Abstract
Background: Patients with malignant pleural effusions (MPEs) have limited life expectancy. This study aims to investigate the feasibility of intrapleural perfusion with hyperthermic chemotherapy (IPHC) under video-assisted thoracoscopic surgery on MPE patients.
Methods: MPE patients were enrolled in the study and treated with IPHC. The treatment response was classified as complete response (CR, no re-accumulation of pleural fluid for 4 weeks), partial response (PR, re-accumulation above the post-IPHC level but below the pre-IPHC level for four weeks), no response (NR; re-accumulation or above the pre-IPHC level). The change of Karnofsky performance score (KPS) and tumour markers were also recorded. Follow-up was done every two weeks during first month and monthly thereafter until death.
Results: Eighty patients included 46 males and 34 females were included in the study. The total response rate was 100%, with 71.3% of CR and 28.7% of PR. The KPS scores were significantly elevated and the level of tumour markers in pleural effusion were dramatically decreased after IPHC. The median survival was 16.8 months ranged from 2.1 to 67.4 months. One-year and two-year survival rates were 82.5% and 23.8%, respectively. There were no serious clinical compilations during IPHC treatment.
Conclusions: IPHC is a safety, effective and promising approach for MPE patients. It provides well survival benefit and minor toxicities.
Introduction
Malignant pleural effusion (MPE) is defined as the identification of malignant cells in plural fluid or on pleural biopsy, which is often caused by primary pleural malignancies or tumours with pleural metastases. It is estimated that over 150 000 MPE cases occur in the United States each year [Citation1]. It is a seriously common complication of advanced malignant diseases. The 8th TNM classification for lung cancer classifies the presence of MPE as M1a, indicating an extremely poor prognosis [Citation2]. The 30-day mortality is 29–50% and the median survival is 3–12 months [Citation3,Citation4]. Currently, there is no standard treatment for MPE patients. The present treatments for MPE included the drainage of pleural effusion, intrapleural chemotherapy and systemic chemotherapy. These approaches are usually palliative, with purpose to relieve symptoms, reduce discomfort, or raise the patient’s quality of life, but the therapeutic outcomes remain to be unsatisfactory.
Intrapleural perfusion with hyperthermic chemotherapy (IPHC) is a promising approach by irrigating the pleural space with a hyperthermic liquid combined with a chemotherapeutic agent such as cisplatin using specially devised extracorporeal circuits [Citation5]. The supraphysiologic temperature will decrease malignant cell survival and enhance the antitumour effects of local intrapleural chemotherapy. Isik et al. reported MPE patients achieved a median survival of 15.4 months treated with IPHC following surgical interventions [Citation6]. Another study reported by Ba et al. showed pleural effusion was controlled in 100% of patients and mean KPS score was increased by 40% after IPHC, besides, MPE patients treated with IPHC significantly prolonged survival to a median time of 12.9 months [Citation7]. Although several reports have demonstrated the effectiveness of IPHC, but only few MPE patients adopt this therapy in clinical settings. Insufficient perfusion due to pleural adhesions and re-surgery as separation of diagnosis and treatment might be the reasons. IPHC under video-assisted thoracoscopic surgery (VATS) simultaneously combined advantages of local surgery, chemotherapy and thermotherapy, which could achieve both aims of diagnosis and treatment. However, there have been few reports of the use of this combination in the literature. This study aims to investigate the feasibility of IPHC under VATS on MPE patients.
Materials and methods
Patients with MPE who received IPHC under VATS from February 1999 to March 2012 were enrolled in this study. Eligibility criteria were the following: patient with metastatic pleural malignancies proved by pleural cytology and/or biopsy; moderate to large MPE measured by X-ray, ultrasound, or computed tomography; expected survival time longer than 3 months; Karnofsky performance status (KPS) more than 50; sufficient liver function and renal function; no previous intrapleural therapy; and no chemotherapy or radiotherapy received within 3 months prior to enrolment. All patients had sufficient cardiorespiratory function to tolerate general anaesthesia, with normal blood routine examination and creatinine level. All patients underwent a complete pre-operative history and physical examination, including computed tomographic scan of the chest and abdomen, magnetic resonance imaging of the brain and general bone scintigraphy to exclude extrathoracic metastasis. The written informed consent was signed before treatment for each patient. The study was approved by the Scientific Research Board of Hangzhou First People’s Hospital. The exclusion criteria were bilateral MPE or extrathoracic metastases.
