Abstract
Purpose: To evaluate the safety and efficacy of the clinical application of hydrodissection under ultrasound (US) guidance for assisting percutaneous microwave ablation (MWA) for the treatment of renal cell carcinomas (RCCs) adjacent to the intestinal tract.
Materials and methods: From April 2014 to December 2016, clinical data from 24 patients with 25 RCCs were retrospectively analysed. Percutaneous MWA under the assistance of US-guided hydrodissection were performed to treat RCCs with a mean maximal diameter of 3.80 ± 1.60 cm because the distance between the index tumour and the adjacent intestinal tracts were less than 0.5 cm on imaging. The separation success rate of the hydrodissection, technique efficacy rate of the MWA, local tumour progression (LTP), complications, and renal function including serum creatinine (Cr) and blood urea nitrogen (BUN) were assessed.
Results: In total, 28 sessions of hydrodissection and MWA procedures were performed (one procedure in 22 patients and two procedures in 3 patients), and the separation success rate was 100% (28/28). The technique efficacy rate was 100% (25/25), and no LTP occurred. One patient exhibited a major complication (4.2%). Minor complications in 5 patients (20.8%) and side effects in 12 patients (50.0%) occurred. Compared with the pre-MWA levels, there were no significant differences in serum Cr and BUN 1-day post-MWA and at the last follow-up.
Conclusions: US-guided hydrodissection assistance for percutaneous MWA could be a safe and effective alternative for selected patients with RCCs adjacent to the intestinal tracts and can achieve good local tumour control and renal function preservation.
Introduction
Renal cell carcinoma (RCC) is the second most common malignant tumour of the urinary system, accounting for approximately 1.6% of all new cancer cases and 0.8% of all deaths in China [Citation1]. With the development of modern imaging technology including ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI), the proportion of asymptomatic sporadic RCCs has exhibited an upward trend and the mean maximum diameter of new RCCs has been reduced to less than 4 cm [Citation2]; therefore, clinical therapeutic methods used to treat patients with RCC have changed from traditional radical nephrectomy to nephron-sparing surgery for the majority of patients. Partial nephrectomy, which is one of the most common nephron-sparing surgeries, can achieve good local tumour control and renal function preservation and has become the standard treatment for patients with RCC at stage T1 [Citation3,Citation4]. For patients with RCC who may not be surgical candidates because of their advanced age [Citation5] or comorbidities [Citation6], nephron-sparing, image-guided thermal ablation (TA), such as radiofrequency ablation (RFA), cryoablation, and microwave ablation (MWA), have become the first-line treatment [Citation7]. TA treatment in selected patients with RCC has demonstrated good long-term curative effects on local tumour control that are similar to those observed after partial nephrectomy [Citation8], and is considered a safe and effective minimally invasive treatment for RCCs in patients with a solitary kidney [Citation9] or renal dysfunction [Citation10].
Compared with partial nephrectomy, TA treatment exhibits a low incidence of major complications in the treatment of RCCs [Citation4], but intestinal injury remains a major complication after TA [Citation11], including RFA [Citation12], cryoablation [Citation13], and MWA [Citation14], due to the anatomical features of the kidneys. The risk of intestinal injury is 5-fold higher following ablation of tumours located in the ventral side of the kidney than that following ablation of tumours located in the dorsal side of the kidney [Citation15]. Patients suffering from intestinal injury following TA may require an unexpected nephrectomy and colostomy, and their quality of life and renal function could be affected. Thus, it is necessary to explore various methods that could effectively be employed to minimise the occurrence of bowel injury complications after ablation. Using RFA to treat patients with RCCs, hydrodissection was shown to be beneficial for reducing intestinal injury complications [Citation16]. In this study, the safety and efficacy of US-guided hydrodissection in assisting percutaneous MWA for the treatment of RCCs adjacent to intestinal tracts were retrospectively assessed.
