Abstract
Objective: We performed a systematic review and meta-analysis to evaluate the safety of radiofrequency ablation (RFA) for the treatment of benign thyroid nodules and recurrent thyroid cancers.
Materials and methods: Ovid-MEDLINE, EMBASE, and Library of Cochrane databases were searched up to 12 July 2016 for studies on the safety of RFA for treating benign thyroid nodules or recurrent thyroid cancers. Pooled proportions of overall and major complications were assessed using random-effects modelling. Heterogeneity among studies was determined using the χ2 statistic for the pooled estimates and the inconsistency index I2.
Results: A total of 24 eligible studies were included, giving a sample size of 2421 patients and 2786 thyroid nodules. 41 major complications and 48 minor complications of RFA were reported, giving a pooled proportion of 2.38% for overall RFA complications [95% confidence interval (CI): 1.42%–3.34%] and 1.35% for major RFA complications (95% CI: 0.89%–1.81%). There were no heterogeneities in either overall or major complications (I2 = 1.24%–21.79%). On subgroup analysis, the overall and major complication rates were significantly higher for malignant thyroid nodules than for benign thyroid nodules (p = 0.0011 and 0.0038, respectively).
Conclusions: RFA was found to be safe for the treatment of benign thyroid nodules and recurrent thyroid cancers.
Introduction
Radiofrequency ablation (RFA) is a promising treatment modality for various tumours, particularly liver cancer [Citation1–4]. For thyroid diseases, ultrasound-guided RFA has shown good results for benign thyroid nodules [Citation5–10], including autonomously functioning thyroid nodules [Citation11–13]. For benign thyroid nodules, RFA effectively ameliorates symptoms and cosmetic problems by reducing the thyroid nodule volume by 84.8% [Citation5,Citation12,Citation14,Citation15]. Recent case series suggest that ultrasound-guided RFA as an alternative treatment for recurrent thyroid carcinoma in patients who are at a high risk of complications from surgery or who refuse to undergo repeated surgeries [Citation16–21]. According to a recent meta-analysis of ultrasound-guided RFA treatment for locally recurrent thyroid cancer, the therapeutic success is 100%, with a serum thyroglobulin reduction of 71.6% [Citation16]. The revised American Thyroid Association guideline suggests ultrasound-guided RFA as a useful alternative to surgical resection of metastatic thyroid cancer [Citation22].
Although RFA has been shown to be an excellent treatment modality for benign thyroid nodules and recurrent thyroid cancers, several RFA-associated complications have been reported, including voice change, skin burns, haematoma formation, and transient hyperthyroidism [Citation5,Citation14,Citation19,Citation20,Citation23–27]. After a Korean group published a large population multicenter study of complications of thyroid RFA in 2012 [Citation24], many studies on thyroid RFA have been published. Although the types and incidence rates of complications have been assessed, previous studies were limited by their retrospective designs, small numbers of patients, and lack of systematic evaluation. Therefore, it is an opportune time to collect current data regarding the use of RFA for treating thyroid disease.
To the best of our knowledge, our present systematic review and meta-analysis is the first study to assess the safety of RFA for the treatment of thyroid nodules. We evaluate the types and incidence rates of complications associated with RFA for the treatment of benign thyroid nodules and recurrent thyroid cancers and compare the results with those of other treatment options for thyroid nodules.
Materials and methods
Literature search strategy
A computerised search of the MEDLINE, EMBASE, and Library of Cochrane databases was performed to identify relevant original literature on complications of RFA for the treatment of benign thyroid nodules or recurrent thyroid cancers. The following search terms were used: (thyroid nodule OR thyroid cancer OR thyroid carcinoma OR thyroid malignancy) AND (radiofrequency ablation OR radio-frequency ablation OR RF ablation OR RFA). Our search was limited to studies published in English up to 12 July 2016. To identify other suitable articles, the bibliographies of the articles were screened.
Inclusion criteria
Studies (or subsets of studies) investigating RFA for the treatment of benign thyroid nodules or recurrent thyroid cancers were eligible for inclusion. Studies (or subsets of studies) satisfying all of the following criteria were included: (a) population: patients underwent image-guidance RFA for treatment of a benign thyroid nodule or recurrent thyroid cancer in the neck; (b) benign thyroid nodule or recurrent thyroid cancer was confirmed by ultrasound-guided fine-needle aspiration or core needle biopsy prior to RFA; and (c) outcomes: complication rates were reported. For studies that met all of the inclusion criteria but had insufficient outcome data, attempts were made to contact the study authors to obtain these data.
Exclusion criteria
The following exclusion criteria were applied: (a) review articles, case reports, editorials, letters, comments, and conference proceedings; (b) studies with overlapping patients and data; (c) studies with no information on complications or side effects; and (d) studies combining other therapies with RFA (i.e. ethanol ablation [EA], radioactive iodine). Two reviewers (S.R.C. and C.H.S.) with 4 years of experience in performing systematic reviews and meta-analysis independently selected the studies in the literature using a standardised form. If the two reviewers could not reach consensus, the study was reviewed by a third reviewer (J.H.B.) with 24 years of clinical experience in performing thyroid ultrasound and 15 years of experience in performing thyroid RFA.
