Abstract
Background: Microwave ablation (MWA) is an effective treatment for severe secondary hyperparathyroidism (SHPT), but it can also be used for mild-to-moderate secondary hyperparathyroidism (SHPT). In this randomised, controlled study, the efficacy of MWA in the treatment of mild-to-moderate hyperparathyroidism is investigated.
Materials and methods: We assessed outcomes 12 months after the randomisation of 28 patients with mild-to-moderate SHPT. The subjects received either MWA plus calcitriol or calcitriol alone. The primary end-points were the rate of achieving target levels of intact parathyroid hormone (iPTH), changes in iPTH levels and the rate of patients developing severe SHPT.
Results: Primary end points: the overall rates of achieving target levels of iPTH were comparable between the MWA and calcitriol alone groups (24% vs. 22%, p = 0.85). However, the rate of iPTH <150 pg/mL (lower limit of the target range) in the MWA group was higher than that in the calcitriol alone group (23% vs. 8%, p = 0.02). The mean iPTH level in the MWA group after MWA was lower than that in the calcitriol alone group (373.09 ± 322.31 vs. 552.28 ± 361.87 pg/mL, p < 0.001). There was a significant difference in the change in iPTH levels over time within the MWA group (p < 0.001) but not in the calcitriol alone group. Only one patient developed severe SHPT in the MWA group, while six patients in the calcitriol alone group developed severe SHPT (p = 0.04).
Conclusions: Compared with calcitriol alone, MWA plus calcitriol decreases iPTH levels and prevents the progression of mild-to-moderate SHPT.
Introduction
Secondary hyperparathyroidism (SHPT) is one of the most common complications experienced by haemodialysis patients and increases their risk of mortality [Citation1]. The level of intact parathyroid hormone (iPTH) is the most important measure of efficacy in the treatment of SHPT, but few patients achieve target levels. Twenty-nine per cent of patients in the Dialysis Outcomes and Practice Patterns Study reached target levels of iPTH levels [Citation2]. In Chinese haemodialysis patients, the incidence was approximately 27% [Citation3]. According to the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, the iPTH range for mild-to-moderate SHPT is between 300 and 600 pg/mL [Citation4]. The main treatment modality for mild-to-moderate SHPT is calcitriol, but the level of control in treated patients is low. More than 21% of haemodialysis patients still demonstrate uncontrolled mild-to-moderate SHPT, and more than 64% of SHPT patients demonstrate mild-to-moderate disease [Citation2]. Therefore, new treatment methods for mild-to-moderate SHPT must be developed.
Microwave ablation (MWA) is a minimally invasive technique that has been used for the treatment of severe SHPT since 2010 [Citation5] and may also be an effective treatment for mild-to-moderate SHPT. Both MWA and parathyroidectomy are used to treat severe SHPT. Compared with parathydoidectomy, MWA appears to offer several advantages. First, MVA produces little trauma compared to parathyroidectomy. As a result, in cases of recurrence, MVA can be used to ablate SHPT repeatedly, while the formation of scar tissue following parathyroidectomy makes a second surgery more difficult. Second, a total parathyroidectomy results in very low iPTH levels, which may lead to adynamic bone disease. However, since MWA is performed under ultrasound guidance, a portion of residual parathyroid tissue may be preserved, potentially preventing a permanent reduction in levels of iPTH. In this randomised, controlled, exploratory trial, we study the efficacy and safety of MWA in cases of mild-to-moderate SHPT.
Methods
Study design
This was a 12-month, single-centre, prospective, randomised, controlled, exploratory trial. We compared the efficacy of MWA plus calcitriol and that of calcitriol alone in patients with mild-to-moderate SHPT. The patients were randomly assigned into MWA and control groups. First, we screened all patients on chronic haemodialysis by iPTH levels between 1 January 2015 and 31 March 2015 in our Hemodialysis Center. Patients with iPTH levels of 300–600 pg/mL had an ultrasound examination of the parathyroid gland. Patients were eligible for inclusion if parathyroid nodules could be detected on ultrasound. All patients provided informed consent and were age-matched (±1 year; 1:1 pairwise matching). If the patients failed to be matched by age, they were excluded. Each pair of cases was assigned the number 1 or 2 at random using Excel software, and patients with the random number “1” were allocated to the control group, while patients with the number “2” were allocated into the MWA group. In accordance with the KDOQI guidelines, the subjects in the MWA group were treated with MWA first and later with calcitriol, while those in the calcitriol group were treated with calcitriol.