Blood routine, renal and liver function test were repeated every other day. Each patient had a pre-drainage baseline posteroanterior (PA) radiograph. Post-IPHC PA radiographs were obtained at the following time: immediately, 3 days, 7 days, 30 days and every month after IPHC. If PA radiographs were not available, thoracic ultrasound was also permitted. The treatment response was classified as follows: (1) complete response (CR; no re-accumulation of pleural fluid after IPHC for at least four weeks), (2) partial response (PR; re-accumulation above the post-IPHC level but below the pre-IPHC level for at least four weeks), and (3) no response (NR; re-accumulation or above the pre-IPHC level) [Citation8].
Surgical procedure
VATS was performed under general anaesthesia with double-lumen endotracheal tubes ventilation on each patient. Electrocardiogram was utilised to monitor the vital signs. And end tidal CO2 (etCO2), invasive blood pressure and urine output were also monitored. The patient was set to 90° of lateral position. A 10 mm incision was made at the sixth intercostal space along the middle axillary line and trocar was inserted into the pleural cavity. Then, thoracoscope was placed through the port and applied to obtain a preliminary exploration of pleural cavity, such as the extent of pleural adhesion and tumour involvement. Meanwhile, another 10 mm port was made at the fourth intercostal space along the anterior axillary line. Thoracoscopic equipments were inserted to break up the intrathoracic adhesions and remove all fibrinoid membrane restraining lung re-expansion under VATS. During the surgery, tumour tissues and normal pleural tissues were achieved for biopsy. After withdrawing all thoracoscope instruments, 32 and 28 F chest tubes were, respectively, placed in the above two ports: one for perfusion outlet and another for perfusion inlet. The tubes were transiently fastened with loose, 7-silk sutures to the chest wall and connected to a standard extracorporeal circuit (heat exchanger, roller pump and reservoir) (Supplementary Figure S1). During surgery, the patients were provided with sufficient fluid to prevent drug-induced nephrotoxicity. Only for patients with large amount of MPE and hypoalbuminaemia, we will prepare plasma before surgery in case of protein loss.
Perfusion technique
The extracorporeal circuit was constituted with a standard roller pump, a heat exchanger and reservoir. Before perfusion, the circuit was primed with 3000–4500 ml of normal saline. Then, the heating circuit system gradually started, until reaching a temperature of approximately 45 °C on the heat exchanger. The cisplatin (200 mg/m2, based on the body area of patients) was added. After that the pleural space began to perfuse, the lungs of each patient were partial collapse. And the flow rate was between 1000 and 1500 ml/min. And the pleural temperature was monitored by a directly intrapleural thermometer to achieve a stable 43 °C in the pleural cavity. The total dose of cisplatin was added as follows: 260 mg in eight patients, 280 mg in 14 patients, and 300 mg in 30 patients, and 320 mg in 20 patients and 340 mg in eight patients. In order to prevent post-operative pulmonary oedema, as well as renal and liver impairment, 500 mg of methylprednisolone and 800 mg of amifostine were, respectively, administered intravenously during IPHC. Eventually, each patient favourably received IPHC for one hour. At the end of perfusion, the fluid in the pleural cavity was drained as completely as possible. To observe the change of pleural carcinomatous nodules and normal pleural tissue after IPHC, three were randomly selected. The forceps was meanwhile used to remove a little pleural tumour tissue and normal pleural tissue for further light and electron microscope. Then, thoracoscopic exploration was again to confirm no active bleeding, pulmonary air leakage. A 32 F chest tube in the midaxillary line through the sixth intercostal space was placed in the thoracic cavity, which was connected to a water-sealed drainage system. Tumour makers were measures again 24 h after IPHC.
