Abstract
Objective: To evaluate the outcomes of percutaneous microwave ablation (MWA) and explore the prognostic factors for the survival of patients with intrahepatic cholangiocarcinoma (ICC).
Methods: A total of 107 patients (age: mean 58.0 years, range 15–85 years) with 171 ICCs (maximum size ≤5 cm, tumour number per patient ≤3) who underwent MWA for ICC during January 2009 to February 2016 were selected, and their clinical and pathological data were collected and reviewed. The MWA-associated mortality, major complication rate and survival were evaluated. The prognostic factors for survival in patients with ICC were analysed with univariate and multivariate analyses.
Results: The median follow-up after MWA was 20.1 months (2.8–63.5 months). There was no procedure-associated death. The overall procedure-associated major complication rate was 2.8%. The median PFS after MWA was 8.9 months; PFS rates after 6, 12, 18 and 24 months were 67.4%, 41.5%, 18.2% and 8.7%. The median OS was 28.0 months; OS rates after 1, 3 and 5 years were 93.5%, 39.6% and 7.9%. Child-Pugh class A and less tumour number were identified as factors predictive of prolonged PFS (HR for Child–Pugh class: 2.62, p = 0.001; HR for tumour number: 2.07, p = 0.002) and OS (HR for Child–Pugh class: 4.14, p < 0.001; HR for tumour number: 1.95, p = 0.024).
Conclusions: Percutaneous ultrasound-guided MWA is safe and effective for ICC. Child–Pugh class A and less tumour number predict prolonged PFS and OS in patients with ICC treated by MWA.
Introduction
Intrahepatic cholangiocarcinoma (ICC) accounts for 10–15% of all primary liver cancers and is the second most common primary liver malignancy in humans, only behind hepatocellular carcinoma (HCC) [Citation1]. Although the frequency of ICC worldwide is considerably less than HCC, the morbidity of ICC is rising around the world due to uncertain reasons over the last few decades [Citation2–4]. And, ICC often presents higher invasiveness and poorer survival than HCC. Patients with ICC can only survive about six months after initial diagnosis or recurrence with palliative treatment [Citation5,Citation6]. Currently, radical excision is the only treatment method to cure ICC. Unfortunately, only about 20–40% of patients with ICC are surgical candidates due to multiple metastases and/or poor liver function [Citation7]. Even after attempted curative resection, more than 60% of ICC will relapse and five-year survival is reported to range from 20% to 40% [Citation8,Citation9]. Nonsurgical treatments such as chemotherapy, radiotherapy have shown limited benefit for survival or quality of life [Citation10,Citation11].
Over the past decade, image-guided ablative therapies, especially radiofrequency ablation (RFA), have already been applied in the management of patients with ICC as an acceptable alternative for surgical resection because of lower level of invasiveness and acceptable clinical outcomes [Citation12–16]. Compared with RFA, microwave ablation (MWA) offers many theoretical advantages, including less dependence on electrical conductivity of tissue, less ablation time, higher intratumoural temperature, and larger and more homogenous and uniform ablation zone [Citation17–19]. Since few studies have investigated the outcomes of MWA in patients with ICC, we undertook a retrospective study to explore the clinical and survival outcomes of ultrasound-guided percutaneous MWA treatment for ICC.
Materials and methods
Patients
All patients were selected from the inpatients of our hospital during January 2009 to March 2016. Their clinical and pathological data were collected and reviewed. Inclusion criteria for this study included: histologically proven ICC, primary () or recurrent after surgery (); maximum size of ICCs ≤5 cm and number of ICCs ≤3; all MWA was carried out percutaneously and guided by ultrasonography (US) with curative intention. Patients having ICC with vascular invasion or extrahepatic metastases were excluded. This retrospective study was approved by our institutional review board. Written Informed consent for the treatment procedures was acquired from each patient.
Figure 1. A 63-year-old male patient undergoing percutaneous microwave ablation for primary intrahepatic cholangiocarcinoma (a). Enhanced MR images show a 2.1 cm lesion with heterogeneous enhancement (arrows) located in right posterior lobe before microwave ablation; Enhanced MR images four months (b) and 13 months (c) after ablation show a non-enhanced area with a clear margin in corresponding site (arrows), and no new lesion was detected.