Data extraction
We extracted the following data from the selected studies onto standardised data forms: (a) study characteristics: authors, year of publication, hospital or medical school, duration of patient recruitment, sample size, and study design; (b) patients’ demographic and clinical characteristics: mean age, sex, thyroid function status, RF techniques (e.g. use of transisthmic approach, moving shot technique, or hydrodissection; mean ablation time and number; mean RF power and duration; type of RF electrode; gauge of RF electrode), clinical experience of operator, nodule characteristics (size, composition, and pathology), and (c) major and minor complications. One reviewer (S.R.C.) extracted the data from the selected studies, and two reviewers (C.H.S. and J.H.B.) verified the accuracy of the extracted data. Complication rates were calculated per person.
Definitions of complications
Major and minor complications were as defined by the Society of Interventional Radiology [Citation28,Citation29]. A major complication was defined as one that, if left untreated, might threaten the patient’s life, lead to substantial morbidity or disability, or result in a lengthened hospital stay. In this study, major complications included a transient or permanent voice change, rupture of a treated nodule, hypothyroidism, brachial plexus injury, Horner’s syndrome, shoulder weakness, and severe Graves’ ophthalmopathy. All other complications, such as haematoma, vomiting, skin burns, and severe pain that need medication to relieve it, were considered minor. Side effects were defined as untoward consequences that did not require therapy or prescription medications; they included mild, transient postprocedural pain, heat sensation, and neck swelling and discomfort.
Quality assessment
The methodological quality of the included studies was independently assessed by the two study reviewers (S.R.C. and C.H.S.) using tailored questionnaires and criteria provided by the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) [Citation30].
Data synthesis
The pooled proportions of overall and major complications were used as the main indices for this meta-analysis. We also carried out subgroup meta-regression analysis according to the benignity of the nodule and the origin of the study. Meta-analytic pooling was conducted by the inverse variance method for calculating weights. Pooled proportions and 95% confidence intervals (CIs) were obtained by Der Simonian-Laird random-effects modelling [Citation31–33].
Heterogeneity among studies was determined by the χ2 statistic for pooled estimates (p < 0.05, indicating significant heterogeneity) and the inconsistency index I2 (0–40%, may not be important; 30–60%, may represent moderate heterogeneity; 50–90%, may represent substantial heterogeneity; 75–100%, may represent considerable heterogeneity) [Citation34]. Publication bias was visually assessed by funnel plots, and statistical significance was evaluated by Egger’s test [Citation35]. We used R version 3.3.1 (The R Foundation for Statistical Computing) with the “metafor” package to perform the statistical analyses.
Results
Literature search
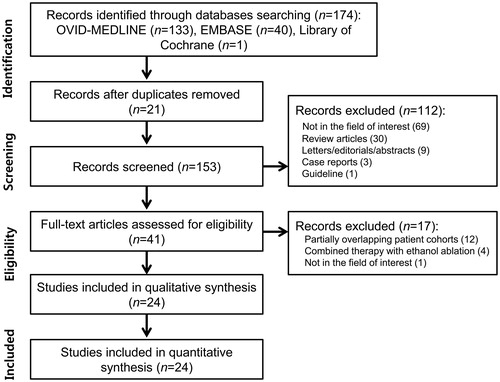
Our study selection process is illustrated in . The literature search of the Ovid-MEDLINE, EMBASE, and Library of Cochrane databases generated 174 initial articles, 21 of which were eliminated after removal of duplicates. Of the remaining 153 articles, we excluded 112 after reviewing the titles and abstracts: 69 articles that were not in the field of interest; 30 review articles; 9 letters, editorials, or conference abstracts; 3 case reports; and 1 guideline. The full texts of the remaining 41 articles were retrieved. An additional search of the bibliographies of these articles identified no further eligible studies. Of these 41 articles, 17 were further excluded after a review of the full text: 12 studies with partially overlapping patient cohorts [Citation6,Citation7,Citation36–45], 4 studies with combined RFA/EA therapy [Citation46–49], and 1 study that was not in the field of interest [Citation50]. In the case of patient overlap, we included the study with the larger number of patients. Finally, 24 eligible studies, with a total sample size of 2421 patients and 2786 thyroid nodules, were included in our systematic review and meta-analysis [Citation5,Citation8,Citation11,Citation12,Citation14,Citation15,Citation17–19,Citation21,Citation23–27,Citation51–58].
Characteristics of the included studies
The characteristics of the 24 included studies are detailed in . The 24 original articles included 12 retrospective studies [Citation5,Citation11,Citation14,Citation17–20,Citation24,Citation26,Citation27,Citation55,Citation57], 9 prospective studies [Citation8,Citation12,Citation13,Citation15,Citation23,Citation25,Citation52,Citation53,Citation56], and 3 studies with unclear study design [Citation21,Citation51,Citation58]. The QUADAS-2 quality of the included studies was moderate overall, and all of the studies satisfied at least four of the seven items (. All included studies had clear descriptions of the RF technique and equipment. Nine studies had a high risk of bias in patient selection because of the retrospective nature of the studies [Citation5,Citation11,Citation14,Citation17,Citation24,Citation26,Citation27,Citation55,Citation57], and three studies had an unclear risk of bias [Citation21,Citation51,Citation58]. The time period between imaging and the reference standard was mentioned in all studies, and details of the complications were clearly described in all studies.
