Abstract
Introduction: CRS and HIPEC confer survival benefit in selected patients with peritoneal metatases (PM). Accurate preoperative assessment of disease burden and exclusion of distant metastases are crucial in selecting the appropriate patient. We evaluate the utility of PET-CT scans in comparison with CT and MRI scans in patients considered for CRS and HIPEC.
Methods: Data were retrospectively collected from patients who had been discussed for CRS and HIPEC between January 2011 and December 2015, at our institutional multidisciplinary tumour board. Patients who underwent PET-CT scan were included. Results of PET-CT were compared against traditional imaging. Patient and tumour factors were analysed to identify those who were most likely to benefit from PET imaging.
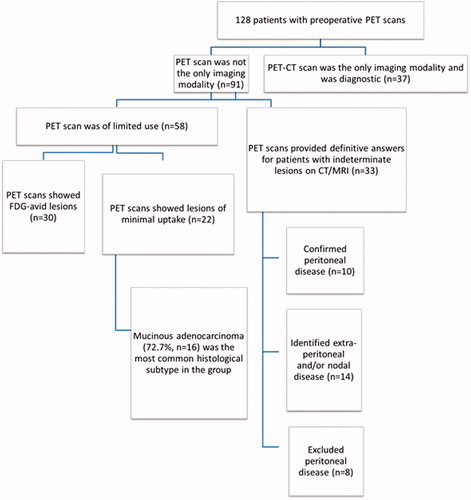
Results: Four hundred and seven patients were considered for CRS and HIPEC. PET-CT was performed for 128(31.4%) patients: being the only imaging modality in 37 and used as an adjunct in 91. In the latter group, it was not beneficial in 58 patients as it provided no additional information (n = 33) or showed lesions of minimal FDG uptake (n = 25). In 33 patients, PET-CT provided definitive answers for indeterminate lesions seen on CT and MRI, confirmed the diagnosis of peritoneal disease in 10 patients (30.3%), identified extra-peritoneal disease and/or nodal metastases in 15 (45.5%) and excluded peritoneal disease in 8 (24.2%). The usefulness of PET-CT was predicted by tumour histology (p = .009), with non-mucinous tumours benefitting the most.
Conclusion: Our results suggest that PET-CT can be used as an adjunct to CT and/or MRI scans, when lesions on the CT/MRI scans are indeterminate, and that it is most useful in patients with non-mucinous tumours.
Introduction
Peritoneal metastases (PM) are thought of as a harbinger of dismal prognosis. Patients with peritoneal disease were traditionally treated with a palliative intent, but since the 1990s, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been increasingly performed to treat PM, changing the management of PM entirely. In selected patients with appendiceal, colorectal, ovarian, primary peritoneal and peritoneal mesothelioma malignancies, this combined modality procedure has been shown to improve survival [Citation1–8]. This dual-modality works on the principal that CRS removes all macroscopic disease and HIPEC targets the residual microscopic disease. Achieving complete cytoreduction has been shown repeatedly to the most important prognostic factor for survival [Citation9–11], whereby the outcomes of patients with residual disease ≥2.5 mm drop dramatically [Citation12].
As with all complex surgical procedures, patient selection is paramount in CRS-HIPEC. Pre-operatively, it is imperative to exclude extra-abdominal metastases and assess the ability to achieve optimum cytoreduction. Although there have been some studies demonstrating feasibility of concomitant liver or lung metastatectomy during CRS and HIPEC, the general consensus is that this aggressive modality should be reserved for those with peritoneal-only disease [Citation1,Citation4]. Additionally, a proportion of patients (25–48%) who are planned for CRS-HIPEC do not successfully complete the surgery, due to extensive disease noted intra-operatively. These patients are deemed to having undergone an opened-closed surgery [Citation13,Citation14]. In our own institution, the opened-closed rate is 13% [Citation15], and hence a significant proportion of patients are subjected to an unnecessary laparotomy. Furthermore, excluding patients from resection at the time of laparotomy often results in delay to systemic chemotherapy.
In an attempt to reduce rates of opened-closed surgery, some institutions have explored the role of diagnostic laparoscopy in the pre-operative setting [Citation16,Citation17]. However, laparoscopy is not without risks and in patients with PM, visualisation is often suboptimal due to adhesions from previous surgery. As a result, most surgeons rely on pre-operative imaging to estimate the extent of disease and assess resectability. Computed tomography (CT) and magnetic resonance imaging (MRI) are the most commonly utilised imaging modalities. However, the sensitivity of these modalities can drop to as low as 9% [Citation18] in low-volume peritoneal disease. Imaging scores have also been suggested to enhance patient selection, but these are often based on CT scans and have their limitations [Citation19–21].