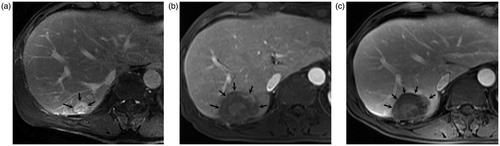
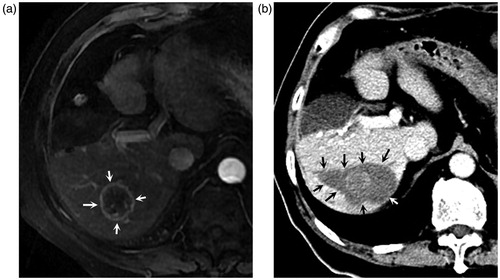
Figure 2. A 73-year-old male patient undergoing percutaneous microwave ablation for recurrent intrahepatic cholangiocarcinoma eight months after partial hepatectomy. (a) Enhanced MR images eight months after partial hepatectomy show a 3.5 cm lesion with peripheral enhancement (arrows) during arterial phase located in right posterior lobe; (b). Enhanced CT obtained one month after microwave ablation show a non-enhanced area with a clear margin in corresponding site (arrows), demonstrating complete ablation.
MWA procedure
All patients were treated with a cooled-shaft system (KY-2000, Kangyou Medical Instruments, Nanjing, China), which was capable of producing a maximum of 100 W of power at 2450 MHz. This system had 21-gauge thermocouple needles, which could be percutaneously placed at a designated location to monitor temperature in real time. After administration of local anaesthetic, US-guided biopsies were performed via the same skin incision that was made for the microwave antenna placement. Microwave antennae (15-gauge, 1.9 mm external diameter, 18 cm long) were then inserted under US guidance. A general anaesthetic was applied after all insertions, and microwave was then emitted. An output setting of 60 W for 300 s was routinely used during ablation sessions. If the heat-produced hyperechoic microbubbles did not completely cover the whole tumour, extended microwave emission was applied until the desired range was reached. After the MWA treatment of the tumour, needle track cauterisation was performed to avoid possible seeding of tumour cells [Citation20]. So far, a session of MWA was completed.
Follow-up and outcomes measures
All patients were followed up with contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). Follow-up imaging was performed one and three months after MWA and then roughly 3–6-month intervals. When the target lesion was revealed as a non-enhanced necrotic area on the contrast-enhanced imaging one month after MWA, the MWA procedure was judged to be successful. An irregular or nodular peripheral enhancement at the ablation margin on one-month follow-up imaging was considered a residual tumour, for which an additional session of MWA was underwent. Follow-up was closed at the time of death or last visit of the patient.
The end points of this study were progression-free survival (PFS) and overall survival (OS). Local tumour recurrence was defined as presence of enhanced lesions at or adjacent to the prior MWA site which had been previously documented as complete ablation. New contrast-enhancing focal lesions at intrahepatic or extrahepatic sites were considered new metastases and defined as intrahepatic progression and extrahepatic progression, respectively. And the objective disease progression included local recurrence, intrahepatic progression and extrahepatic progression. PFS was calculated from the date of first session of MWA treatment for ICC to the date of objective disease progression or the last date of follow-up (in patients who did not suffer from objective disease progression). OS was calculated from the date of first session of MWA treatment for ICC to the date of death or last date of follow-up. Mortality related to thermal ablation was defined as death within 30 days after ablation. Major complications were defined as events which caused substantial morbidity and disability that increased the level of care, or led to hospital admission, or substantially prolonged the hospital stay [Citation21].
Statistics
The quantitative data was expressed as mean ± standard deviation, and qualitative data was expressed as frequency. PFS and OS were estimated by the Kaplan–Meier method. Univariate analyses were performed using log-rank test. Multivariate analyses were performed using the Cox proportional hazards model. For all analyses, p values was bilateral and <0.05 was considered statistically significant. All data were analysed with SPSS, version 19 (SPSS Inc., Chicago, IL, USA).