Follow-up
Follow-up was done every two weeks during the first month and monthly thereafter. The patients who died of non-tumour related diseases or who were lost to follow-up within the first month were excluded from the study. Chest radiographs or ultrasound were obtained. All patients were followed until they died. Computed tomographic scan was performed every 6 months. Follow-up time ranged from 3 to 60 months, with an average of (36.0 ± 3.2) months.
Statistics
The measurements were represented as mean and standard deviations. Simple descriptive methods were used along the study. Pairwise t-test was used for serum tumour markers and KPS. Survival analysis was done with the Kaplan–Meier method. IBM SPSS version 22.0 (IBM SPSS, Inc., Chicago, IL) was used for these calculations. p < 0.05 was considered to be statistically significant.
Results
Patient characteristics
Of the 80 patients, 46 were males and 34 were females; the median age was 61 years, ranging from 31 to 81 years. Sixty-five patients were diagnosed with lung cancer, six with breast cancer, three with lymphoma, three with colon cancer, two with diffuse malignant mesothelioma and one with adenocarcinoma of unknown origin. All the patients had received thoracentesis and 25 had received additional bleomycin sclerotherapy, but all failed with pleural fluids accumulation in a short during time. Details are in .
Table 1. Patient characteristics (n = 80).
Clinical efficacy and antitumour activity
All the patients re-evaluated pleural fluids after IPHC. The total response rate was 100%, with 71.3% of CR and 28.7% of PR. The mean amount of fluid drainage was 480 ± 120 ml (260–1380 ml). The average time from start of IPHC to chest tube removal was 5.5 ± 1.5 days (3–7 days) and the average hospital stay was 8.5 days. Symptoms relief or disappear were observed in all patients. The mean KPS score was significantly elevated from 61.8 ± 11.8 to 82.5 ± 9.7 after IPHC (+33.5%, p < 0.001). The level of CEA, Cyfra21–1 and NSE in pleural effusion were 42.5 ± 6.7 ng/ml, 35.8 ± 3.2 ng/ml and 29.4 ± 5.7 U/ml, respectively; these markers were dramatically decreased to 12.7 ± 3.1 ng/ml, 6.3 ± 2.5 ng/ml and 10.5 ± 3.8 U/ml after IPHC (). In addition, further observation on pleural carcinomatous nodules revealed tumour cells necrosis, chromatin condensation, apoptotic body occurrence and nuclear fragmentation after IPHC ().
Figure 1. The changes of CEA, Cyfra21–1 and NSE in pleural effusions before and 24 h after IPHC. IPHC intrapleural perfusion with hyperthermic chemotherapy; *p values less than 0.01.
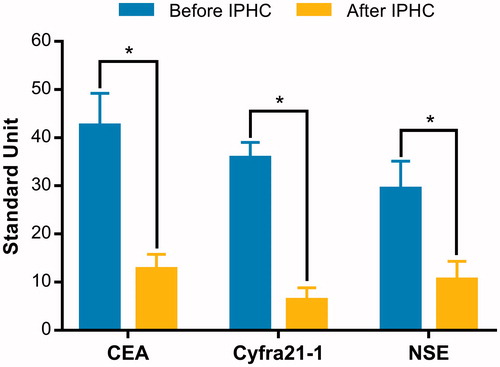
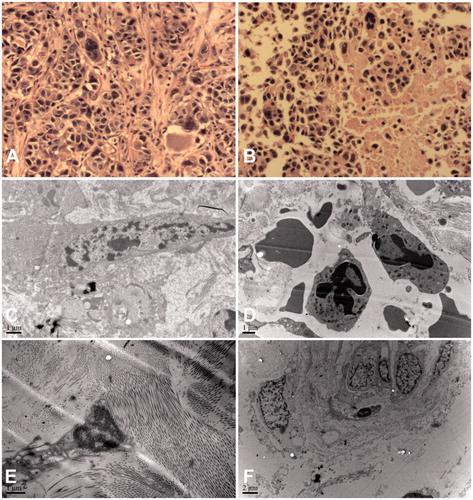
Figure 2. The morphologic and ultrastructure of pleural tumour tissue before and immediately after IPHC. (A) Tumour specimen before IPHC showed piles of tumour cells (200 ×); (B) The same tumour specimen after IPHC exhibited extensive necrosis (200 ×); (C) Pleural carcinomatous nodules found large and irregular nucleus before IPHC by electron microscopic; (D) The chromatin began to concentrate accompanied with apoptotic body after IPHC; (E and F) Normal pleural tissues showed no ultrastructure change by electron microscopy before and after IPHC. IPHC intrapleural perfusion with hyperthermic chemotherapy.
