) 0.2 cm from the ipsilateral carotid artery. For nodules located in the isthmic region (i.e. those close to or near the trachea), in order to avoid thermal injury, treatment head was tilted slightly at an angle such that the treatment beam did not point directly to the trachea. A laser-based movement detector enabled immediate power interruption when the patient moved or swallowed during ablation. To avoid skin burn, the skin was cooled by a balloon (filled with 10 °C liquids) at the tip of the treatment head. All ablations started at 204 J/pulse and increased up to 280 J/pulse until hyperechoic marks appeared at the focal point. The total energy delivered to each nodule (in KJ) and the time taken (in minutes) for that delivery was automatically recorded by the device’s computer. Oral diet was resumed immediately afterwards and patients were discharged from hospital 2 h after treatment.
Biochemistry/laboratory
Serum TSH and T4 levels measurements were performed by the same analyser (ADVIA® Centaur system, Siemens Medical Solutions Diagnostics) in the same laboratory at our institution. The normal reference values for TSH were 0.35–4.78 mIU/L and for FT4 were from 12 to 23 pmol/L.
Statistical analysis
Continuous variables were expressed as median (range) and groups were compared using the Mann–Whitney U test. Changes in TSH and FT4 over time from baseline were evaluated by Wilcoxon signed-rank test. All statistical analyses were conducted using SPSS version 18.0 (SPSS, Inc., Chicago, IL). p values ≤0.05 were considered statistically significant.
Results
Of the 83 patients who underwent a complete ablation of their benign thyroid nodule, six (7.2%) had a suppressed TSH at baseline, three (3.6%) patients had two or more nodules ablated and two (2.4%) patients were found to have markedly raised anti-thyroid auto-antibodies suggestive of lymphocytic thyroiditis. After excluding these patients, 72 (%) patients were analysed. shows their baseline characteristics. Most patients (83.3%) suffered from local pressure symptoms like intermittent choking on swallowing and a subjective feeling of pressure when lying flat. Only 12 (16.7%) patients had pure cosmetic concerns. Among these patients, 55 (76.4%) patients never underwent previous thyroid resection (i.e. had an intact thyroid gland) (group I) while 17 (23.6%) had one lobe surgically removed (i.e. previous lobectomy) (group II). The median (range) interval from previous lobectomy to HIFU ablation treatment was 7.48 (2.50–13.10) years. Most of these lobectomies were on the right side (13/17, 76.5%). No patients required thyroxine replacement before treatment. Most patients had nodule shrinkage and symptom improvement in the first 6 months ().
Table 1. Baseline patient characteristics.
Table 2. Treatment parameters and efficacy over 6 months.
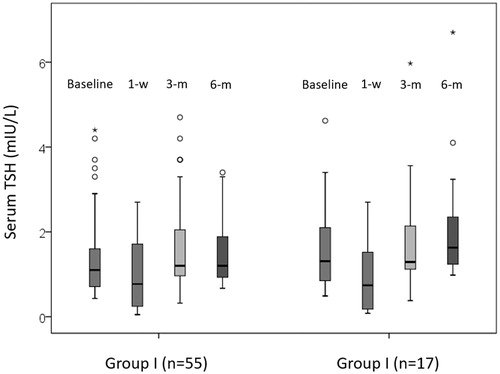
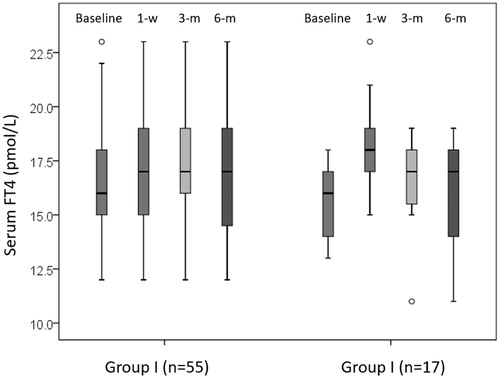
Relative to baseline, the overall serum TSH significantly dropped at 1 week (from 1.16 to 0.76 mIU/L, p < 0.001) but remained similar at 3 months (p = 0.125) and 6 months (p = 0.249). Consistent with this, serum FT4 significantly rose at 1 week (from 16.0 to 17.8 pmol/L, p < 0.001) but not at 3 months (p = 0.245) and 6 months (p = 0.089). There was one (1.4%) patient who developed hypothyroidism (with a FT4 of 11 pmol/L) at 3 and 6 months. Interestingly, that patient belonged to group II but did not suffer any clinical manifestations of hypothyroidism (Patient No. 1, respectively, in ). At 1 week, 27 (37.5%) had a suppressed TSH.