Materials and methods
Patients
From April 2014 to December 2016, clinical data from 24 patients, including 18 males and 6 females with a mean age of 68.3 ± 9.8 years (ranging from 51 to 85 years) were retrospectively evaluated and are listed in . One patient had 2 tumours; thus, in total, 25 RCCs were ablated using US-guided percutaneous MWA as follows: 14 lesions were in the right kidney, and 11 lesions were in the left kidney. All tumours were histopathologically proven by 18-gauge core needle biopsies (CNB) under US guidance before the ablation, including 24 renal clear cell carcinoma and one renal papillary cell carcinoma. The mean maximum diameter of the RCCs was 3.80 ± 1.60 cm (ranging from 0.9 to 7.5 cm). The inclusion criteria for the patients were as follows: (1) the maximum diameter of the RCC was less than 8 cm, and the tumour could be detected by US or contrast-enhanced US (CEUS) imaging; (2) the distance between the index tumour and the adjacent bowel was less than 0.5 cm on the pre-operative CT or MRI images [Citation17]; (3) no renal vein embolus and extrarenal metastases were detected; (4) the prothrombin time, prothrombin activity and platelet counts were less than 25 s, greater than 40% and greater than 40 cells ×109, respectively; and (5) the patients were non-surgical candidates due to comorbidities in important organs, such as the heart, brain, lung, liver, and kidney, or the patients refused surgical resection. This study was approved by our Institutional Review Board, and written informed consent was obtained from each patient prior to the treatment.
Table 1. Clinical features of the patients and tumours.
Hydrodissection procedure
A colour Doppler US Acuson Sequoia 512 device (Siemens Medical Solutions, Mountain View, CA) with 3.0–4.5 MHz phased array multifrequency transducers was used to perform the hydrodissection, CNB and percutaneous MWA procedures.
After the administration of local anaesthesia (i.e. 1% lidocaine) at the puncture site, a 16-gauge intravenous catheter (BD Angiocath, Sandy, UT), which was 15 cm in length, was used to puncture the tissue between the index renal tumour and the proximal intestinal tract under US guidance. Several millilitres of a saline solution were rapidly injected, and then, a small local separation of the liquid between the tumour and the bowel was present. The saline was continuously injected, while the tip of the catheter was advanced along the local liquid separation under US guidance until the separation was successfully achieved, which was defined as a separation of at least 1 cm between the tumour and the adjacent tract on the US image. The outer catheter was retained for saline instillation, which occurred immediately after the inner stylet was removed. For large tumours, an additional hydrodissection procedure could be performed at another site to separate the index tumour and the bowel. Because it can be difficult to separate the tumour from the intestinal tract due to previous abdominal surgery, saline could be injected into the perirenal adipose capsule to achieve a successful separation. The total amounts of the injected and instilled saline were recorded after the MWA.
CNB and MWA prodecures
An MWA System (KY-2000; Kangyou Medical, Nanjing, China) was used to produce the highest power output of 100 W at 2450 MHz. A cooled-shaft needle antenna (15-gauge diameter and 18 cm length) with an active 0.5 cm or 1.1 cm tip was applied in this study. In general, for tumours less than 2.0 cm in diameter, a single antenna was advanced, whereas two or more antennas were required for tumours with diameters of 2.0 cm or greater. CNB was arranged before the MWA procedure to minimise the risk of bleeding because most of the RCCs presented a rich blood flow supply. When possible, the biopsy and ablation did not pass the liquid area caused by the hydrodisplacement.
All patients were treated under intravenous anaesthesia and a combination of Propofol (Diprivan; Zeneca Pharmaceuticals, Wilmington, DE) and Ketamine (Shuanghe Pharmaceuticals, Beijing, China) was administered by an anaesthesiologist. First, CNB was performed using an 18-gauge automatic cutting needle. Then, the antenna was percutaneously advanced into the index tumour under US guidance. After the placement of all antennas was carefully verified, microwave output was emitted at a power of 50 W or 60 W. The ablation was ceased when the hyperechoic zone covered the entire index tumour on US imaging, and then, a needle tract cauterisation was routinely performed during the antenna withdrawal to minimise bleeding and tumour seeding along the needle track. Contrast-enhanced imaging, such as CEUS, contrast-enhanced CT or MRI, was routinely employed 1–3 days after the procedure to evaluate the treatment response. An irregular peripheral enhancement of the ablated lesion indicated a possible residual unablated tumour; consequently, an additional MWA session was repeated within 1 week.
Follow-up
The technique efficacy, i.e. complete ablation, was defined as no enhancement of the entire tumour on contrast-enhanced imaging, including CT, MRI or CEUS, 1-month post-ablation. If complete ablation was confirmed, routine contrast-enhanced imaging to detect the local tumour progression (LTP) or distant metastases and laboratory tests measuring serum creatinine (Cr) and blood urea nitrogen (BUN) to assess renal function were performed on the 3rd and 6th month after the surgery and then at 6-month intervals.