Results
Patients’ characteristics
A total number of 107 patients with 171 ICCs were eligible for the inclusion criteria and included in this study. Patients’ primary characteristics are shown in . Among these, comorbidities including hypertension, diabetes mellitus, and/or previous cerebral infarction presented in 46 patients. Sixty patients suffered from recurrences of ICC after radical resection and received MWA treatment for their recurrent tumours within 1–2 months after a diagnosis of recurrence, which was determined by contrast-enhanced CT, MRI or ultrasound. Residual tumours were observed in 12 patients (12 of 171 tumours), as confirmed by one-month follow-up imaging. All residual tumours were successfully ablated with a second session of MWA.
Table 1. Patients’ primary characteristics.
Procedure-related death and major complications
There was no MWA procedure-related death. For the whole patients cohort, major complications related to the MWA procedure were observed in three patients (2.8%), including two with pleural effusion, one with liver abscess. Those patients with pleural effusion were treated by catheter drainage. The patient with liver abscess was treated by catheter drainage combined with antibiotic treatment. All the three patients’ symptoms were apparently relieved after treatment. No major complications led to permanent sequelae.
Survival outcomes
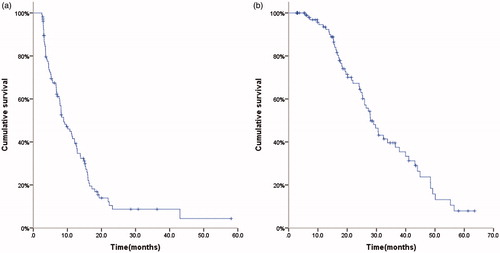
After a median follow-up of 20.1 months (2.8–63.5 months), 83 patients presented with progression. Among them, 58 patients deceased. The median PFS after MWA was 8.9 months (95%CI 6.5–11.3 months); PFS rates after 6, 12, 18 and 24 months were 67.4%, 41.5%, 18.2% and 8.7% (). The median OS was 28.0 months (95%CI 23.7–32.2 months); OS rates after 1, 3 and 5 years were 93.5%, 39.6% and 7.9% ().
Prognostic factors
In order to assess prognostic factors for survival outcomes in patients with ICC treated by MWA, we subjected 11 clinical parameters (age, gender, HBV infection, HCV infection, comorbidities, primary or not, Child–Pugh class, CA19–9 level, tumour number, maximum tumour size and tumour differentiation) into univariate analyses. The results of univariate analysis showed that Child–Pugh class and tumour number significantly influenced both PFS and OS (). Patients with Child–Pugh A class had significantly longer PFS (11.2 vs. 6.6 months, p = 0.005) and OS (32.4 vs. 16.7 months, p < 0.001) than those with Child–Pugh B class. Likewise, patients with single tumour had significantly longer PFS (12.6 vs. 4.6 months, p = 0.015) and OS (33.8 vs. 24.2 months, p = 0.002) than those with multiple tumours.
Table 2. The results of univariate analysis using log-rank test.
We then subjected factors with p values of univariate analyses less than 0.200 into multivariate analysis to identify the independent prognostic factors for PFS and OS. HBV infection, primary or not, Child–Pugh class and tumour number were included in the multivariate analysis of PFS, while primary or not, Child–Pugh class and tumour number included in that of OS (). The results suggested that both Child–Pugh class and tumour number were identified as the independent prognostic factors for PFS (hazard ratio [HR] for Child–Pugh class: 2.62, p = 0.001; HR for tumour number: 2.07, p = 0.002) and OS (HR for Child–Pugh class: 4.14, p < 0.001; HR for tumour number: 1.95, p = 0.024).
Table 3. The results of multivariate analysis using cox proportional hazards model.
Discussion
Because of its minimally invasiveness and reliable effectiveness, recent guidelines have suggested RFA as a standard treatment for patients with HCC within the Milan criteria but not suitable for surgical therapy [Citation22]. Recent guidelines also indicate that ablation may provide survival benefits for patients with ICC if surgery is not an option [Citation23]. However, although RFA has been the most studied, whether MWA is safe and effective in patients with ICC is rarely reported.