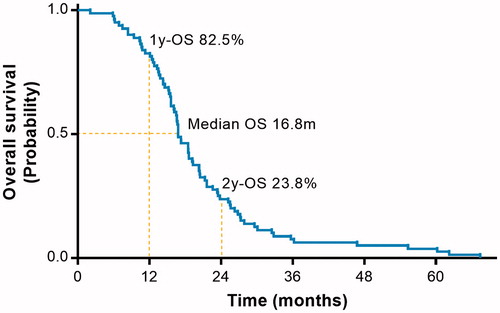
At the time of last follow-up, all the patients have died. Survival time ranged from 2.1 to 67.4 months, with a median survival of 16.8 months (). One-year and two-year survival rates were 82.5% and 23.8%. Four patients died within 6 months after IPHC. One died of primary disease progression, two died of brain metastases, and one died of systemic multiple metastases. No patients died of ipsilateral uncontrolled pleural effusions. Three patients survived more than 60 months.
Safety and tolerability
There were no serious clinical compilations observed during IPHC treatment. The three most occurring adverse effects were mild to moderate gastrointestinal reactions (25 patients), fever (12 patients) and mild bone marrow suppression (6 patients). All symptoms were alleviated after appropriate treatment ().
Table 2. Adverse effects after IPHC.
Case presentations
A 50-year-old woman with MPE caused by lung adenocarcinoma was hospitalised for unknown chest tightness. A posteroanterior chest radiograph showed a large, free-flowing left pleural effusion. After diagnosing with metastatic lung adenocarcinoma by thoracoscopic biopsy, IPHC was directly performed. The patient recovered quite well without any discomforts at discharge. Three months later, no residual fluid was seen on a chest radiograph and the patient’s dyspnoea disappeared. Follow-up with subsequent 7 months, no effusion in the chest was confirmed by chest radiograph ().
Figure 4. A case of a 50-year-old female with MPE from lung adenocarcinoma received IPHC. (A) A posteroanterior chest radiograph showed a large pleural effusion before treatment; (B) No residual fluid was seen on the chest radiograph 3 months after IPHC; (C) No effusion in the chest was confirmed 10 months after IPHC. IPHC intrapleural perfusion with hyperthermic chemotherapy.
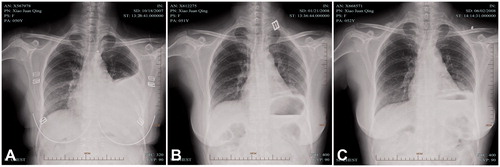
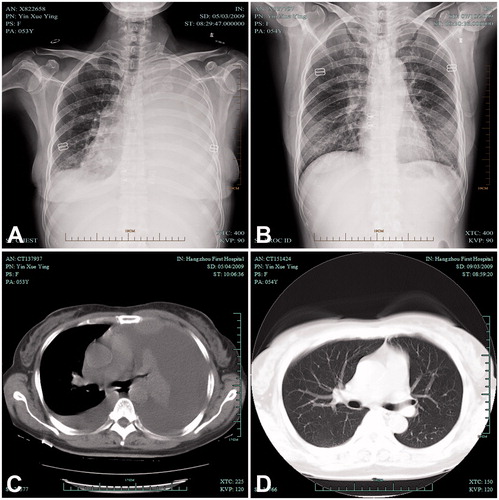
A 53-year-old woman was admitted to hospital for repeated dyspnoea at rest. A posteroanterior chest radiograph showed a large, left pleural effusion and computed tomogram at the subcarinal level showed parasternal intrathoracic mass, accompanied with left pulmonary atelectasis and a small, right pleural effusion. She was also suggested to receiving thoracoscopy. During operation, removal of pleural effusion, the tumour tissue was excised to confirm as lymphoma. Then, ICHPC was administered with successful control of pleural effusions. Two months later, chest radiograph revealed complete expansion of left lung without any residual effusions. After another 4 months, computed tomogram examination showed bilateral effusion disappearance and the mass in the left lower hemithorax was also absent ().