Table 3. Patients who had one lobe surgically removed before high intensity focused ultrasound (HIFU) ablation.
Comparison of serum TSH and FT4 between groups I and II
Serum TSH level was comparable between the two groups at baseline, 1 week and 3 months. However, serum TSH appeared to be significantly higher in groups II than I at 6 months (1.63 vs. 1.20 mIU/L, p = 0.008). For serum FT4, there were no significant differences at baseline, 1 week, 3 and 6 months between the two groups (p > 0.05) .
Table 4. First 6 months changes in thyroid function following one-sided HIFU ablation between patients with ablated nodule volume <30% of total glandular volume and with ablated nodule volume ≥30% of total glandular volume.
First 6 months changes in serum TSH and FT4 in group I
Relative to baseline, serum TSH level significantly dropped at 1 week (1.10–0.77 mIU/L, p = 0.006). However, there were no significant differences at 3 months (p = 0.184) and 6 months (p = 0.827) from baseline (see ). At 1 week, 20 (36.4%) patients had a suppressed TSH but by the 3 and 6 months, this number dropped to 3 (5.5%) and 0 (0.0%), respectively. Relative to baseline, serum FT4 significantly rose at 1 week (from 16.0 to 17.0 pmol/L, p = 0.025) but not at 3 months (p = 0.121) or at 6 months (p = 0.091) (see ). However, there were no patients who were biochemically hyper- or hypo-thyroid in the first 6 months in group I.
First 6 months changes in serum TSH and FT4 in group II
In group II, relative to baseline, serum TSH also significantly lowered at 1 week (1.31–0.74 mIU/L, p = 0.003) but interestingly, at 6 months, serum TSH became significantly higher than baseline (1.31–1.63 mIU/L, p = 0.016) (see ). In terms of patients with a suppressed TSH, the proportion at baseline, 1 week, 3 months and 6 months were 0.0, 41.2, 0.0 and 0.0%, respectively.
Relative to baseline, serum FT4 significantly rose at 1 week (from 16.0 to 18.3 pmol/L, p < 0.001) but remained similar at 3 months (p = 0.055) and 6 months (p = 0.409) (see ). In terms of patients having a FT4 outside the normal biochemical reference in the first 6 months, there was one (5.9%) patient (Patient No. 1) who had hypothyroidism (with a FT4 of 11 pmol/L) at 3 and 6 months (see ).
Relationship between first 6 months thyroid function and ablation volume ratio
The overall median ablation volume ratio was 29.9 (5.00–64.0)%. Interestingly, using the median (30%) as the cut-off, no patients in the <30% ablation volume ratio developed hyper or hypothyroidism in the first 6 months while 1/33 (3.0%) patients in the >30% ratio group developed hypothyroidism. This patient had one of the highest ratios among the cohort (64.00%, respectively) (see ). Because total gland volume was significantly less in group II, the median ablation volume ratio in group II was significantly higher than group I (48.0 vs. 29.9%, p < 0.001).
compares the first 6 months changes in TSH and FT4 between those with <30% ablation volume ratio (n = 39) and with ≥30% ablation volume ratio (n = 33). In the group with <30% ratio, relative to baseline, serum TSH remained similar at 1 week (p = 0.153), 3 months (p = 0.198) and 6 months (p = 0.294). Similarly, serum FT4 did not change significantly at 1 week (p = 0.182), 3 months (p = 0.122) and 6 months (p = 0.235). In the group with ≥30% ratio, relative to baseline, serum TSH significantly dropped at 1 week (from 1.39 to 1.28 mIU/L, p = 0.018) but remained similar at 3 months (p = 0.784) and at 6 months (p = 0.557). Serum FT4 rose significantly at 1 week (from 16.0 to 18.5 pmol/L, p < 0.001) but not at 3 months (p = 0.175) and 6 months (p = 0.144).
