Abstract
Objective: Osteoblastoma (OB) is a painful, rare, benign bone tumour usually observed in young populations, and this condition involves the spine in up to one-third of cases. We sought to focus on the minimally invasive treatment of spinal OB with radiofrequency ablation (RFA) under computed tomography (CT) guidance. When performed near the spinal cord, surgery can lead to instability of the spine, sometimes requiring additional interventions to stabilise the segments involved, and can cause the precocious onset of arthrosis or other degenerative diseases.
The results were evaluated both clinically and with the aid of diagnostic imaging techniques during a 5-year follow-up study.
Materials and methods: Eleven patients affected by spinal OB were treated in a single session with biopsy and CT-guided RFA. Pre- and post-evaluations of the patients were performed both clinically and with CT and magnetic resonance imaging (MRI).
Results: Complete success in terms of pain relief was achieved in all patients. Additional treatments were not required in any patients. There were no complications. During follow-up, neither complications nor pathological findings related to the treatment were observed.
Conclusions: Our experience demonstrates that RFA for spinal OB is safe and effective. One of the main advantages of this technique is represented by its lower grade of invasiveness compared with that for potentially hazardous surgical manoeuvres.
Introduction
Osteoblastoma (OB) is a rare, benign bone tumour accounting for 1% of all primary bone tumours [Citation1–4]. Appearing in the 2nd or 3rd decades of life, OB is more commonly found in males, with a male to female ratio of 2:1. OB is four times less frequent than osteoid osteoma (OO) and most commonly involves the spine (sacrum in up to one-third of cases) and long bones. OB is histologically similar to OO but differs in size [Citation5,Citation6]; lesions exceeding 1.5 cm are usually highly suggestive of OB [Citation7]. OB is biologically more aggressive than OO and can also infiltrate extraskeletal tissues. Malignant transformation in 12–25% of lesions has been described in the literature [Citation7–9].
Spinal OB most commonly affects the posterior aspects of the spine (lamina, pedicles or spinous processes). The main clinical manifestations are of a neurological nature and include progressive focal or radicular pain exacerbated by movement. One possible consequence of spinal OB is progressive painful scoliosis [Citation1,Citation7]. Pain is usually less severe at night compared with that in OO. Nevertheless, the need to administer nonsteroidal anti-inflammatory drugs for long periods of time makes it crucial to identify alternative and resolutive treatments.
Computed tomography (CT)-guided radiofrequency ablation (RFA) is accepted as the gold standard for OO, even when presenting in the spine, where, if necessary, it is used with thermal protection techniques [Citation10,Citation11]. Surgical excision is still considered the curative treatment for OB depending on its size and closeness to the sensitive structures of the spine; however, it is possible that these tumours may recur after surgery (in up to 50% of cases in the presence of aggressive OB) [Citation2]. Surgical limitations include invasiveness and difficulty in performing a complete resection, especially when operating on highly vascularised tumours.
The effectiveness and safety of percutaneous RFA in the treatment of OB presenting in the spine have already been described in the literature [Citation12], but to our knowledge, this is the first study focussing only and specifically on this topic. Treating spinal lesions is quite challenging due to the presence of the spinal cord. Thus, long-term follow-up studies based on clinical and imaging data enable assessment of the structural and morphological changes secondary to OB treatments.
Materials and methods
Selection criteria and ethical policy
Between March 2009 and April 2015, 17 patients presented with symptoms suggestive of spinal OB. After clinical evaluation and CT and magnetic resonance imaging (MRI) examinations, the diagnosis of OB was confirmed in 11 patients. The radiological criteria for diagnosis included the presence of a radiolucent lesion exceeding 1.5 cm in diameter on plain film [Citation1–4] and the presence of a lytic lesion with or without central calcifications on CT. In OB, the sclerotic rim is usually less evident than in OO, and MRI shows a focal lesion surrounded by remarkable oedema [Citation1,Citation2]. The 11 patients showing these radiological findings were included in the study. For each case, the final decision to perform RFA was made by an interdisciplinary team made up of an interventional radiologist and orthopaedic and spinal surgeons.