Figure 5. A case of a 53-year-old female with MPE received IPHC. (A and C) A posteroanterior chest radiograph and CT showed a large pleural effusion on the left; (B) Pleural fluid was completed disappeared 2 months after IPHC confirmed by chest radiograph; (D) No pleural fluid re-accumulated 6 months after IPHC confirmed by CT. IPHC intrapleural perfusion with hyperthermic chemotherapy; CT computed tomography.
Discussion
Current treatments for MPE are complicated and usually take multiple procedures for diagnostic and therapeutic purposes, such as diagnostic thoracentesis (often more than once), pleural biopsies (closed or thoracoscopic) followed by therapeutic thoracentesis, surgical or bedside pleurodesis (when MPE recurs) and indweling pleural catheter (IPC) insertion or further thoracenteses if pleurodesis failed [Citation1]. Although many non-surgical diagnostic methods exist, there is still 15–25% of pleural effusions remained undiagnosed [Citation9]. Boutin and coauthors reported that figure was 21.5% [Citation10]. Repeated thoracentesis increase the diagnostic rate but cause both physically and psychologically traumatic to the patient and a burden to the healthcare system. Palliative pleurodesis is commonly used for the treatment of MPE. But the fluid control rate is only 75% at 1 month and 50% by 6 months [Citation11]. Besides, pleurodesis is associated with many complications such as pain, fever and acute respiratory distress syndrome [Citation12,Citation13]. IPC allows ambulatory fluid drainage, and minimised the need for hospitalisation and costs [Citation14]. It has been increasingly employed for patients with symptomatic MPE [Citation15,Citation16], particularly for those who failed after pleurodesis or were unsuitable for pleurodesis. But IPC also has severe complications including infection, blockage symptomatic loculations, catheter track metastases [Citation17–19]. Moreover, none of these approaches provide a significant survival benefit except symptoms relief. In Isık’s study, the median survival was only 6 months in talc pleurodesis group and 8 months in pleurectomy group. One-year survival in two groups was 0.6% and 0.8%, respectively [Citation6]. Therefore, it is of great importance to remove the pleura fluids and simultaneously destroy metastatic pleural lesions to prevent recurrence and prolong survival.
VATS has advantages of both diagnosis and treatment. In this study, all suspected MPE patients were directly recommended to receive thoracoscopy. Elimination of loculated pleural effusions and pulmonary decortication were performed to facilitate lung re-expansion during thoracoscopy, which improved the pulmonary functions and prevented pleural effusions recurrence. Macroscopic abnormalities were obvious under VATS, where accurate biopsy could achieve from the parietal pleura. This procedure not only decreased time delay in pathologic diagnosis, but also obtained enough fresh tissue specimens for molecular and gene test. Once intraoperative pathologic findings reported as MPE, IPHC subsequently began to perform in the patient for 1 h. All procedures were finished under VATS through only two 10 mm port incisions, which minimised the harm of surgery. All patients of this study recovered well. IPHC under VATS characterised with less pain, rapid recovery and less hospitalised time, which was therefore suitable for the borderline patients with limited cardiopulmonary function, elder or malnourished patients.