The present retrospective study showed that there was no procedure-associated death in patients with ICC treated by MWA, which was a significant decrease from the reported perioperative mortality rates range from 1.2% to over 7% [Citation24–26]. The rate of procedure-associated major complications was significantly lower than that of post-operation, which was reported to be range from 11% to 58% [Citation27,Citation28]. The survival of ICC patients was a significant improvement from that after palliative treatment alone and comparable with that after radical resection [Citation29–32]. These results suggest that MWA is less invasive than surgical treatment and is safe and effective for ICC.
In addition, the present study analysed the prognostic factors for the survival of ICC patients treated with MWA. It’s worth noting that age had no significant impact on both PFS and OS, suggesting that MWA is safe and effective in both young and old ICC patients. So percutaneous MWA provides another choice other than palliative treatment for old patients who are intolerant for radical resection due to poor physical condition or various comorbidities. Furthermore, the results indicated that Child-Pugh class A and less tumour number were identified as factors predictive of prolonged PFS and OS. Less tumour number is considered to represent less invasive biologic behaviour of the tumour, and therefore correlated with improved survival. Child–Pugh class A was reported as a significant predictor of longer PFS and OS in patients with HCC undergoing sorafenib therapy [Citation33,Citation34], suggesting that patients with good hepatic reserve benefit more from sorafenib. Nevertheless, the mechanism behind the correlation was far from clear. The current study revealed similar significance of Child–Pugh classification in patients with ICC treated with MWA. Possible explanation for this correlation may be that liver plays an important role in immune surveillance and defence [Citation35,Citation36]. Good hepatic reserve indicates that immune surveillance and defence by the liver is unimpaired or mildly impaired, thus enabling immune system to eliminate the residual and migrating tumour cells which are the major causes of tumour progression. Hence, progression will be delayed in patients with good hepatic reserve. However, further research is needed to uncover the specific mechanism.
In contrast to previous report [Citation8,Citation37], PFS and OS has not been shown to be predicted by tumour size and tumour differentiation in this study. The underlying reason may be attributed to one of the inclusion criteria of maximum size of ICC ≤5 cm in the current study. It has been concluded in the study of Xu et al. [Citation16] that acceptable survival can be achieved in ICCs measuring <5 cm which were treated with percutaneous ultrasound-guided thermal ablation by means of MWA or RFA. With larger sample number of more than 100 patients, the results of current study added evidence to the conclusion of the above mentioned study drawn from a relative small group of 18 patients. Poor tumour differentiation is usually proved to be associated with shorter OS in ICC patients treated with surgery or RFA [Citation8,Citation37]. However, the influence of tumour differentiation on the survival of ICC patients treated with MWA seemed to be not the case in this study. Further research is needed to confirm whether it is the therapeutic approaches that lead to the different results between these studies.
The limitations of our study included the retrospective design and short duration of patient follow-up. It lacks a comparison with other therapy methods. Large, multi-institutional randomised-controlled studies are required to provide more convincing evidence for the role of MWA in patients with unresectable ICC.
Conclusions
In conclusion, percutaneous ultrasound-guided MWA is safe and effective for ICC. In consideration of its zero mortality, low complication rate and acceptable survival, MWA may be an alternative treatment approach for ICC ≤5 cm in patients who are not candidates for surgery. Child–Pugh class A and less tumour number predict longer PFS and OS in patients with ICC treated by MWA. Further studies to compare the outcomes between MWA and other treatments for ICC are necessary in the future.
Acknowledgements
This study was supported by the Ministry of Science and Technology Support Program of China [grant no. 2013BAI01B01] and the National Natural Science Foundation of China [grant nos. 81430039, 81627803].
Disclosure statement
No conflict of interest exits in the submission of this manuscript. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Razumilava N, Gores GJ. (2014). Cholangiocarcinoma. Lancet 383:2168–79.
- Shaib Y, El-Serag HB. (2004). The epidemiology of cholangiocarcinoma. Semin Liver Dis 24:115–25.
- Khan SA, Toledano MB, Taylor-Robinson SD. (2008). Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 10:77–82.
- Shaib YH, Davila JA, McGlynn K, El-Serag HB. (2004). Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase?. J Hepatol 40:472–7.
- Anderson CD, Pinson CW, Berlin J, Chari RS. (2004). Diagnosis and treatment of cholangiocarcinoma. Oncologist 9:43–57.