The following inclusion criteria were applied: (i) CT and MRI or plain X-rays and MRI suggesting OB; (ii) spinal location of the lesion, with involvement of the dorsal aspects; (iii) lesion diameter ≥1.5 cm (up to 2.5 cm of maximum diameter) with a round or oval morphology; and (iv) clinical symptomatology (pain) highly suggestive of OB.
The exclusion criteria were as follows: (i) non-symptomatic lesion; (ii) uncertain characterisation of the lesion with imaging; (iii) extraspinal location of the lesion; and (iv) lesions with irregular morphology not treatable with the interventional radiology technique.
The patients treated consisted of four females and seven males with a mean age of 26 years (range, 18–45). The lesions were mainly located in the thoracic and lumbar spine (). Clinically, the patients complained of pain and limitations in daily activities. The visual analogue scale (VAS) questionnaire was administered prior to the procedure and resulted in a mean value of 7.5 (range 6–9).
Table 1. Demographic table and characteristic of lesions.
Written informed consent was obtained the day before treatment from all patients, who also received a detailed explanation of the procedure. The study was approved by the institutional review board and was performed according to the principles of the Declaration of Helsinki.
Procedure
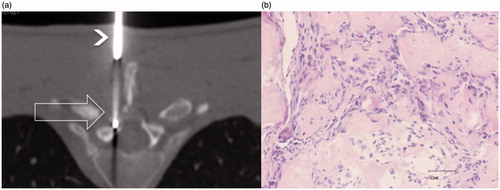
All patients underwent a routine pre-procedural screening including a medical history, physical and basic laboratory analysis, and anesthesiological evaluation. All procedures were performed by one interventional radiologist with 10 years of experience and one resident in radiology, both with experience in musculoskeletal interventional radiology. RFA was performed by means of a multi-detector CT scanner with the patients under deep sedation in the prone position on the CT table. A broad-spectrum antibiotic was administered prior to the procedure. Two grounding pads were placed on the thighs to inhibit the transmission of current through the patient. Multiplanar reconstructions were obtained to spot the lesion and define the access. The latter was identified using laser and skin markers. After local anaesthesia (8–10 ml mepivacaine) was applied along the whole pathway, from the skin up to the periosteum, the procedure began with an incision of the skin made with a scalpel. The access to the lesion in all cases was performed with a 13 G coaxial bone biopsy needle (MadisonTM LAURANE Medical, Le Pradet, France), using Fluoro-CT to check the pathway. Once in the nidus, a bone biopsy was performed. The penetration cannula was left and used as a placeholder, and a 17 G radiofrequency electrode (RFA 1510 cool tip, RF Medical Co., Seoul, Korea) was inserted through the cannula with the active tip within the nidus ().
Figure 1. (a) CT axial slice (low resolution due to the low dose used with Fluoro-CT to reduce the dose), RFA needle inside the lesion (large unfilled arrow), and the bone biopsy needle (arrowhead) used as a guide; (b) core biopsy histology revealing disorganised trabeculae of immature bone consistent with OB (haematoxylin–eosin).
Different sizes of the active tip of the RFA needles were used (up to 2 cm) depending on the lesion size. When the tip was in the correct position (inside the lesion, along the major axis), the penetration cannula was partially withdrawn to prevent heat propagation along the needle. The size of the active tip was chosen based on the lesion size. The aim was to determine whether the expected area of ablation was slightly wider (few millimetres) than the lesion. It was not possible to obtain a safety margin around the lesion repeatable for all lesions due to the particular location (in the vertebra), as it was irregular and close to sensitive structures. Only one needle at time was used, and if needed, it was repositioned after a cycle when the lesion was larger than the expected ablation area. A temperature-controlled radiofrequency generator was used, and a temperature of 90° was reached for 6 min to perform the ablation, according to the manufacturer’s instructions. In no case was the area of ablation in contact or at a distance less than 5 mm from the sensitive structures (spinal cord or spinal roots).
At the end of the procedure, in the evening and the day after, 4 mg of corticosteroid (betamethasone 4 mg) was administered to all patients to reduce treatment-induced inflammation. In addition, a continuous infusion of morphine, gastroprotective and antiemetic drugs was performed for 12 h to avoid post-procedural pain and guarantee a night’s rest. All patients were discharged the day after the procedure. All patients were requested to stay in the hospital one night before and one night after treatment.