According to the definition of complications and side effects published for the standardisation of terminology and reporting criteria of image-guided tumour ablation [Citation18], major and minor complications and side effects related to the procedures were recorded and managed after the ablation. The detailed time and reason of death were recorded.
Statistical analysis
GraphPad Prism software version 5.0 for windows (GraphPad Software, San Diego, CA) was applied to generate a graph and calculate and statistically analyse the data. The measurement data are presented as the mean ± SD. Unpaired t-tests (two sides) were used to compare the mean values of the patient’ serum Cr and BUN levels at 1-day post-MWA and the last follow-up pre-MWA. A p value less than 0.05 was considered statistically significant.
Results
Efficacy of hydrodissection in assisting MWA
One session was performed to ablate 88% of the RCCs (22/25), and an additional session was repeated for the remaining three RCCs because a residual ablated tumour was detected on contrast-enhanced imaging within 3 days after the MWA. In total, 28 ablation sessions were performed, and the mean number of treatment sessions was 1.1 ± 0.3 (ranging from 1 to 2). The separating success rate of the hydrodissection with the saline injection was 100% (28/28), and the mean volume of injected saline was 631.1 ± 384.7 ml (ranging from 150 to 1570 ml) per session. Regarding the MWA procedure, the mean needle insertion, microwave power output, ablation time and ablation energy for RCC tumours were 3.1 ± 1.6 (ranging from 1 to 7), 52.4 ± 4.4 W (ranging from 50 to 60 W), 1189.6 ± 661.0 s (ranging from 300 to 2680 s) and 62.8 ± 36.1 kJ (ranging from 15 to 158 kJ), respectively. The hydrodissection and ablation parameters are listed in .
Table 2. Parameters of the hydrodissection and ablation procedure.
The mean follow-up period was 14.9 ± 10.1 months (ranging from 1 to 30 months), the technique efficacy rate of the MWA was 100% (25/25), and all 25 ablated tumours exhibited complete ablation on contrast-enhanced imaging 1 month after the MWA (). All patients survived, and no evidences of LTP, distal metastases or neoplasms related to the procedure of hydrodisplacement during the follow-up period.
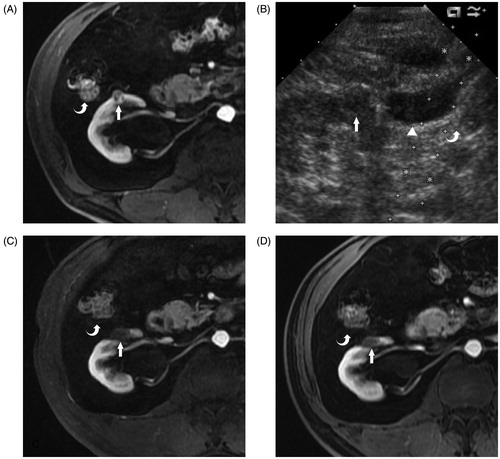
Figure 1. A 67-year-old man with histopathologically a proven clear cell carcinoma renal tumour on the right side. (A) Transverse contrast enhanced MRI reveals a 1.3-cm hypoenhancement tumour (arrow) located on the ventral side of the right kidney adjacent to the colon (curve arrow) before the MWA. (B) After the saline injection hydrodissection (solid triangle), the colon (curve arrow) is successfully separated from the renal tumour (arrow) on US imaging. (C) Transverse contrast-enhanced MRI 3 months after the MWA reveals that the tumour adjacent to the colon (curved arrow) is completely ablated (arrow). (D) Transverse contrast-enhanced MRI 6 months after the MWA reveals the completely ablated tumour (arrow) adjacent to the colon (curved arrow) shrinkage.
Complications and side effects
All complications and side effects encountered in this study are listed in . The observed complications included one major complication (4.2%) of massive right pleural effusion; 5 minor complications (20.8%), including haemoglobinuria in one patient, gross haematuria in one patient and microscopic haematuria in three patients; and 12 side effects (50%), including abdominal distension in three patients, post-ablation syndrome with mild to moderate fever from 37.3 to 38.6 °C in seven patients and mild pain in two patient.
Table 3. Complications and side effects.