Figure 2. Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criteria for the included studies.
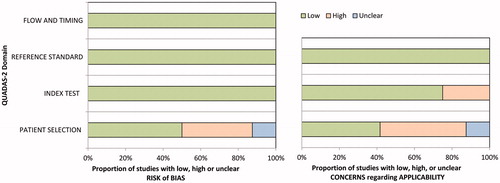
Table 1. Characteristics of the included studies.
There were more case series from Asian countries (n = 14; South Korea 10, China 4) than from non-Asian countries (n = 10; Italy 6, Turkey 2, Germany 1, United States 1). The mean patient age was 49.1 years (range: 19–72.5 years). There were 16 studies of RFA for benign thyroid nodules, which included 2540 nodules in 2245 patients [Citation5,Citation8,Citation11–15,Citation23–25,Citation51,Citation53,Citation55–58], and 8 studies of recurrent thyroid cancer, which included 246 nodules in 176 patients [Citation17–21,Citation26,Citation27,Citation52]. The 2540 benign thyroid nodules included 118 hot nodules on thyroid scan. The mean volume of the treated nodules was 10.3 ml (range: 0.05–27.7 ml). The RFA electrodes used in the studies were an expandable needle in three studies (four hooks in two studies and nine hooks in one study) and a straight-type internally cooled electrode in 21 studies with 0.5- to 2-cm active tips. Of the 24 studies, 20 used a moving shot technique [Citation59]. The mean number of RFA sessions was less than 1.5 in 91.7% of the studies (22 of 24 studies), and the mean duration of RFA was 7.3 min (range: 2.7–12.6 min). The experience of the RFA operator was noted in 10 studies by various descriptions (2–18 years of experience, 10–3000 cases, and well-trained operator in 1 study).
Pooled proportions of overall and major RFA complications
Eighty-nine complications of RFA were reported among 2786 thyroid nodules in 2421 patients. The overall complication rate was 2.38% (95% CI: 1.42%–3.34%; I2 = 21.79%) (, ). In the funnel plots and Egger’s test, a significant publication bias was noted for overall RFA complications (p < 0.0001) (). The rate of major complications was 1.35% (95% CI: 0.89%–1.81%; I2 = 1.24%) (). A significant publication bias was also noted for major complications (p = 0.0049) (). There were no heterogeneities in either overall or major complications.
Figure 3. Forest plots to show the pooled proportions of (A) overall complications and (B) major complications of radiofrequency ablation.
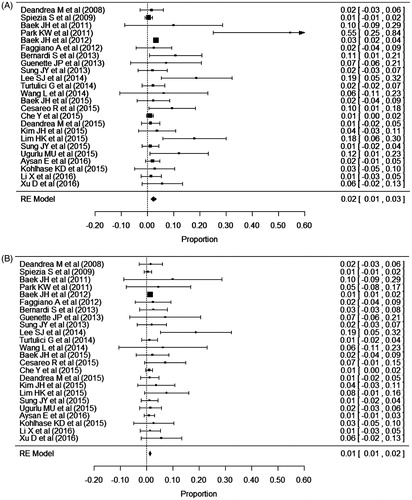
Figure 4. Funnel plot to assess publication bias of (A) overall complications and (B) major complications of radiofrequency ablation.
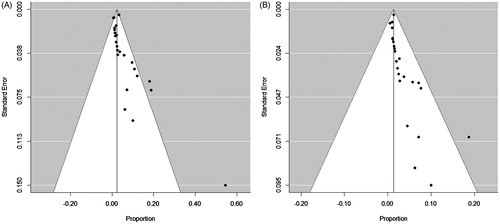
Table 2. Summary of the meta-analytic pooled proportions of overall and major RFA complications.
Major complications
Forty-one major complications were reported, including voice change, nodule rupture, permanent hypothyroidism, and brachial plexus injury. Thirty-one patients had a transient voice change [Citation5,Citation14,Citation19,Citation20,Citation23–27]; 12 patients complained of a voice change immediately after or during the RFA, and 25 patients recovered within 2–3 months. Four patients (three with recurrent cancers and one with a benign nodule) had a permanent voice change [Citation17,Citation18,Citation20,Citation25]. Thus, the overall incidence of voice change (transient or permanent) after RFA was 1.44% (35/2421). The incidence of voice change was higher in patients with recurrent thyroid cancers (7.95%, 14/176) than in patients with benign thyroid nodules (0.94%, 21/2245).