Outcome and follow-up
Technical success was defined as the correct placement of the active tip within the nidus and performance of a complete RFA.
The primary clinical outcome was defined as pain relief achieved after the procedure (evaluated by means of the VAS) and the interruption of painkiller drug administration. The first clinical follow-up visit was set at one week after the procedure to avoid possible alterations of the clinical results caused by the inflammatory reaction that inevitably follows thermal ablation. Additional follow-up studies were performed at 6 and 12 months to confirm the primary clinical outcome in all patients. All follow-up studies were based on clinical evaluation and CT and/or MR imaging. To collect long-term results, all patients were called several times between 2015 and 2016. Clinical and imaging results were collected over a 5-year period.
MRI data were obtained with a 1.5 T unit (Signa, GE, Little Chalfont, UK). All examinations were performed including standard sequences (T1-weighted spin-echo (SE), T2-weighted TSE, short tau inversion recovery (STIR) and T1-weighted SE) after intravenous injection of contrast agent. The contrast agent was used only at the first follow-up visit at 6 months.
Analysis of the results of MR images was performed considering the following findings: (a) disappearance of the oedema of bone and soft tissues and (b) contrast enhancement (C.E.) in the nidus. On CT images, the presence of calcifications was investigated.
Results
All procedures were conducted successfully from a technical point of view, and there were no major complications [Citation13]. Skin burns were not present at the site of RFA needle access or around the grounding pads. A slight sensation of swelling and mild pain at the site of ablation was reported as side effects by eight out of 11 patients the day after the treatment upon being discharged from the hospital. These symptoms required anti-inflammatory drugs for pain relief (administered only for two days) in four patients.
The histological samples confirmed the diagnosis of OB in eight out of 11 cases; in the other three cases, the tissue samples were considered inadequate for histological diagnosis, and diagnosis was made based on the images alone.
Primary clinical outcome
All patients (100%) showed a good response to the treatment one week after the procedure, with complete disappearance of pain and related symptoms (mean value of VAS at 1 week was 0.3; range: 0–1). Neither complaints of functional impairment nor requests for pain killers were reported.
A mean VAS value equal to 0 was reported during the first follow-up study at 6 months. No recurrence of disease or mid-term complications were observed. No patients took any medication for treatment-induced pain. The second follow-up at 12 months confirmed these findings.
The long-term results also confirmed these data. The patients underwent follow-up for a mean of 43.6 months (four of them were controlled 60 months after treatment, three after 48 months, one after 36 months, two after 24 months and one after 12 months).
Imaging outcome
Considerable reduction of oedema (quantitatively, to a greater extent than 50%) and the absence of contrast enhancement were observed at the first MRI follow-up at 6 months in eight patients (72%). At the following visit (1 year), complete disappearance of oedema both in the bone and in the soft tissues near the lesion was observed in all cases (100%).
By CT imaging, a sclerotic process (ossification) of the lesion was found. In all cases, at the 6-month follow-up, ossification of the nidus of the treated lesion was observed, which was confirmed in all cases at the subsequent 1-year follow-up. In the long-term follow-up studies, in three cases (two at 60 months and one at 48 months), complete restoration of the bone to its original condition was observed: the tumour had completely disappeared. In the remaining eight patients, even though partial bone remodelling was detected, it was not possible to observe complete disappearance of the lesion during the follow-up period ().
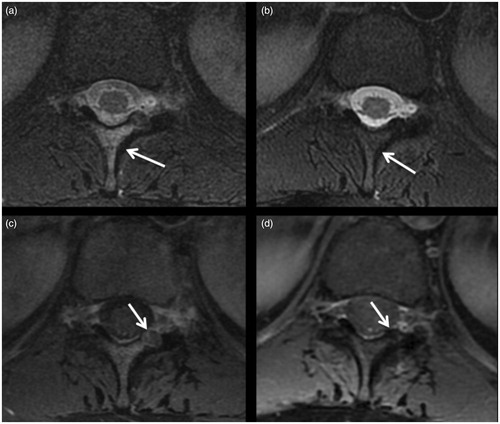
Figure 2. OB (arrow) of the posterior arc of D6: (a) before treatment; (b) follow-up 6 months after treatment showing sclerosis of the lesion; (c, d) follow-up after 24 and 60 months, respectively, showing the slow progression of bone remodelling toward disappearance of the treated lesion.