- Puhalla H, Schuell B, Pokorny H, et al. (2005). Treatment and outcome of intrahepatic cholangiocellular carcinoma. Am J Surg 189:173–7.
- Tan JC, Coburn NG, Baxter NN, et al. (2008). Surgical management of intrahepatic cholangiocarcinoma – a population-based study. Ann Surg Oncol 15:600–8.
- Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. (2014). Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg 149:565–74.
- Yamamoto M, Ariizumi S. (2011). Surgical outcomes of intrahepatic cholangiocarcinoma. Surg Today 41:896–902.
- Park SY, Kim JH, Yoon HJ, et al. (2011). Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol 66:322–8.
- Saxena A, Bester L, Chua TC, et al. (2010). Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 17:484–91.
- Carrafiello G, Lagana D, Cotta E, et al. (2010). Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol 33:835–9.
- Kim JH, Won HJ, Shin YM, et al. (2011). Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 196:W205–9.
- Bale R, Schullian P, Haidu M, Widmann G. (2013). Stereotactic radiofrequency ablation (SRFA) of intrahepatic cholangiocellular carcinomas: a minimal invasive alternative to liver resection. Wien Med Wochenschr 163:128–31.
- Zhang SJ, Hu P, Wang N, et al. (2013). Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol 20:3596–602.
- Xu HX, Wang Y, Lu MD, Liu LN. (2012). Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol 85:1078–84.
- Wright AS, Sampson LA, Warner TF, et al. (2005). Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 236:132–9.
- Yu J, Liang P, Yu X, et al. (2011). A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol 79:124–30.
- Brace CL. (2009). Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences?. Curr Probl Diagn Radiol 38:135–43.
- Zhang K, Yu J, Zhou F, et al. (2016). Impact of timing and cycles of systemic chemotherapy on survival outcome of colorectal liver metastases patients treated by percutaneous microwave ablation. Int J Hyperthermia 32:531–8.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 273:241–60.
- Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD (2005). Management of hepatocellular carcinoma. Hepatology 42:1208–36.
- Bridgewater J, Galle PR, Khan SA, et al. (2014). Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60:1268–89.
- Endo I, Gonen M, Yopp AC, et al. (2008). Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 248:84–96.
- Lang H, Sotiropoulos GC, Sgourakis G, et al. (2009). Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg 208:218–28.
- Jonas S, Thelen A, Benckert C, et al. (2009). Extended liver resection for intrahepatic cholangiocarcinoma: a comparison of the prognostic accuracy of the fifth and sixth editions of the TNM classification. Ann Surg 249:303–9.
- Choi SB, Kim KS, Choi JY, et al. (2009). The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 16:3048–56.
- Ellis MC, Cassera MA, Vetto JT, et al. (2011). Surgical treatment of intrahepatic cholangiocarcinoma: outcomes and predictive factors. HPB (Oxford) 13:59–63.
- Wu ZF, Wu XY, Zhu N, et al. (2015). Prognosis after resection for hepatitis B virus-associated intrahepatic cholangiocarcinoma. World J Gastroenterol 21:935–43.
- Jutric Z, Johnston WC, Hoen HM, et al. (2016). Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford) 18:79–87.
- Spolverato G, Yakoob MY, Kim Y, et al. (2015). The impact of surgical margin status on long-term outcome after resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol 22:4020–8.
- Park J, Kim MH, Kim KP, et al. (2009). Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: a large-scale observational study. Gut Liver 3:298–305.
- Lee S, Kim BK, Kim SU, et al. (2014). Clinical outcomes and prognostic factors of patients with advanced hepatocellular carcinoma treated with sorafenib as first-line therapy: a Korean multicenter study. J Gastroenterol Hepatol 29:1463–9.
- Nishikawa H, Nishijima N, Enomoto H, et al. (2017). Predictive factors in patients with hepatocellular carcinoma receiving sorafenib therapy using time-dependent receiver operating characteristic analysis. J Cancer 8:378–87.
- Racanelli V, Rehermann B. (2006). The liver as an immunological organ. Hepatology 43:S54–S62.
- Jenne CN, Kubes P. (2013). Immune surveillance by the liver. Nat Immunol 14:996–1006.
- Fu Y, Yang W, Wu W, et al. (2012). Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol 23:642–9.