Discussion
HIFU ablation has been shown to be a safe and less-invasive treatment for symptomatic benign thyroid nodules and our reported 6 months efficacy appeared to be consistent with that of the literature [Citation19]. However, despite being a focused treatment with thermal damage confining mostly to the target itself, it is known that the energy in the beam could damage the normal parenchyma on its way to the target area. Also when given at higher intensity, off-focus high energy destruction as well as non-linear propagation and cavitation effects may occur [Citation17]. As a result, it is theoretically possible that HIFU ablation of a benign thyroid nodule may induce transient thyroid dysfunction due to energy dissipated to the surrounding parenchyma. We hypothesised that the amount of underlying thyroid parenchyma and ablation size may influence the risk of thyroid dysfunction following single HIFU ablation. To our knowledge, this is the first study to address this issue and to formally assess the first 6 months changes in thyroid function after single HIFU ablation.
In our study, there were several interesting findings. First, similar to other forms of thermal ablation [Citation10–12], our data found that thyroid dysfunction following HIFU ablation occurred infrequently (1.4%). In our cohort, there was only one (1.4%) patient (Patient No. 1 in ) who fulfilled the criteria of hypothyroidism at 3 and 6 months following HIFU ablation and that patient did not suffer any clinical manifestations of hypothyroidism. However, since this patient already had one lobe surgically removed previously, it is possible that the overall thyroid function was impaired even before HIFU ablation. This was supported by the fact that this patient’s TSH level before ablation was already in the upper range of the normal reference (see ). Another finding worth highlighting was the fact that none (0/55) of the patients with an intact thyroid gland (i.e. group I) after a single HIFU ablation developed hypothyroidism in the first 6 months. This is consistent with the finding from previous studies [Citation7,Citation13]. This information is clinically relevant because knowing the fact that requiring thyroxine supplementation rarely occurs helps certain patients to decide on ablation over surgical resection in the first place.
Although some studies have reported several cases of hypothyroidism following other forms of thermal ablation [Citation11,Citation20], it remains unknown if they were a direct result of thermal ablation effect itself or an ongoing thyroid process (such as thyroiditis) [Citation10,Citation11,Citation20]. Given that none of our patients had elevated anti-thyroid autoantibodies to begin with, we believe the ablation itself may have had some effect on underlying thyroid function leading to hypothyroidism. However, it should be reiterated that this hypothyroid patient (Patient No. 1 in ) did have one thyroid lobe surgically removed.
Given that hypothyroidism happens rarely with only one patient fulfilling the criteria of hypothyroidism, it is difficult to conclude the possible association between thyroid reserve, extent of ablation and hypothyroidism. Also because there were patients with a smaller total thyroid volume or with a similar ablation volume ratio who did not develop hypothyroidism 6 months after ablation (), we believe our findings were not able to support our initial hypothesis.
Nevertheless, we believe our findings were somewhat in agreement with our initial hypothesis that the patients’ thyroid reserve and relative extent of ablation might play a role in determining post-ablation thyroid dysfunction.
Nevertheless, given that this was a relatively small study evaluating thyroid function after HIFU ablation, we advocate regular monitoring of thyroid function in the first few years following treatment.