Renal function
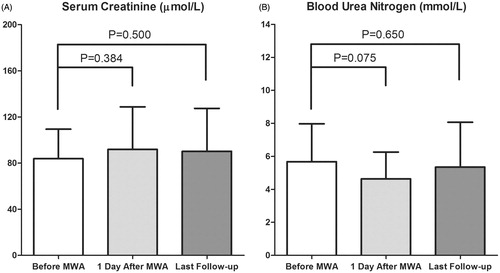
The mean serum Cr and BUN levels before the MWA, 1 day after the MWA and at the last follow-up were 83.9 ± 25.6 μmol/L (ranging from 49.2 to 139.6 μmol/L) and 5.68 ± 2.29 mmol/L (ranging from 2.89 to 12.25 mmol/L); 91.9 ± 36.7 μmol/L (ranging from 52.0 to 224.7 μmol/L) and 4.63 ± 1.62 mmol/L (ranging from 2.33 to 8.73 mmol/L); 90.1 ± 37.3 μmol/L (ranging from 53.4 to 223.4 μmol/L) and 5.34 ± 2.72 mmol/L (ranging from 2.18 to 16.10 mmol/L), respectively. Compared with the mean levels prior to the MWA, there were no significant differences in the mean serum Cr () and BUN () levels 1 day after the procedure and at the last follow-up.
Figure 2. The changes in renal function. (A) There was no significant difference in the mean serum creatinine level 1 day after the MWA (p = 0.384) and at the last follow-up (p = 0.500) compared with that prior to the MWA. (B) No significant differences in the mean blood urea nitrogen were observed 1 day after the MWA (p = 0.075) and at the last follow-up (p = 0.650) compared with that before the MWA.
Discussion
To minimise the major complications associated with bowel injury after ablation for the treatment of RCCs adjacent to intestinal tracts, several non-invasive and minimally invasive methods have been adopted clinically. Pre-operative intestinal preparation and maintaining patients in the left or right lateral position during the procedure have been commonly used during percutaneous ablation of RCCs. However, these techniques were not always able to achieve an effective separation between the bowel and the index tumour. Under US guidance, one or more thermocouple temperature needles were advanced into the tissue between the tumour and the adjacent bowel for real-time monitoring to avoid overheating the gastrointestinal tracts, which has been reported to be an effective method for reducing injury in the gastrointestinal tracts during ablation procedures [Citation17]. However, obvious limitations were noted as follows: (1) the temperature distribution in the entire intestinal tracts adjacent to the index tumour might not be accurately monitored in real time by one or more of the thermocouple measuring dots and (2) the procedure is operator-dependent.
US-guided hydrodissection has been applied to assist tumour ablation of liver [Citation19–21], kidney [Citation22], and mediastinal [Citation23] tumours and benign prostatic hyperplasia [Citation24], and the key purpose of the technique is to create a liquid gap between the index tumour or the target organ and important adjacent structures, such as the gastrointestinal tracts, gallbladder, nerves and large vessels, through a precise puncture and liquid injection. Thus, the index tumour can be ablated safely and effectively. The results of this retrospective study showed that the hydrodisplacement technique was easy to perform and achieved a 100% separation success rate. Then, 25 RCCs adjacent to intestinal tracts in 24 patients were successfully treated by the percutaneous MWA with a technique efficacy rate of 100%. Additionally, no deaths, local recurrence, distant metastasis and neoplasms related to the hydrodisplacement procedure occurred during the follow-up; however, further studies with a long-term follow-up would be more convincing. Therefore, this preliminary clinical study demonstrated the efficacy of hydrodissection in clinical applications for assisting percutaneous MWAs of RCCs that are near the intestinal tracts.
Successful separation is not always achieved using hydrodissection in MWA of hepatic tumours that are near the gastrointestinal tracts in some patients with post-operation abdominal adhesion [Citation19]. However, in our experience, if the renal fascia and tracts could not be successfully separated, successful separation between the index RCC and the adjacent intestinal tracts could be achieved by administering saline into the perirenal adipose capsule between the index tumour and the bowels. Thus, the presence of the perirenal adipose capsule provides an alternative for achieving separating success with the hydrodissection procedure because of the specific anatomical features of the kidney.