The study was approved by the Institutional Review Board of Beijing Friendship Hospital, Capital Medical University (number: BJFH-EC/2014–101) and was in compliance with the Declaration of Helsinki. This study was registered with ClinicalTrials.gov (number: NCT02332135).
Study participants
All patients with mild-to-moderate SHPT undergoing haemodialysis in our centre between 1 January 2015 and 31 March 2015 were eligible for this study. We used iPTH levels of 300–800 pg/mL as the criteria for a diagnosing moderate SHPT in accordance with the KDOQI guidelines.
Inclusion criteria: (1) age: 18–75 years; (2) iPTH levels: 300–800 pg/mL; (3) parathyroid nodules detectable on ultrasound.
Exclusion criteria: (1) previous parathyroid surgery including all ablations; (2) previous treatment with a calcimimetic within 1 month; (3) recent neck surgery within 3 months; (4) pregnancy or lactation.
Study procedure
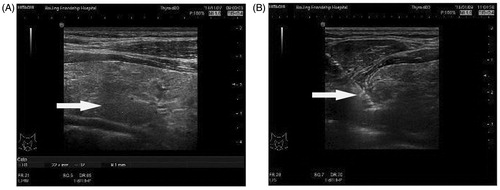
A single ultrasound specialist performed the MWA. The microwave therapy apparatus was a KY2000 ablation system (Nanjing Kangyou Applied Research Institute, Nanjing, China) operating at a frequency of 2450 MHz and equipped with a 2 mm outside diameter antenna with a 5 mm tip. The procedure was as follows (). First, an ultrasound of the parathyroid was performed to determine the blood supply to the parathyroid nodule and the ablation path. Second, after sterilising the neck, local anaesthesia using 2% lidocaine hydrochloride was applied. Next, 5 mL of 2% lidocaine hydrochloride was diluted in 15 mL of normal saline and injected into the area around the parathyroid nodule to develop a heat insulation layer to protect the adjacent nerve and blood vessel. Third, an ablation needle was inserted into the parathyroid tissue under ultrasound guidance. Ablation was subsequently performed. The MWA power used was between 25 and 35 watts according to the target nodule size. When hypoechoic signals covered the entire nodule and no flow signal was detected, ablation was terminated.
Follow-up
Blood samples for serum iPTH, calcium, phosphorus and alkaline phosphatase were obtained from all subjects at 0 and 2 months, and every 2 months thereafter until month 12. In addition, to monitor the efficacy and safety of MWA, the patients in the MWA group had an additional blood sample taken for iPTH levels 1 week following MWA. The follow-up was discontinued in cases of death or the development of severe SHPT. The definition of severe SHPT was [Citation4]: persistent serum levels of iPTH >800 pg/mL associated with hypercalcaemia and/or hyperphosphataemia that were refractory to medical therapy for 2 months.
Endpoints
Primary endpoints: (1) the percentage of patients who achieved target iPTH levels during the study period, (2) the change in iPTH levels after MWA: a comparison of iPTH levels after MWA between the two groups and the change over time of iPTH levels within each group, and (3) the percentage of subjects who developed severe SHPT.
Secondary endpoints: (1) calcitriol weekly dosage per patient, (2) overall percentage of patients who achieved target levels of serum calcium and phosphorus, and (3) changes in serum calcium and phosphorus after MWA.
Statistical analysis
Data were analysed using the Statistical Package for the Social Sciences (SPSS version 22.0, Chicago, IL). All continuous variables with a normal distribution are reported as the means and standard deviations. Variables with a non-normal distribution are reported as medians and interquartile ranges. Categorical variables are reported with frequencies.
A paired-sample t-test was used to compare the variables with a normal distribution between two groups: age, dialysis age, baseline iPTH level and size of parathyroid nodules. The Wilcoxon test was used when the distribution was skewed: follow-up time. Fisher’s exact test was used to compare categorical variables between the two groups. A mixed linear model was used to compare iPTH levels between groups and the change over time in serum iPTH levels within each group, where group and time were used as the fixed effects in this model, respectively. Because there was one additional iPTH examination in the MWA group (1 week after MWA), iPTH levels at this time point were only used for a statistical description and not for comparison between the two groups.
Results
Study patients
Of the 314 patients receiving haemodialysis between 1 January 2015 and 31 March 2015 in our Hemodialysis Center, 28 (9%) were enrolled in this study. Additional details are presented in . The clinical baseline data for these 28 subjects are shown in .