Four patients were diagnosed with nodule rupture after RFA (0.17%, 4/2421), including one patient with rupture and abscess formation [Citation14,Citation24]. The nodule ruptures occurred 7, 22, 30, and 50 days after treatment. Two patients recovered after conservative treatment and one patient recovered after antibiotic treatment; the one patient with abscess formation underwent lobectomy. Permanent hypothyroidism occurred in one patient [Citation24]. One case of hypothyroidism was detected 6 months after RFA with a complaint of gradual neck bulging. Before and after RFA, this patient’s serum concentration of antithyroid peroxidase antibody was more than 1000 IU/ml. One patient had a brachial plexus injury [Citation24]. She complained of numbness and decreased sensation in the fourth and fifth fingers of her left hand and gradually recovered during the next 2 months.
Minor complications
Forty-eight minor complications were reported, including pain, haematoma, vomiting, skin burns, and transient thyroiditis. Sixteen patients complained of pain during or after RFA treatment [Citation5,Citation12,Citation25,Citation26,Citation52]. Six patients did not complete the treatment because of severe pain, and the remainder required additional treatment to reduce pain after RFA. Fifteen patients had haematomas, which were located in the perithyroidal, subcapsular, and intranodular areas [Citation24]. Most haematomas disappeared completely within 1 or 2 weeks. Nine patients had vomiting after RFA [Citation24]. Treatment with antiemetics resulted in improvement within 1–2 days. Various degrees of skin burns occurred in seven patients [Citation23,Citation24,Citation52,Citation55]; six patients had a first-degree burn and one had a second-degree burn. All patients with burns recovered within 1 month. One patient had painless thyroiditis with thyrotoxicosis 3 months after the procedure, which resolved spontaneously within 30 days [Citation5].
Subgroup analyses
The summary of subgroup analyses for the primary outcomes is presented in . For malignant nodules, the overall complication rate was 10.98% (95% CI: 4.82%–17.15%), which was significantly higher than the rate of 2.11% for benign nodules (95% CI: 1.15%–3.06%) (p = 0.0011). The rate of major complications of 6.71% (95% CI: 3.05%–10.36%) for malignant nodules was also significantly higher than the rate of 1.27% (95% CI: 0.81%–1.73%, p = 0.0038) for benign nodules. There were no significant differences between studies conducted in Asian and non-Asian countries in the rates of overall complications (p = 0.7286) or major complications (p = 0.5856).
Table 3. Subgroup analysis according to nodule characteristics and origin of the study.
Discussion
In the present meta-analysis, the overall complication rate of RFA was 2.38% (95% CI: 1.42%–3.34%), and the rate of major complications was 1.35% (95% CI: 0.89%–1.81%). No patients had any life-threatening RFA-related complications. Only five patients (0.21%, 5/2421) had sequelae after treatment complications: one with thyroid lobectomy due to nodule rupture with abscess formation and four with permanent vocal cord palsy. Considering these results, we suggest that RFA is a safe modality for the treatment of benign thyroid nodules and recurrent thyroid cancers.
In the subgroup analysis, the rate of complications after RFA was significantly higher for malignant thyroid nodules than for benign thyroid nodules. This may be due to the absence of a safety area around recurrent tumours, whereas benign thyroid nodules are usually surrounded by normal thyroid parenchyma. Other possible variables affecting the complication rate of RFA are the origin of the study, the operator’s experience, the duration or number of RFA session, and the type of equipment. In the subgroup analysis, there were no significant differences in complication rates between Asian and non-Asian studies. Information about the operator’s experience was available for 11 of the 24 studies, but the descriptions were too variable to permit comparisons among studies. All included studies used linear electrodes except three studies (four-hook electrode in two studies and nine-hook electrode in one study), and the majority of studies (22 of 24 studies) reported that the mean number of RFA sessions was less than 1.5. Thus, the type of equipment and mean the numbers of RFA sessions are unlikely to significantly affect the complication rate. Information on the mean duration of RFA was available for 12 of the 24 studies the mean duration was 7.8 min (range: 2.7–12.6 min).
Voice change is the most common major complication of RFA. We identified 35 patients (35/2421, 1.45%) with voice change. However, only four patients had a permanent voice change. The incidence of voice change after RFA is higher for recurrent thyroid cancers (7.95%, 14/176) than for benign thyroid nodules (0.94%, 21/2245), possibly because of the absence of a safety area around recurrent tumours. Voice changes are likely due to dysfunction of the recurrent laryngeal nerve, which can be caused by both thermal injury and haemorrhage [Citation24]. Thermal injury to the recurrent laryngeal nerve may be prevented by using the moving shot technique and the transisthmic approach in benign nodules [Citation38–40,Citation46,Citation60]. Use of the moving shot technique, the transisthmic approach, and undertreatment of the danger triangle may minimise recurrent laryngeal nerve injury. Another possible cause of voice changes in patients undergoing RFA is vagus nerve damage [Citation61]. The vagus nerve is located within the carotid sheath, usually between the common carotid artery and the internal jugular vein. However, it may also be located adjacent to the thyroid gland, or a bulging thyroid nodule may alter the location of the vagus nerve [Citation61]. Therefore, when performing RFA, operators should be aware of the location of the vagus nerve and any possible variations in its route.