RFA directly heats tissue near the electrode and is guided by the basic principle of the Joule effect, which is produced by frictional agitation at the ionic level that is caused by electrical current generated from the radiofrequency generator [Citation25]. Thus, the instilled solution used in hydrodissection is 5% dextrose solution (D5W) instead of saline to prevent the ions inform the radiofrequency current from flowing to unexpected tissue. The excessive use of D5W in hydrodissection procedures might increase the risk of hyponatremia [Citation26] in the patients, and D5W must be carefully applied in diabetes mellitus patients. The basic principle of MWA, which directly heats tissue near the antenna, is dielectric hysteresis (rotating dipoles) [Citation25], which differs from the Joule heating mechanism of RFA. Thus, 0.9% saline can be used in hydrodissection procedures because there is no current flowing to the surrounding tissue that could cause postoperative electrolyte imbalance in the patients.
The puncture needles used in hydrodissection were varied in diameter ranging from 16-gauge to 20-gauge [Citation22,Citation27]. In this study, a 16-gauge intravenous catheter (15 cm length) was used. Its needle sheath was soft, could be safely kept in the abdomen to instil saline during the MWA procedure and was easily removed after the ablation. Although the diameter of the needle was relatively large, no complications including bleeding, haematoma, and important structural damage, were detected during and after the procedure.
Major and minor complications and side effects were detected in 1 (4.2%), 5 (20.8%) and 12 cases (50%), respectively. The incidence of complications was similar to that reported after RFA [Citation27], cryoablation [Citation28] and MWA [Citation14] procedures for the treatment of RCCs. One major complication occurred in an 81-year-old male patient who has suffered from radical nephrectomy in the left kidney due to clear cell carcinoma for 7 years. One day after the ablation of a pathologically proven clear cell carcinoma tumour located in the upper pole of right kidney, massive right pleural effusion was detected, and 3-day catheter drainage was required. A 51-year-old female patient exhibited haemoglobinuria after her right renal tumour (7.5 cm in diameter; proven clear cell carcinoma) was administered. A sodium bicarbonate injection (Shuanghe Pharmaceuticals, Beijing, China) was administered once, and the colour of her urine returned to normal the following morning. Hemoglobinuria was commonly observed after ablation of a large volume tumour, and one of the possible reasons involved damage to numerous red blood cells during the ablation session. The urine tests of the patients with gross haematuria and microscopic haematuria became normal within 7 days without medication, and a likely explanation of the haematuria might be that the tumours were near the renal pelvis. Regarding the side effects, three patients complained of abdominal distension with no remedy and exhibited recovery 2–4 h after the procedure, and this side effect considered related to the use of more than 1300 ml in the saline instillation during the hydrodissection. Seven patients exhibited post-ablation syndrome, presenting a mild to moderate fever of 37.3 to 38.6 °C, and recovered within 2–5 days after the MWA. Two of these patients were administered acetaminophen, and the remainder recovered with no therapy. Mild pain located laterally of the ablated lesion was reported by 2 patients (8.3%), who recovered within 1–2 days without analgesics. The incidence of post-ablation syndrome appeared high in this study, which may be related to the large mean diameter of the RCCs those were ablated, and patients’ body temperature increased due to absorption fever caused by the large volume of necrotic tissue after the MWA. Moreover, due to the large diameter RCCs, in this study, the ablation parameters (), such as ablation time and antenna insertions, were greater than those used in a previous report [Citation16]. In addition to the abdominal distention reported by three patients, other complications and side effects were not considered to be related to the hydrodissection procedure. In addition, no significant differences were observed in the mean serum Cr and BUN levels 1 day after the procedure and at the last follow-up compared with those prior to the MWA. The complications, side effects and changes in serum Cr and BUN post-ablation demonstrated the safety of the percutaneous MWA treatment for patients with RCCs near the intestinal tracts under the assistance of US-guided hydrodissection.
Because this was a preliminary clinical study, there were several instinctive limitations in this study, including a single participating centre, small-scale enrolment, no control group and a short-term follow-up period. The clinical application of this technique in further studies with large-scale patients and long-term follow-up could be more encouraging.
Conclusions
In conclusion, US-guided hydrodissection assistance in percutaneous MWA treatment is a safe and effective alternative for selected patients with RCCs adjacent to the intestinal tract and can achieve good local tumour control and renal function preservation.
Acknowledgements
This study was supported by the National Scientific Foundation Committee of China [grant Nos. 81430039 and 81627803].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen W, Zheng R, Baade PD, et al. (2016). Cancer statistics in China, 2015. CA Cancer J Clin 66:115–32.
- Hancock SB, Georgiades CS. (2016). Kidney cancer. Cancer J 22:387–92.