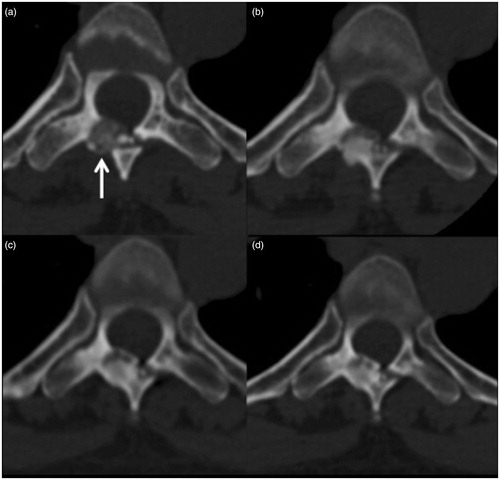
Figure 3. Same case as in : (a) pre- and (b) post-treatment T2 FAT SAT MRI, showing bone oedema around the lesion (arrowheads) and disappearance during follow-up.
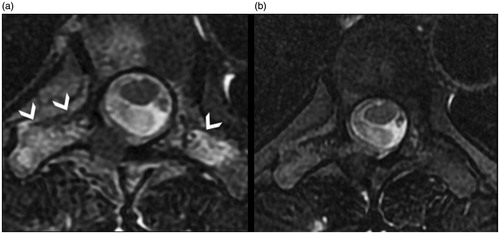
Figure 4. (a) OB of the lamina of D11 (arrow); (b) ossification of the nidus treated (arrow) with the sign of the pathway of the nidus (black arrowhead); (c, d) coronal reconstructions along the plane hatched on the axial images with evidence of ossification of the treated lesion.
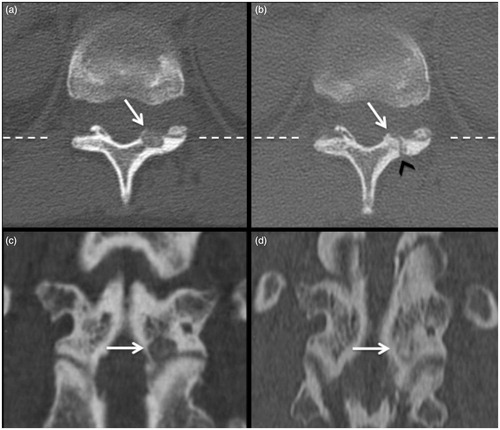
Figure 5. Same case as in : (a) pre- and (b) post-treatment T2 FAT SAT MRI, showing in (a) the presence of bone oedema before treatment, which has disappeared in (b); (c) pre- and (d) post-treatment T1 FAT SAT with C.E., showing disappearance of C.E. in the nidus of the lesion (arrow) after treatment.
Discussion
RF thermal ablation is a safe and effective method for the treatment of OO and OB appearing in various localisations [Citation10,Citation14,Citation15]. These tumours may affect the spine, although treatments for spinal OO are more commonly described in the literature than for spinal OB [Citation9]. This difference is likely because OB tumours are larger in size and more aggressive, they require more radical ablative approaches. The possibility of relapse or procedural failure makes spinal OB treatment unpopular among interventional radiologists. On the other hand, besides being more invasive, surgery requires longer hospitalisation times and does not always guarantee clinical success after the first intervention. Sometimes, additional surgical procedures are needed to achieve stabilisation of the spine [Citation9]. The spinal location poses a real challenge to both the surgeon and interventional radiologist, who are well aware not only of the risks due to the close proximity to sensitive and important structures, such as the spinal cord, but also that every instance of damage to the spine can lead to the premature evolution of spondylarthrosis with consequent painful symptomatology and functional impairment.
The aims of this study were to evaluate the effectiveness and safety of the treatment of spinal OB with CT-guided RFA and to evaluate the long-term results on the basis of imaging findings. Throughout the lifetime, the spine is subjected to continuous evolution. Therefore, it is important to understand whether minimally invasive treatments may provoke morphological and structural changes, possibly leading to a precocious onset of arthrosis or other degenerative diseases.