By intrapleural chemotherapy, tumour lesions can directly expose to high-concentration drugs with less systemic toxicities. Hyperthermia is a highly effective cancer treatment [Citation20], particularly when combined with chemotherapy, radiotherapy or immunotherapy [Citation21,Citation22]. It can inhibit DNA repair, promote intracellular accumulation of chemical agents, alter cellular Ca2+ homoeostasis, induce cell apoptosis and cycle arrest, increase membrane permeability and rearrange the cytoskeleton [Citation23–25]. Combination of hyperthermia and antitumour drugs produce a synergistic effect [Citation26]. Recently, IPHC have been regarded as a promising treatment with survival benefit by many researchers [Citation6,Citation7,Citation27]. While few patients have received IPHC because the long-term therapeutical effect, toxicity and safety were still unknown and need more evidence. This study showed 80 patients who received IPHC under VATS provided significantly better survival (median survival of 16.8 months) compared to those who received either pleurectomy or talc pleurodesis reported in the literature. The one-year, two-year survival was 82.5% and 23.8%, respectively. No patients died in perioperative period. The pleura fluid in most patients completely disappeared except two suffered fluids re-accumulation after three months. Moreover, further examination on carcinoma pleural lesions confirmed tumour necrosis and no change with paracarcinoma tissues after IPHC, suggesting IPHC had an evident killing effect on the metastatic pleural lesions. It might explain most patients did not observe effusion reoccurrence. As to debulking VATS, most patients in our study had diffuse tumour lesions in pleura membrane rather than solitary lesion in peripheral lung, which was hard to perform wedge excision. Considering the inevitable pulmonary function damage by debulking VATS and the satisfying effusion control, we did not undertake debulking VATS. Migliore et al. [Citation28] showed two lung patients treating by debulking VATS combination with IPHC and the survival was >18 and >7 months, respectively. The two patients received subsequent TKI or RT. The author provided a well experience, while the number of patients is small. Whether debulking VATS combination with IPHC can further improve survival need more clinical data.
Benefits of most treatments are limited by dose-dependent or dose-independent toxicity. Our study revealed that IPHC was associated with high incidences of mild-to-moderate gastrointestinal reactions and a small number of patients complained of fever, chest distress and weakness. But all these discomforts were alleviated after supportive treatment. Another serious concern was nephrotoxicity when the patient administered high dose of cisplatin during IPHC. To prevent the occurrence of renal dysfunction, a dose of 200 mg/m2 of cisplatin was applied to all subjects, and 500 mg of methylprednisolone and 800 mg of amifostine were administered intravenously during IPHC. Three patients had a moderate increase in urea and creatinine levels and were successfully controlled by intravenous fluid treatment. The toxicities of IPHC are considered completely anticipatable and acceptable. Eight patients more than 75 years old and six patients with a KPS of 50 were safely performed. In addition, the KPS of patients were dramatically improved when pleural effusions disappeared. The total time in hospital stay was shortened, with an average of 8.5 days in this study, and total hospitalised fees were also reduced.
There are some limitations in this study. First, this is a retrospective study with small number of cases. Then, heterogeneity exists between different tumour sources of MPE. Finally, this is a single-arm study and we compared our results with publications, which inevitably caused bias.
In this study, the pleura effusion of 80 patients was successfully controlled by IPHC under VATS. This therapy markedly improved KPS and extended the patient’s survival superior to literatures. Therefore, we believed IPHC under VATS was a promising strategy for patients with MPE. VATS can effectively clear effusion and break up those intrathoracic adhesions and fibrinoid membrane which restrain lung re-expansion; IPHC eradicated tumour cells to prevent effusion re-accumulation. Of course, this is a retrospective study with deficiencies, but we hope our data can provide an attempt or inspiration to clinicians. Nevertheless, rigorously randomised control trials should be required before it is widely used.
Supplementary File
Download Zip (10.5 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Thomas R, Francis R, Davies HE, Lee YC. (2014). Interventional therapies for malignant pleural effusions: the present and the future. Respirology 19:809–22.
- Goldstraw P, Chansky K, Crowley J, et al. (2016). The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 11:39–51.
- Antunes G, Neville E. (2000). Management of malignant pleural effusions. Thorax 55:981–3.
- Tattersall M. (1998). Management of malignant pleural effusion. Aust N Z J Med 28:394–6.
- Tan G, Chia C, Kumar M, et al. (2017). 201 consecutive cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) procedures in a single Asian tertiary centre. Int J Hyperthermia 33:288–94.