- Ghandour RA, Danzig MR, McKiernan JM. (2015). Renal cell carcinoma: risks and benefits of nephron-sparing surgery for T1 tumors. Adv Chronic Kidney Dis 22:258–65.
- Wang S, Qin C, Peng Z, et al. (2014). Radiofrequency ablation versus partial nephrectomy for the treatment of clinical stage 1 renal masses: a systematic review and meta-analysis. Chin Med J (Engl) 127:2497–503.
- Low G, Huang G, Fu W, et al. (2016). Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol 8:484–500.
- Vetterlein MW, Jindal T, Becker A, et al. (2016). Small renal masses in the elderly: contemporary treatment approaches and comparative oncological outcomes of nonsurgical and surgical strategies. Investig Clin Urol 57:231–9.
- D’Andrea D, Shariat SF, Klatte T. (2016). Update on ablative therapies of renal tumors. Curr Opin Urol 26:410–16.
- Georgiades C, Rodriguez R. (2013). Renal tumor ablation. Tech Vasc Interv Radiol 16:230–8.
- Lin Y, Liang P, Yu XL, et al. (2014). Percutaneous microwave ablation of renal cell carcinoma is safe in patients with a solitary kidney. Urology 83:357–63.
- Lin Y, Liang P, Yu XL, et al. (2016). Percutaneous microwave ablation of renal cell carcinoma is safe in patients with renal dysfunction. Int J Hyperthermia 15:1–6.
- Park BK, Kim CK. (2009). Complications of image-guided radiofrequency ablation of renal cell carcinoma: causes, imaging features and prevention methods. Eur Radiol 19:2180–90.
- Kim KR, Thomas S. (2014). Complications of image-guided thermal ablation of liver and kidney neoplasms. Semin Intervent Radiol 31:138–48.
- Shimizu K, Mogami T, Michimoto K, et al. (2016). Digestive tract complications of renal cryoablation. Cardiovasc Intervent Radiol 39:122–6.
- Dong X, Li X, Yu J, et al. (2016). Complications of ultrasound-guided percutaneous microwave ablation of renal cell carcinoma. Onco Targets Ther 9:5903–9.
- De Filippo M, Bozzetti F, Martora R, et al. (2014). Radiofrequency thermal ablation of renal tumors. Radiol Med 119:499–511.
- Howenstein MJ, Sato KT. (2010). Complications of radiofrequency ablation of hepatic, pulmonary, and renal neoplasms. Semin Intervent Radiol 27:285–95.
- Yu J, Liang P, Yu XL, et al. (2012). US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology 263:900–8.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology 273:241–60.
- Zhang M, Liang P, Cheng ZG, et al. (2014). Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia 30:134–41.
- Yan K, Yang W, Chen MH, et al. (2016). Clinical application of hepatic wedge ablation for treating liver malignancies of the inferior margin: a new ablation technique. Int J Hyperthermia 20:1–9.
- Meloni MF, Chiang J, Laeseke PF, et al. (2017). Microwave ablation in primary and secondary liver tumours: technical and clinical approaches. Int J Hyperthermia 33:15–24.
- Klapperich ME, Abel EJ, Ziemlewicz TJ, et al. (2017). Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology 11:160592.
- Garnon J, Koch G, Caudrelier J, et al. (2016). Percutaneous image-guided cryoablation of challenging mediastinal lesions using large-volume hydrodissection: technical considerations and outcomes. Cardiovasc Intervent Radiol 39:1636–43.
- Faber K, de Abreu AL, Ramos P, et al. (2015). Image-guided robot-assisted prostate ablation using water jet-hydrodissection: initial study of a novel technology for benign prostatic hyperplasia. J Endourol 29:63–9.
- Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. (2011). Principles of and advances in percutaneous ablation. Radiology 258:351–69.
- Jiang L, Krishnasamy V, Varano GM, Wood BJ. (2016). Hyponatremia following high-volume D5W hydrodissection during thermal ablation. Cardiovasc Intervent Radiol 39:146–9.
- Wah TM, Irving HC, Gregory W, et al. (2014). Radiofrequency ablation (RFA) of renal cell carcinoma (RCC): experience in 200 tumours. BJU Int 113:416–28.
- El Dib R, Touma NJ, Kapoor A. (2012). Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: a meta-analysis of case series studies. BJU Int 110:510–16.