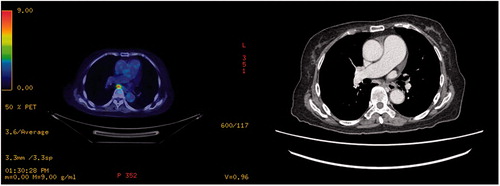
PET scans work on the basis that tumour cells are metabolically deranged and hence take up more of the glucose analogue fluorodeoxyglucose (FDG). Increasingly, they are being utilised during the pre-operative assessment of patients planned for CRS-HIPEC, to exclude extra-abdominal metastases, and to assess indeterminate lesions seen on CT/MRI. Nevertheless, the PET-CT is costly, might not provide additional information, and hence may not be necessary for all patients being considered for CRS-HIPEC.
The aim of our study is first, to evaluate the utility of PET-CT imaging in the pre-operative assessment of patients being considered for CRS-HIPEC, specifically to assess if PET-CT had resulted in change in management in our patients and secondly, to identify the group of patients who would most likely benefit from a PET-CT scan.
Methods
The study was carried out with the approval of the Centralized Institutional Review Board of the Singapore Health Services. Patients presented at the weekly multidisciplinary tumour boards (MDTB) for consideration of CRS-HIPEC and underwent a PET-CT between January 2011 to December 2015 were included in the study. Demographic and clinicopathogical data were retrospectively collected for these patients from electronic and clinical charts.
At our institution, patients with PM who are being considered for CRS and HIPEC are put up for discussion at the weekly MDTB where medical, surgical, radiation oncologists, pathologists and oncologic radiologists are present. All imaging scans, whether done within our institution or externally, are evaluated by our radiologist and/or nuclear medicine physician who specialised in oncologic imaging. All lesions seen on scans are reviewed and the surgical risks discussed. Patients with extensive disease such as extensive small bowel or mesentery involvement, porta hepatitis infiltration were usually deemed not suitable for surgical resection.
The patients are evaluated for the presence of extra-abdominal disease via a CT scan of the thorax, while the extent of peritoneal disease is examined on a CT or MRI scan of the abdomen and pelvis. In cases where lesions are indeterminate on CT/MRI scans, a 18-FDG PET-CT may be recommended and the patient is reviewed again after the PET-CT results are out. An indeterminate lesion on CT or MRI scan is defined as a lesion without clear radiological features of malignancy but remains suspicious in the known clinical context of cancer, for example a lymph node that was not enlarged by size criteria.
In some cases, a PET-CT scan may have been performed as part of the initial staging work-up and is the only imaging the patient receives.
Imaging
18-FDG PET/CT
Patients were fasted for 6 h before scans. Blood glucose levels were checked to ensure that they were below 11 mmol/L. They received an intravenous injection of approximately 10 mCi of 18F-FDG 60 to 75 min before a whole body PET-CT scan was performed. This included a 16-slice CT scan with either oral or IV contrast (depending on clinician preference) for attenuation correction and anatomical correlation. FDG PET emission scan duration was for 2–3 min per bed position depending on patient size. Both the 18F-FDG PET and CT component of the images were read together by one nuclear medicine physician and one radiologist. Both were not blinded to clinical data. In cases of discrepancy, diagnosis was reached by consensus.
MRI
Patients were fasted for 4 h before scans. The MR abdomen and pelvis studies were preformed after receiving a single intravenous dose of gadolinium contrast (Dotarem). Anti-spasmodic agents are not routinely administered at our institution. The patients were scanned with either 1.5 T or 3 T scanners. The abdominopelvis imaging included 5 mm cuts from the base of lungs to the iliac crest/upper thighs. T1 and T2 weighted sequences were obtained with post contrast dynamic imaging of the imaged region. Fat suppressed sequences (DIXON) and diffusion imaging were also performed to aid in lesion detection and characterisation. B values of 0, 100 and 600 were used.