Laser ablation (LA) is another thermal ablation technique used to treat thyroid lesions. A recent large retrospective study reported that 8 of 1531 patients (0.5%) had a voice change after LA of a benign thyroid nodule [Citation62], which is similar to the findings of our meta-analysis (0.94%, 21/2245). LA was also associated with minor complications of haematoma in eight patients (0.5%) and skin burn in one patient (0.1%), rates similar to those of this study (0.54% and 0.25%, respectively) [Citation62]. One meta-analysis of the efficacy of LA and RFA demonstrated a significantly higher volume reduction after RFA compared with LA (RFA: 77.8%, 95% CI: 67.7–88.0 vs. LA: 49.5%, 95% CI: 26.7–72.4), despite the smaller number of treatment sessions [Citation63]. Possible explanation for the superior efficacy of RFA is the use of the moving-shot technique and straight, internally cooled electrodes, which allow the entire tumour to be treated safely and effectively [Citation40,Citation63]. However, this meta-analysis study limited by small number of included studies and majority of study about LA were from same author. Recently, Mauri et al. reported a study directly comparing the two techniques performed by the same operator [Citation64]. They found that RFA and LA are similarly feasible, safe, and effective in treating benign thyroid nodules when performed by the same equipment [Citation64]. For the treatment of metastatic thyroid carcinoma in the neck, only three studies have been reported [Citation65–67]. Of total 54 lymph nodes which treated with LA, two cases have transient voice change. Other major complications including permanent voice change have not been reported.
Voice changes after EA are very rare [Citation53,Citation68–72]. Although the exact incidence of voice changes after EA is unclear, because no systematic reviews have examined this question, it is lower than the incidence of voice changes after RFA [Citation16]. A possible mechanism for voice changes is leakage of ethanol outside the thyroid gland causing damage to the recurrent laryngeal nerve [Citation56]. Given that the therapeutic efficacy of EA is not inferior to that of RFA for the treatment of cystic or predominantly cystic thyroid nodules and that EA is simpler and less expensive than RFA [Citation53,Citation56], EA could be used as first-line treatment for cystic or predominantly cystic thyroid nodules [Citation42,Citation53]. However, in predominantly solid nodules, the treatment outcome of EA is relatively poor, with a success rate of 38.3%, which is believed to be due to poor diffusion of ethanol in solid tissue and early washout of ethanol because of the abundant vascularity of solid nodules [Citation73,Citation74]. A few studies have been reported about the efficacy and safety of EA for treatment of metastatic thyroid carcinoma in the neck [Citation18,Citation37,Citation72,Citation75–78]. Of seven original articles, transient voice change was occurred in two cases [Citation76,Citation77]. Other major complications including permanent voice change have not been reported. The rate of local progression after ablation is known to be higher in EA than RFA (23.8% vs. 0%).
Because of the complexity of thyroid and neck anatomy, surgery in this region has a relatively high incidence of recurrent laryngeal nerve injury. The reported rate of permanent recurrent laryngeal nerve injury after conventional total thyroidectomy ranges from 0% to 4%, whereas the incidence of transient recurrent laryngeal nerve injury ranges from 0% to 16% [Citation79]. The rate of permanent recurrent laryngeal nerve injury is similar to that of subtotal thyroidectomy (0.6%–2.1%) [Citation80] and is higher after repeat surgery (0–12%) [Citation81,Citation82]. Thus, voice problems seem to be more frequent after surgery than after minimally invasive therapies. Other complications, such as hypocalcaemia and hypothyroidism, are also common. The reported rate of permanent hypocalcaemia ranges from 0% to 3.1%, whereas that of hypothyroidism is as high as 75% [Citation14,Citation79]. Parathyroid injury during RFA has not yet been reported, and hypothyroidism has been reported in only one patient [Citation24].
Rupture of the treated thyroid nodule is the second most common major complication of RFA (0.17%, 4/2421). It may present as sudden neck bulging and pain at the RFA site. Ultrasound or computed tomography usually shows breakage of the thyroid capsule with bulging of the tumour into the anterior neck. Rupture can occur as a result of expansion of the tumour from delayed haemorrhage or a tear in the tumour wall or as a result of postprocedural massaging of the neck [Citation41]. Initial conservative management with simple compression and antibiotics and/or analgesics may suffice. However, drainage or surgical excision may be required if symptoms progress [Citation24,Citation41].
Other possible serious complications of RFA have been suggested, including injury to the oesophagus, trachea, and other nerves (spinal accessory, sympathetic ganglion, and phrenic nerves) [Citation60,Citation83]. Horner’s syndrome after EA has been reported as a consequence of damage to the middle sympathetic cervical ganglion from leakage of injected ethanol [Citation84,Citation85]. The spinal accessory nerve or the phrenic nerve can be damaged during ablation of metastatic lymph nodes located in the lateral neck. To prevent these potential serious complications, the operator should know the anatomy of the neck and always trace the tip of the electrode during RFA [Citation61,Citation86,Citation87]. In addition, for treatment of recurrent tumours close to the neck nerves, hydrodissection with continuous fluid infusion has been introduced as a safe technique to prevent thermal damage to the nerve [Citation26,Citation83].