Despite our findings, there are several shortcomings which should be acknowledged. First, since this was a study conducted in a single institution, referral and selection biases could have influenced some of the results. Second, since there were too few cases of thyroid dysfunctions, a meaningful factor analysis was not possible. Third, a longer-term follow-up period would have been necessary to establish the relationship between extent of ablation and subsequent thyroid function. Lastly, since our study did not evaluate thyroid autoimmunity following treatment, autoimmunity leading to hypothyroidism was still a possibility.
Conclusion
Hypothyroidism following single HIFU ablation occurred very uncommonly (1.4%) and did not seem to carry any clinical significance. Given that only one patient who developed hypothyroidism following single HIFU ablation had a history of lobectomy and a large ablation relative to the total gland volume, it remains unclear how patients with different amount of parenchyma and relative extent of ablation might affect subsequent thyroid function.
Disclosure statement
All authors had nothing to disclose. No competing financial interests exist.
References
- Gharib H, Papini E, Garber JR, et al. (2016). AACE/ACE/AME task force on thyroid nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the diagnosis and management of thyroid nodules – 2016 Update. Endocr Pract 22:622–39.
- Haugen BR, Alexander EK, Bible KC, et al. (2016). 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133.
- Durante C, Costante G, Lucisano G, et al. (2015). The natural history of benign thyroid nodules. JAMA 313:926–35.
- Gharib H, Hegedüs L, Pacella CM, et al. (2013). Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab 98:3949–57.
- Sung JY, Baek JH, Kim KS, et al. (2013). Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology 269:293–300.
- Wong KP, Lang BH. (2013). Use of radiofrequency ablation in benign thyroid nodules: a literature review and updates. Int J Endocrinol 2013:428363.
- Korkusuz H, Sennert M, Fehre N, et al. (2014). Local thyroid tissue ablation by high-intensity focused ultrasound: effects on thyroid function and first human feasibility study with hot and cold thyroid nodules. Int J Hyperthermia 30:480–5.
- Kovatcheva RD, Vlahov JD, Stoinov JI, Zaletel K. (2015). Benign solid thyroid nodules: US-guided high-intensity focused ultrasound ablation - initial clinical outcomes. Radiology 276:597–605.
- Lang BH, Woo YC, Wong CK. (2017). High intensity focused ultrasound (HIFU) treatment for symptomatic benign thyroid nodules: a prospective study. Radiology. [Epub ahead of print]. doi: 10.1148/radiol.2017161640
- Baek JH, Lee JH, Valcavi R, et al. (2011). Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol 12:525–40.
- Baek JH, Moon WJ, Kim YS, et al. (2009). Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg 33:1971–7.
- Baek JH, Lee JH, Sung JY, et al. (2012). Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology 262:335–42.
- Korkusuz H, Sennert M, Fehre N, et al. (2015). Localized thyroid tissue ablation by high intensity focused ultrasound: volume reduction, effects on thyroid function and immune response. Fortschr Röntgenstr 187:1011–5.
- Lang BH, Wong CK, Wong KP, et al. (2017). Effect of thyroid remnant volume on the risk of hypothyroidism after hemithyroidectomy: a prospective study. Ann Surg Oncol. [Epub ahead of print]. doi: 10.1245/s10434-016-5743-9
- Verloop H, Louwerens M, Schoones JW, et al. (2012). Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab 97:2243–55.
- Chu KK, Lang BH. (2012). Clinicopathologic predictors for early and late biochemical hypothyroidism after hemithyroidectomy. Am J Surg 203:461–6.
- Chauhan S, Lowe MJ, Davies BL. (2001). A multiple focused probe approach for high intensity focused ultrasound based surgery. Ultrasonics 39:33–44.
- Cibas ES, Ali SZ. NCI Thyroid FNA State of the Science Conference. (2009). The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol 132:658–65.
- Kovatcheva R, Zaletel K. (2017). High-intensity focused ultrasound for thyroid nodule ablation: the evidence to date. Rep Med Imaging 10:9–16.
- Kim C, Lee JH, Choi YJ, et al. (2016). Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. [Epub ahead of print]. doi:10.1007/s00330-016-4690-y