Technological developments in recent years have enabled great precision in delivering energy for thermal ablation [Citation14]. The energy delivered by the needle creates a rise in temperature around the tip. The manufacturer precisely defines the dimension of the ablation area (from 0.5 to 3 or more cm of diameter) to guarantee that the whole area is ablated. The tissues surrounding the target area, where the temperature is significantly decreased, are safe or at most subjected to sublethal and temporary damage. This is particularly true in the bone, especially in the cortical bone, which has an insulating property such that the temperature significantly decreases as the distance from the needle tip increases [Citation16]. Meticulous care is required when positioning the needle tip within the nidus of the lesion. In fact, even the smallest tumoural portion left untreated can cause relapse. To ensure that the whole lesion is treated, it is recommended to leave a safety margin, i.e. an area of healthy tissue, around the wound. However, due to the irregular vertebral borders and the close proximity of the spinal cord and nerves, it was not possible in our experience to standardise a safety margin around the lesion that could be repeated in all cases (5 mm or more). In every patient, however, the ablation area was guaranteed by the needle, and we were able to cover the entire lesion with a few millimetres of margin.
In our study, excellent results were observed in terms of clinical outcome. We know from experience the importance of the meticulous planning of this procedure. For example, it is of fundamental importance to place the needle exactly in the centre of the nidus to avoid damaging the sensitive structures along the pathway and around the lesion. This approach likely justifies the lack of major complications observed in our experience.
The use of short-term corticosteroid therapy immediately after treatment limited the onset of inflammatory reactions induced by thermal ablation, and the patients could be rapidly discharged from the hospital.
Moreover, the clinical results were confirmed by imaging. In particular, in the MRI follow-up studies performed at 6 and 12 months, the absence of reactive inflammatory processes around the lesions and contrast enhancement was observed. These findings confirmed the success of the procedure.
Quite encouraging results emerged from the long-term analysis. In particular, CT images performed at 12 months showed ossification of the lesion in all patients. The needle pathway through the bone could also be clearly appreciated, demonstrating coherently with MR images that the lesion was replaced by bone (ossification) after treatment. Observing the evolution of the target area over time, progressive bone remodelling was appreciated. In three patients controlled at least 48 months after treatment, complete restoration of the treated bone segment was observed. In two patients submitted to the same control in terms of time (48 months), a similar process was found. These findings demonstrate that RF thermal ablation does not leave any sign of treatment and does not affect the physiological and morphological evolution of the spine.
To our knowledge, the bone remodelling process reflects the characteristics of the bone segment involved. In fact, if the bone segment is weight-bearing (vertebral articular process), the progressive remodelling of the bone seems to last at least 4 years, after which time it will be possible to appreciate the disappearance of the lesion with complete restoration of the bone segment to its original condition. Conversely, in non-weight-bearing bone segments, the sclerosis of the nidus remains and the bone remodelling process is slower.
Some authors encourage the use of thermal protection techniques (e.g. gas or hydrodissection) when operating near the spinal cord and/or the nerve roots. We did not use these techniques because when the patient was ready for the procedure on the CT table, the target lesion was not in contact or at a distance greater than 5 mm from sensitive structures. Nevertheless, we are fully aware of the medico-legal importance of thermal protection, which helps reduce complications.
This study was conducted retrospectively focussing on RFA alone without any comparison with other techniques, which can be considered a limitation of this study. One explanation for our approach is that RFA is the most diffuse, safe and effective technique for the treatment of OB. Moreover, OB is rare, and our intent was to focus attention on a sub-population of tumours with a specific localisation. For these reasons, we treated all of the lesions with one single method (RFA) to render our results as numerically significant as possible. Nevertheless, we are fully aware that a prospective comparison of RFA with other techniques would have been desirable, even though similar reports have been described in the literature [Citation5].
The treatments applied required radiation exposure. In fact, CT is the best choice for optimal bone visualisation and the comfort of the operator (the MRI gantry is too narrow to allow accurate positioning of the needle). For this reason, attention must be paid to reduce exposure for patients as well as operators (Fluoro-CT, virtual navigation, etc.).