This study has some limitations. First, other variables that might affect the complication rate of RFA, such as operator experience and mean ablation time, were not evaluated by subgroup analysis because it was difficult to extract accurate data for our meta-analysis. Second, although we classified complications according to the revised American Thyroid Association guideline, some classifications may be uncertain because of the retrospective nature of the studies. Third, we only included articles in English, which could result in overestimation or underestimation of the results. Finally, we excluded grey literature, such as letters, case reports, conference abstracts, and unpublished data, which may have caused a publication bias.
In conclusion, our present systematic review with meta-analysis provides a thorough summary of the current literature on complications after RFA for benign thyroid nodules and recurrent thyroid cancers. The results suggest that RFA has an acceptable complication rate for the treatment of these conditions.
Declaration of interest
No potential conflict of interest was reported by the authors.
References
- Choi H, Loyer EM, DuBrow RA, et al. (2001). Radio-frequency ablation of liver tumors: assessment of therapeutic response and complications. Radiographics 21:S41–54.
- Dupuy DE, Goldberg SN. (2001). Image-guided radiofrequency tumor ablation: challenges and opportunities – part II. J Vasc Interv Radiol 12:1135–48.
- Lencioni R, Cioni D, Bartolozzi C. (2001). Percutaneous radiofrequency thermal ablation of liver malignancies: techniques, indications, imaging findings, and clinical results. Abdom Imaging 26:345–60.
- Livraghi T, Solbiati L, Meloni MF, et al. (2003). Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226:441–51.
- Bernardi S, Dobrinja C, Fabris B, et al. (2014). Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol 2014:934595.
- Huh JY, Baek JH, Choi H, et al. (2012). Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session – prospective randomized study. Radiology 263:909–16.
- Kim YS, Rhim H, Tae K, et al. (2006). Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 16:361–7.
- Spiezia S, Garberoglio R, Milone F, et al. (2009). Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 19:219–25.
- Russ G. (2016). Risk stratification of thyroid nodules on ultrasonography with the French TI-RADS: description and reflections. Ultrasonography 35:25–38.
- Shin JH, Baek JH, Chung J, et al. (2016). Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 17:370–95.
- Sung JY, Baek JH, Jung SL, et al. (2015). Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid 25:112–17.
- Ugurlu MU, Uprak K, Akpinar IN, et al. (2015). Radiofrequency ablation of benign symptomatic thyroid nodules: prospective safety and efficacy study. World J Surg 39:961–8.
- Faggiano A, Ramundo V, Assanti AP, et al. (2012). Thyroid nodules treated with percutaneous radiofrequency thermal ablation: a comparative study. J Clin Endocrinol Metab 97:4439–45.
- Che Y, Jin S, Shi C, et al. (2015). Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. Am J Neuroradiol 36:1321–5.
- Deandrea M, Sung JY, Limone P, et al. (2015). Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid 25:890–6.
- Suh CH, Baek JH, Choi YJ, Lee JH. (2016). Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid 26:420–8.
- Baek JH, Kim YS, Sung JY, et al. (2011). Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. Am J Roentgenol 197:W331–6.
- Guenette JP, Monchik JM, Dupuy DE. (2013). Image-guided ablation of postsurgical locoregional recurrence of biopsy-proven well-differentiated thyroid carcinoma. J Vasc Interv Radiol 24:672–9.
- Kim JH, Yoo WS, Park YJ, et al. (2015). Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology 276:909–18.
- Lee SJ, Jung SL, Kim BS, et al. (2014). Radiofrequency ablation to treat loco-regional recurrence of well-differentiated thyroid carcinoma. Korean J Radiol 15:817–26.
- Wang L, Ge M, Xu D, et al. (2014). Ultrasonography-guided percutaneous radiofrequency ablation for cervical lymph node metastasis from thyroid carcinoma. J Cancer Res Ther 10:C144–9.
- Haugen BR, Alexander EK, Bible KC, et al. (2016). 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133.
- Aysan E, Idiz UO, Akbulut H, Elmas L. (2016). Single-session radiofrequency ablation on benign thyroid nodules: a prospective single center study: radiofrequency ablation on thyroid. Langenbecks Arch Surg 401:357–63.
- Baek JH, Lee JH, Sung JY, et al. (2012). Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology 262:335–42.
- Cesareo R, Pasqualini V, Simeoni C, et al. (2015). Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab 100:460–6.
- Lim HK, Baek JH, Lee JH, et al. (2015). Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol 25:163–70.
- Xu D, Wang L, Long B, et al. (2016). Radiofrequency ablation for postsurgical thyroid removal of differentiated thyroid carcinoma. Am J Transl Res 8:1876–85.
- Burke DR, Lewis CA, Cardella JF, et al. (2003). Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol 14:S243–6.