Figure 2. Randomisation and follow-up. Of 314 patients who underwent randomisation, 14 received MWA initially and then were treated according to the KDOQI guidelines; 14 were treated using calcitriol alone, in accordance with the KDOQI guidelines. In the MWA group, 13 completed the study, and one patient’s follow-up ended early because of the development of severe SHPT. In the control group, eight patients completed the study, and the follow-up of six patients ended early because of the development of severe SHPT.
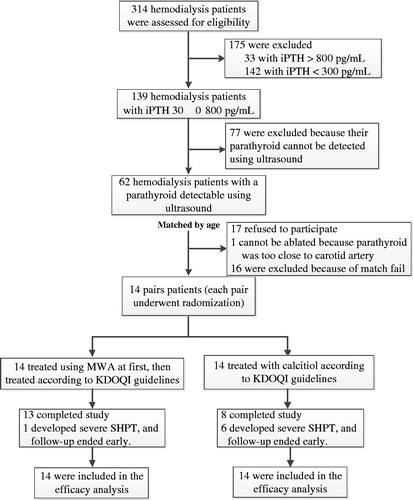
Table 1. Clinical baseline data for the study participants.
Endpoints
Primary endpoints
(1) The percentage of patients who achieved target levels of iPTH: there was no significant difference in the overall percentage of patients who reached target levels of iPTH between the MWA and calcitriol alone group (24% vs. 22%, p = 0.85). However, the percentage of patients who reached iPTH levels <150 pg/mL (lower limit of the target range) in the MWA group was considerably higher than that in the calcitriol alone group (23% vs. 8%, p = 0.02).
(2) The change in iPTH levels: the iPTH levels in the MWA group were higher than that in the calcitriol alone group at baseline (571.74 ± 186.24 vs. 530.11 ± 144.39 pg/mL; p = 0.51), but this difference did not reach statistical significance. The mixed model showed that the mean iPTH level of the MWA group after MWA was lower than that of the calcitriol alone group (373.09 ± 322.31 vs. 552.28 ± 361.87 pg/mL, p < 0.001; ).
Figure 3. Changes in serum levels of intact parathyroid hormone (iPTH). The levels of iPTH in the microwave ablation (MWA) group (B) were lower than those in the calcitriol alone group (A). Upper limit of the target range in iPTH (300 pg/mL). Lower limit of the target range in iPTH (150 pg/mL).
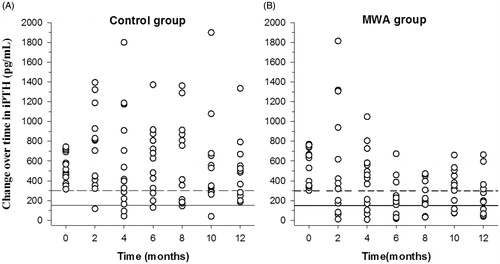
There was a significant difference in the change in iPTH levels over time within the MWA group (p < 0.001). Compared with the baseline, the iPTH levels of the MWA group after MWA were lower, and the difference reached significance after the four follow-up (months 6, 8, 10, and 12; p = 0.002, 0.014, 0.001 and 0.018, respectively). There was no significant difference in iPTH levels over time within the calcitriol alone group (p = 0.34).
(3) The percentage of subjects who developed severe SHPT: only one patient (7%) developed severe SHPT in the MWA group, and six patients (43%) in the calcitriol alone group developed severe SHPT (p = 0.04).
Secondary endpoints
(1) Calcitriol weekly dosage: the calcitriol weekly dosage for the MWA group was lower than that for the calcitriol alone group (1.56 ± 1.49 vs. 4.72 ± 4.72 µg, p = 0.03).
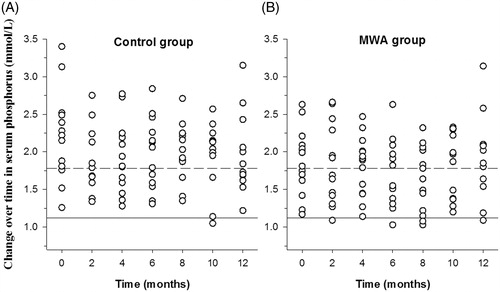
(2) The overall percentage of patient who reached target levels of serum calcium and phosphorus: the percentage of patients who achieved target levels of serum calcium in the MWA group after MWA was higher than that of the calcitriol alone group (40% vs. 24%, p = 0.049). The percentage of subjects who reached target levels of serum phosphorus after MWA was comparable between the MWA and calcitriol alone groups (phosphorus: 37% vs. 38%, p = 0.99).