Conclusions
CT-guided RFA is a safe and effective minimally invasive treatment for spinal OB that does not leave signs of treatment, as demonstrated by our long-term follow-up analysis.
Acknowledgements
The authors wish to thank Angela Martella for translating the manuscript and Luca Ventura (MD, Division of Pathology, San Salvatore Hospital, L’Aquila) for the evaluation of the histological samples.
All procedures in this study were performed in accordance with the Helsinki Declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lucas DR. (2010). Osteoblastoma. Arch Pathol Lab Med 134:1460–6.
- Kumasaka S, Miyazaki M, Tsushima Y. (2015). CT-guided percutaneous cryoablation of an aggressive osteoblastoma: a case report. J Vasc Interv Radiol 26:1746–8.
- Greenspan A. (1993). Benign bone-forming lesions: osteoma, osteoid osteoma, and osteoblastoma. Clinical, imaging, pathologic, and differential considerations. Skeletal Radiol 22:485–500.
- Yalcinkaya U, Doganavsargil B, Sezak M, et al. (2014). Clinical and morphological characteristics of osteoid osteoma and osteoblastoma: a retrospective single-center analysis of 204 patients. Ann Diagn Pathol 18:319–25.
- Chotel F, Franck F, Solla F, et al. (2012). Osteoid osteoma transformation into osteoblastoma: fact or fiction? Orthopaed Traumatol: Surg Res 98:S98–104.
- Youssef BA, Haddad MC, Zahrani A, et al. (1996). Osteoid osteoma and osteoblastoma: MRI appearances and the significance of ring enhancement. Eur Radiol 6:291–6.
- Samdani A, Torre-Healy A, Chou D, et al. (2009). Treatment of osteoblastoma at C7: a multidisciplinary approach. A case report and review of the literature. Eur Spine J 18(Suppl.2):S196–200.
- Boriani S, Amendola L, Bandiera S, et al. (2012). Staging and treatment of osteoblastoma in the mobile spine: a review of 51 cases. Eur Spine J 21:2003–10.
- Weber MA, Sprengel SD, Omlor GW, et al. (2015). Clinical long-term outcome, technical success, and cost analysis of radiofrequency ablation for the treatment of osteoblastomas and spinal osteoid osteomas in comparison to open surgical resection. Skeletal Radiol 44:981–93.
- Rybak LD, Gangi A, Buy X, et al. (2010). Thermal ablation of spinal osteoid osteomas close to neural elements: technical considerations. AJR Am J Roentgenol 195:W293–8.
- Filippiadis DK, Velonakis G, Kostantos C, et al. (2017). Computed tomography-guided radiofrequency ablation of intra-articular osteoid osteoma: a single center’s experience. Int J Hyperthermia. [Epub ahead of print]. doi: 10.1080/02656736.2017.1294711.
- Rehnitz C, Sprengel SD, Lehner B, et al. (2012). CT-guided radiofrequency ablation of osteoid osteoma and osteoblastoma: clinical success and long-term follow up in 77 patients. Eur J Radiol 81:3426–34.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 273:241–60.
- Masciocchi C, Zugaro L, Arrigoni F, et al. (2016). Radiofrequency ablation versus magnetic resonance guided focused ultrasound surgery for minimally invasive treatment of osteoid osteoma: a propensity score matching study. Eur Radiol 26:2472–81.
- Masciocchi C, Arrigoni F, La Marra A, et al. (2016). Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br J Radiol 89:20150356.
- Floridi C, Radaelli A, Abi-Jaoudeh N, et al. (2014). C-arm cone-beam computed tomography in interventional oncology: technical aspects and clinical applications. Radiol Med 119:521–32.
- Carrafiello G, Fontana F, Cotta E, et al. (2011). Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med 116:1059–66.
- Cazzato RL, Garnon J, Ramamurthy N, et al. (2016). Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol 33:140.
- Masciocchi C, Conchiglia A, Gregori LM, et al. (2014). Critical role of HIFU in musculoskeletal interventions. Radiol Med 119:470–5.
- Irastorza RM, Trujillo M, Martel Villagrán J, Berjano E. (2016). Computer modelling of RF ablation in cortical osteoid osteoma: assessment of the insulating effect of the reactive zone. Int J Hyperthermia 32:221–30.