- Lewis CA, Allen TE, Burke DR, et al. (1997). Quality improvement guidelines for central venous access. The Standards of Practice Committee of the Society of Cardiovascular & Interventional Radiology. J Vasc Interv Radiol 8:475–9.
- Whiting PF, Rutjes AW, Westwood ME, et al. (2011). QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–36.
- Suh CH, Park SH. (2016). Successful publication of systematic review and meta-analysis of studies evaluating diagnostic test accuracy. Korean J Radiol 17:5–6.
- Lee J, Kim KW, Choi SH, et al. (2015). Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol 16:1188–96.
- Kim KW, Lee J, Choi SH, et al. (2015). Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-Part I. General guidance and tips. Korean J Radiol 16:1175–87.
- Higgins J, Green S. (2011). Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration. Available from: http://handbook.cochrane.org
- Egger M, Davey Smith G, Schneider M, Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–34.
- Dupuy DE, Monchik JM, Decrea C, Pisharodi L. (2001). Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery 130:971–7.
- Monchik JM, Donatini G, Iannuccilli J, Dupuy DE. (2006). Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg 244:296–304.
- Jeong WK, Baek JH, Rhim H, et al. (2008). Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 18:1244–50.
- Baek JH, Moon WJ, Kim YS, et al. (2009). Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg 33:1971–7.
- Baek JH, Kim YS, Lee D, et al. (2010). Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. Am J Roentgenol 194:1137–42.
- Shin JH, Jung SL, Baek JH, Kim JH. (2011). Rupture of benign thyroid tumors after radio-frequency ablation. Am J Neuroradiol 32:2165–9.
- Sung JY, Kim YS, Choi H, et al. (2011). Optimum first-line treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? Am J Roentgenol 196:W210–4.
- Nakamura S, Nouso K, Kobayashi Y, et al. (2013). The diagnosis of hypovascular hepatic lesions showing hypo-intensity in the hepatobiliary phase of Gd-EOB-DTPA-enhanced MR imaging in high-risk patients for hepatocellular carcinoma. Acta Med Okayama 67:239–44.
- Lim HK, Lee JH, Ha EJ, et al. (2013). Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol 23:1044–9.
- Ji Hong M, Baek JH, Choi YJ, et al. (2015). Radiofrequency ablation is a thyroid function-preserving treatment for patients with bilateral benign thyroid nodules. J Vasc Interv Radiol 26:55–61.
- Lee JH, Kim YS, Lee D, et al. (2010). Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg 34:1488–93.
- Jang SW, Baek JH, Kim JK, et al. (2012). How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol 81:905–10.
- Kim DW. (2012). Sonography-guided ethanol ablation of a remnant solid component after radio-frequency ablation of benign solid thyroid nodules: a preliminary study. Am J Neuroradiol 33:1139–43.
- Yoon HM, Baek JH, Lee JH, et al. (2014). Combination therapy consisting of ethanol and radiofrequency ablation for predominantly cystic thyroid nodules. Am J Neuroradiol 35:582–6.
- Long B, Li L, Yao L, et al. (2015). Combined use of radioiodine therapy and radiofrequency ablation in treating postsurgical thyroid remnant of differentiated thyroid carcinoma. J Cancer Res Ther 11:244. Suppl:C7.
- Deandrea M, Limone P, Basso E, et al. (2008). US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol 34:784–91.
- Park KW, Shin JH, Han BK, et al. (2011). Inoperable symptomatic recurrent thyroid cancers: preliminary result of radiofrequency ablation. Ann Surg Oncol 18:2564–8.
- Sung JY, Baek JH, Kim KS, et al. (2013). Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology 269:293–300.
- Suh CH, Kim SY, Kim KW, et al. (2014). Determination of normal hepatic elasticity by using real-time shear-wave elastography. Radiology 271:895–900.
- Turtulici G, Orlandi D, Corazza A, et al. (2014). Percutaneous radiofrequency ablation of benign thyroid nodules assisted by a virtual needle tracking system. Ultrasound Med Biol 40:1447–52.
- Baek JH, Ha EJ, Choi YJ, et al. (2015). Radiofrequency versus ethanol ablation for treating predominantly cystic thyroid nodules: a randomized clinical trial. Korean J Radiol 16:1332–40.
- Kohlhase KD, Korkusuz Y, Groner D, et al. (2016). Bipolar radiofrequency ablation of benign thyroid nodules using a multiple overlapping shot technique in a 3-month follow-up. Int J Hyperthermia 32:511–16.
- Li XL, Xu HX, Lu F, et al. (2016). Treatment efficacy and safety of ultrasound-guided percutaneous bipolar radiofrequency ablation for benign thyroid nodules. Br J Radiol 89:20150858.
- Ha EJ, Baek JH, Lee JH. (2014). Moving-shot versus fixed electrode techniques for radiofrequency ablation: comparison in an ex-vivo bovine liver tissue model. Korean J Radiol 15:836–43.
- Shin JH, Baek JH, Ha EJ, Lee JH. (2012). Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol 2012:919650.
- Ha EJ, Baek JH, Lee JH. (2015). Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol 16:749–66.