- Isik AF, Sanli M, Yilmaz M, et al. (2013). Intrapleural hyperthermic perfusion chemotherapy in subjects with metastatic pleural malignancies. Respir Med 107:762–7.
- Ba M, Long H, Wang Y, et al. (2013). Intrapleural hyperthermic perfusion using distilled water at 48 degrees C for malignant pleural effusion. J Cancer Res Clin Oncol 139:2005–12.
- Yildirim H, Metintas M, Ak G, et al. (2008). Predictors of talc pleurodesis outcome in patients with malignant pleural effusions. Lung Cancer 62:139–44.
- Beheshtirouy S, Kakaei F, Mirzaaghazadeh M. (2013). Video assisted rigid thoracoscopy in the diagnosis of unexplained exudative pleural effusion. J Cardiovasc Thorac Res 5:87–90.
- Boutin C, Viallat JR, Cargnino P, Farisse P. (1981). Thoracoscopy in malignant pleural effusions. Am Rev Respir Dis 124:588–92.
- Yildirim E, Dural K, Yazkan R, et al. (2005). Rapid pleurodesis in symptomatic malignant pleural effusion. Eur J Cardiothorac Surg 27:19–22.
- Davies HE, Lee YC. (2013). Management of malignant pleural effusions: questions that need answers. Curr Opin Pulm Med 19:374–9.
- Maskell NA, Lee YC, Gleeson FV, et al. (2004). Randomized trials describing lung inflammation after pleurodesis with talc of varying particle size. Am J Respir Crit Care Med 170:377–82.
- Azzopardi M, Porcel JM, Koegelenberg CF, et al. (2014). Current controversies in the management of malignant pleural effusions. Semin Respir Crit Care Med 35:723–31.
- Roberts ME, Neville E, Berrisford RG, et al. (2010). Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 65:ii32–40.
- Simoff MJ, Lally B, Slade MG, et al. (2013). Symptom management in patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e455S–97S.
- Tremblay A, Mason C, Michaud G. (2007). Use of tunnelled catheters for malignant pleural effusions in patients fit for pleurodesis. Eur Respir J 30:759–62.
- Fysh ET, Tremblay A, Feller-Kopman D, et al. (2013). Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest 144:1597–602.
- Thomas R, Budgeon CA, Kuok YJ, et al. (2014). Catheter tract metastasis associated with indwelling pleural catheters. Chest 146:557–62.
- Issels R, Kampmann E, Kanaar R, Lindner LH. (2016). Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: translation into clinical application. Int J Hyperthermia 32:89–95.
- Wust P, Hildebrandt B, Sreenivasa G, et al. (2002). Hyperthermia in combined treatment of cancer. Lancet Oncol 3:487–97.
- Saga T, Sakahara H, Nakamoto Y, et al. (2001). Enhancement of the therapeutic outcome of radio-immunotherapy by combination with whole-body mild hyperthermia. Eur J Cancer 37:1429–34.
- Han SI, Duong HQ, Choi JE, et al. (2008). Hyperthermia switches glucose depletion-induced necrosis to apoptosis in A549 lung adenocarcinoma cells. Int J Oncol 32:851–60.
- Luchetti F, Mannello F, Canonico B, et al. (2004). Integrin and cytoskeleton behaviour in human neuroblastoma cells during hyperthermia-related apoptosis. Apoptosis 9:635–48.
- Kong G, Anyarambhatla G, Petros WP, et al. (2000). Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res 60:6950–7.
- Kodama K, Doi O, Tatsuta M, et al. (1989). Development of postoperative intrathoracic chemo-thermotherapy for lung cancer with objective of improving local cure. Cancer 64:1422–8.
- Shigemura N, Akashi A, Nakagiri T, et al. (2004). Pleural perfusion thermo-chemotherapy under VATS: a new less invasive modality for advanced lung cancer with pleural spread. Ann Thorac Surg 77:1016–21.
- Migliore M, Calvo D, Criscione A, et al. (2015). Cytoreductive surgery and hyperthermic intrapleural chemotherapy for malignant pleural diseases: preliminary experience. Future Oncol 11:47–52.