(3) Change in serum calcium and phosphorus levels: there was no significant difference between serum calcium levels at baseline between the MWA and calcitriol alone groups (2.45 ± 0.27 vs. 2.58 ± 0.25 mmol/L, p = 0.25). There was also no significant difference in serum calcium levels between the two groups after treatment (calcitriol alone group 2.50 ± 0.24 vs. MWA group 2.35 ± 0.25 mmol/L, p± 0.14; ).
Figure 4. Changes in serum levels of calcium. The serum levels of calcium in the microwave ablation (MWA) group (B) were lower than those in the calcitriol alone group (A). Upper limit of the target range in calcium (2.10 mmol/L). Lower limit of the target range in calcium (2.38 mmol/L).
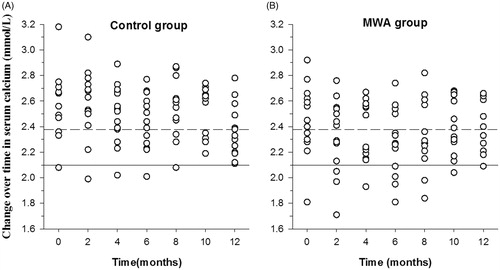
There was no significant difference in serum phosphorus levels between the MWA and calcitriol alone groups at baseline (1.87 ± 0.43 vs. 2.17 ± 0.61 mmol/L, p = 0.19), and no significant difference between them was observed after treatment (calcitriol alone group: 1.97 ± 0.45 vs. MWA group 1.82 ± 0.46 mmol/L, p = 0.16; ).
Safety
The main complications in the MWA group were recoverable hypocalcaemia and hoarseness. Three cases presented with hypocalcaemia and were given oral calcitriol and calcium carbonate. Two cases recovered to the normal serum calcium range at month 4, and the other case recovered at month 8. Three cases presented with hoarseness: one case recovered at month 1, the other two cases recovered at month 3.
Discussion
Our results demonstrate that the rate of achieving target levels of iPTH was comparable between the MWA and calcitriol alone groups, but the iPTH levels of the MWA group were significantly lower than those of the calcitriol alone group. Furthermore, the rate at which subjects developed severe SHPT in the MWA group was significantly lower than in the calcitriol alone group. In our study, MWA was also a safe procedure with no serious complications. The only side effects were hypocalcaemia and hoarseness, both of which resolved.
MWA is a minimally invasive treatment that involves thermal ablation, which causes parathyroid cells to lose their endocrine functionality. MWA is an effective treatment modality for severe SHPT. In one retrospective study, iPTH concentrations decreased from a pre-treatment mean of 1570 pg/mL (651–6800 pg/mL) to 419 pg/mL (21–1500 pg/mL) at a 12-month follow-up [Citation6]. However, MWA has been used for SHPT treatment for only 6 years [Citation5], and no comprehensive list of indications for when to use this technique in the treatment of SHPT has been established to date. Though MWA is a minimally invasive procedure, it has only been applied for the same indication as parathyroidectomy, namely severe SHPT.
However, MWA may also be effective for the treatment of mild-to-moderate SHPT for the following reasons. First, MWA does not result in very low iPTH levels. Because MWA is performed under ultrasound guidance, it is difficult to ablate the parathyroid tissue completely. Since the remaining parathyroid tissue still has endocrine functionality, very low iPTH levels rarely occur after MWA [Citation7]. In this study, the iPTH levels at all follow-ups were higher than 200 pg/mL. Conversely, total parathyroidectomy, performed though a more popular surgical method [Citation8–10], can result in very low iPTH levels, potentially leading to adynamic bone disease. Second, compared with MWA, parathyroidectomy produces a larger area of trauma. Most importantly, the scar tissue around the parathyroid following parathyroidectomy makes a second surgery more difficult in cases of recurrence. Considerably less scar tissue is formed with MWA, making it easier to treat recurrences with either surgery or repeat MWA. Third, although the use of MWA for severe SHPT was based on the same indications recommended for parathyroidectomy, in actuality, the indications for parathyroidectomy for SHPT are not well established [Citation11].
The main international guidelines recommend parathyroidectomy in cases of severe SHPT. However, the definition of severe SHPT varies among the different guidelines. The KDOQI guidelines define severe SHPT as [Citation4]: persistent serum levels of iPTH >800 pg/mL, associated with hypercalcaemia and/or hyperphosphataemia that are refractory to medical therapy. The Japanese Society for Dialysis Therapy guidelines define severe SHPT as iPTH levels >500 pg/mL or whole PTH levels >300 pg/mL [Citation12]. According to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [Citation13], severe SHPT is defined as patients with SHPT who fail to respond to medical/pharmacological therapy and have iPTH levels of more than 600 pg/mL.