- Pacella CM, Mauri G, Achille G, et al. (2015). Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab 100:3903–10.
- Ha EJ, Baek JH, Kim KW, et al. (2015). Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J Clin Endocrinol Metab 100:1903–11.
- Mauri G, Cova L, Monaco CG, et al. (2017). Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia 33:295–9.
- Mauri G, Cova L, Tondolo T, et al. (2013). Percutaneous laser ablation of metastatic lymph nodes in the neck from papillary thyroid carcinoma: preliminary results. J Clin Endocrinol Metab 98:E1203–7.
- Papini E, Bizzarri G, Bianchini A, et al. (2013). Percutaneous ultrasound-guided laser ablation is effective for treating selected nodal metastases in papillary thyroid cancer. J Clin Endocrinol Metab 98:E92–7.
- Mauri G, Cova L, Ierace T, et al. (2016). Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol 39:1023–30.
- Kim DW. (2014). Usefulness of two-stage ethanol ablation in the treatment of benign predominantly cystic thyroid nodules. Endocr Pract 20:548–55.
- Lee SJ, Ahn IM. (2005). Effectiveness of percutaneous ethanol injection therapy in benign nodular and cystic thyroid diseases: long-term follow-up experience. Endocr J 52:455–62.
- Kim DW, Rho MH, Park HJ, Kwag HJ. (2012). Ultrasonography-guided ethanol ablation of predominantly solid thyroid nodules: a preliminary study for factors that predict the outcome. Br J Radiol 85:930–6.
- Cho YS, Lee HK, Ahn IM, et al. (2000). Sonographically guided ethanol sclerotherapy for benign thyroid cysts: results in 22 patients. Am J Roentgenol 174:213–16.
- Heilo A, Sigstad E, Fagerlid KH, et al. (2011). Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J Clin Endocrinol Metab 96:2750–5.
- Kim JH, Lee HK, Lee JH, et al. (2003). Efficacy of sonographically guided percutaneous ethanol injection for treatment of thyroid cysts versus solid thyroid nodules. Am J Roentgenol 180:1723–6.
- Basu N, Dutta D, Maisnam I, et al. (2014). Percutaneous ethanol ablation in managing predominantly cystic thyroid nodules: an eastern India perspective. Indian J Endocrinol Metab 18:662–8.
- Hay ID, Lee RA, Davidge-Pitts C, et al. (2013). Long-term outcome of ultrasound-guided percutaneous ethanol ablation of selected “recurrent” neck nodal metastases in 25 patients with TNM stages III or IVA papillary thyroid carcinoma previously treated by surgery and 131I therapy. Surgery 154:1448–54. discussion 54-5.
- Kim BM, Kim MJ, Kim E-K, et al. (2008). Controlling recurrent papillary thyroid carcinoma in the neck by ultrasonography-guided percutaneous ethanol injection. Eur Radiol 18:835–42.
- Lim CY, Yun JS, Lee J, et al. (2007). Percutaneous ethanol injection therapy for locally recurrent papillary thyroid carcinoma. Thyroid 17:347–50.
- Lewis BD, Hay ID, Charboneau JW, et al. (2002). Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. Am J Roentgenol 178:699–704.
- Lang BH, Wong CK, Tsang JS, et al. (2014). A systematic review and meta-analysis comparing surgically-related complications between robotic-assisted thyroidectomy and conventional open thyroidectomy. Ann Surg Oncol 21:850–61.
- Padur AA, Kumar N, Guru A, et al. (2016). Safety and effectiveness of total thyroidectomy and its comparison with subtotal thyroidectomy and other thyroid surgeries: a systematic review. J Thyroid Res 2016:7594615.
- Kim MK, Mandel SH, Baloch Z, et al. (2004). Morbidity following central compartment reoperation for recurrent or persistent thyroid cancer. Arch Otolaryngol Head Neck Surg 130:1214–16.
- Al-Saif O, Farrar WB, Bloomston M, et al. (2010). Long-term efficacy of lymph node reoperation for persistent papillary thyroid cancer. J Clin Endocrinol Metab 95:2187–94.
- Shin JE, Baek JH, Lee JH. (2013). Radiofrequency and ethanol ablation for the treatment of recurrent thyroid cancers: current status and challenges. Curr Opin Oncol 25:14–19.
- Pishdad GR, Pishdad P, Pishdad R. (2011). Horner’s syndrome as a complication of percutaneous ethanol treatment of thyroid nodule. Thyroid 21:327–8.
- Bartos M, Pomorski L, Narebski J. (1999). The treatment of solitary thyroid nodules in non-toxic goiter with 96% ethanol injections. Wiad Lek 52:432–40.
- Shin JE, Baek JH, Ha EJ, et al. (2015). Ultrasound features of middle cervical sympathetic ganglion. Clin J Pain 31:909–13.
- Hong MJ, Baek JH, Kim DY, et al. (2016). Spinal accessory nerve: ultrasound findings and correlations with neck lymph node levels. Ultraschall Med 37:487–91.