There are several limitations in this study. First, the sample size was small, and the follow-up time was only 12 months. Long-term outcomes should be evaluated in future studies. Second, only a single practitioner performed the MWA procedure. Therefore, it is still unclear whether other practitioners can achieve similar results. Multi-centre studies with a large sample size are needed to verify the efficacy of this technique. Finally, two other thermal ablations, namely laser and radiofrequency ablation, were not performed because MWA was the only ablation technique available to our participants in this study. Thermal ablation procedures include MWA, radiofrequency and laser and are increasingly being used in the management of various diseases, such as thyroid nodules [Citation14,Citation15], metastatic lymph nodes in the neck [Citation16,Citation17] and hyperparathyroidism [Citation18,Citation19]. All three ablations are performed under ultrasound guidance, which reduces complications [Citation20]. Although MWA is faster than radiofrequency and laser, it requires significantly higher energy and may result in more complications. The efficacy of the other ablation techniques may be better than MWA for treating smaller parathyroid glands.
In conclusion, MWA produces a significant decrease in iPTH levels in patients with mild-to-moderate SHPT and prevents the progression to severe SHPT. This study has demonstrated that MWA is effective for the treatment of mild-to-moderate SHPT and should be used in clinical settings.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Melamed ML, Eustace JA, Plantinga L, et al. (2006). Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int 70:351–7.
- Tentori F, Wang M, Bieber BA, et al. (2015). Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 10:98–109.
- Kong X, Zhang L, Zhang L, et al. (2012). Mineral and bone disorder in Chinese dialysis patients: a multicenter study. BMC Nephrol 13:116.
- National Kidney Foundation. (2003). K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42:S1–S201.
- Zhang JQ, Qiu M, Sheng JG, et al. (2013). Ultrasound-guided percutaneous thermal ablation for benign parathyroid nodules. Acad J Second Mil Med Univ 34:362–70 [in Chinese].
- Yu MA, Yao L, Zhang L, et al. (2015). Safety and efficiency of microwave ablation for recurrent and persistent secondary hyperparathyroidism after parathyroidectomy: a retrospective pilot study. Int J Hyperthermia 32:180–6.
- Diao Z, Liu X, Qian L, et al. (2016). Efficacy and its predictor in microwave ablation for severe secondary hyperparathyroidism in patients undergoing haemodialysis. Int J Hyperthermia 32:614–22.
- Puccini M, Carpi A, Cupisti A, et al. (2010). Total parathyroidectomy without autotransplantation for the treatment of secondary hyperparathyroidism associated with chronic kidney disease: clinical and laboratory long-term follow-up. Biomed Pharmacother 64:359–62.
- Shih ML, Duh QY, Hsieh CB, et al. (2009). Total parathyroidectomy without autotransplantation for secondary hyperparathyroidism. World J Surg 33:248–54.
- Jia X, Wang R, Zhang C, et al. (2015). Long-term outcomes of total parathyroidectomy with or without autoimplantation for hyperparathyroidism in chronic kidney disease: a meta-analysis. Ther Apher Dial 19:477–85.
- Lorenz K, Sekulla C, Dralle H. (2013). Surgical management of renal hyperparathyroidism. Zentralbl Chir 138:e47–54.
- Fukagawa M, Yokoyama K, Koiwa F, et al. (2013). Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial 17:247–88.
- KDIGO CKD-MBD Work Group. (2009). KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl S1–S130.
- Pacella CM, Mauri G, Achille G, et al. (2015). Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab 100:3903–10.
- Mauri G, Cova L, Monaco CG, et al. (2017). Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia 33:295–9.
- Mauri G, Cova L, Ierace T, et al. (2016). Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol 39:1023–30.
- Mauri G, Cova L, Tondolo T, et al. (2013). Percutaneous laser ablation of metastatic lymph nodes in the neck from papillary thyroid carcinoma: preliminary results. J Clin Endocrinol Metab 98:E1203.
- Peng C, Zhang Z, Liu J, et al. (2017). Efficacy and safety of ultrasound-guided radiofrequency ablation of hyperplastic parathyroid gland for secondary hyperparathyroidism associated with chronic kidney disease. Head Neck 39:564–71.
- Sormaz IC, Poyanlı A, Açar S, et al. (2017). The results of ultrasonography-guided percutaneous radiofrequency ablation in hyperparathyroid patients in whom surgery is not feasible. Cardiovasc Intervent Radiol 40:596–602.
- Mauri G, Solbiati L. (2015). Virtual navigation and fusion imaging in percutaneous ablations in the neck. Ultrasound Med Biol 41:898.