CT
Patients were fasted for 4 h before scans. Most of our patients undergo CT imaging will receive an intravenous injection of approximately 70 ml of Iohexol (Omnipaque 350), an iodinated contrast, titrated according to body weight. Oral contrast was administered at the discretion of the radiologist, based on comorbidities of the patient and scan indication. All CT scans were performed following institution protocol with either 64/128/192 or 256 slice CT equipment. Thickness of reconstruction was 3 mm for both the thoracic and abdominopelvis regions in both the axial and coronal planes. The radiologists reading the scans were not blinded to clinical data.
Data analysis
Patients, who only had a PET-CT performed as part of the pre-operative assessment, were considered separately from those patients who had both PET-CT and CT/MRI. When available, results of the PET-CT were compared against the CT and/or MRI scans performed within 30 d of the PET-CT. The PET-CT was considered to be ‘beneficial’ if it provided definitive conclusions for indeterminate lesions seen on CT and/or MRI. It was regarded as being ‘non-beneficial’ if it provided no additional information to that revealed by CT and/or MRI.
In patients who underwent surgery, resection specimens were sent for histopathology and were considered the gold standard reference for which all imaging was compared against. However, in patients who had not undergone surgery, the standard of reference was the development of the lesion of interest on subsequent imaging.
Patient and tumour factors were subsequently analysed to identify the group of patients who were most likely to benefit from pre-operative assessment with a PET-CT. Tumour board decisions were also considered to see if PET-CT had resulted in a change in management.
Statistical analysis
Chi-square test was used to assess for correlation between the PET-CT results, origin of tumour and histology. Statistical analyses were performed using SPSS version 21 (SPSS, Chicago, IL) and a p value ≤.05 was considered significant.
Results
We identified 407 patients who were presented at the MDTB for consideration for CRS and HIPEC during the study period. PET-CT was performed in 128 (31.4%) patients. The basic demographics and clinicopathological characteristics of these 128 patients are summarised in .
Table 1. Demographics and clinicopathologic characteristics of the patients who underwent a PET-CT scan.
The majority of our patients were female (66.4%, 85/128), and the median age was 53 years (15–78 years). The majority of our patients were Chinese (71.9%, n = 92). Most of our patients had colorectal cancer (47.7%, n = 61) of which adenocarcinoma (n = 45) was the most common histological subtype, followed by mucinous adenocarcinoma (n = 16). This was followed by ovarian cancer (27.3%, n = 35) of which papillary serous adenocarcinoma (n = 25) was the most common subtype. The other histologies within the ovarian cancers included mucinous, clear cell, endometriod and adenocarcinoma not otherwise specified (NOS). All 16 cases of appendiceal tumours were of mucinous histology.
Of the 128 patients, 54 underwent surgery and post-operative histopathology was used as the gold standard for which the imaging was compared against. The remaining patients were followed up with subsequent scans and those scans were used as standard of reference.
Thirty-seven (28.9%) patients had PET-CT as the only imaging modality and all the scans were diagnostic of peritoneal disease. In this group of patients, 15 were recommended at the multidisciplinary tumour board to undergo CRS and HIPEC. Eleven of these patients underwent successful CRS and HIPEC; three were found at laparotomy to have extensive disease and one patient declined treatment. The other 22 patients were deemed to have unresectable disease at tumour board and were given palliative treatment. All these patients were excluded from subsequent analyses. summarises the impact of PET scans in our patient cohort.
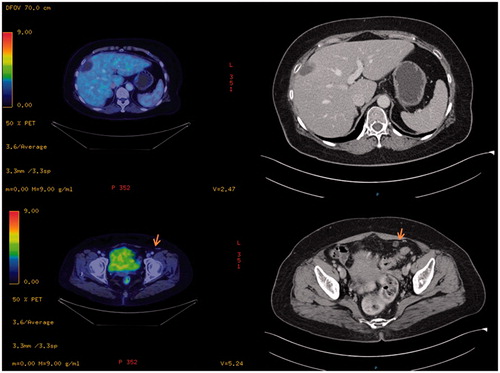
In the remaining 91 patients where PET-CT was used as an adjunct, it was ‘beneficial’ in 33 (36.2%) patients. These patients had indeterminate peritoneal or extra-peritoneal lesions on their CT/MRI scans for which the PET-CT was able to provide clarification. Of these 33 patients, PET-CT confirmed peritoneal-only disease in 10 (30.3%) of the patients and excluded it in eight patients (24.2%). Extra-abdominal disease and/or extra-abdominal lymph nodes were identified in 15 patients (45.5%) and were thus spared from unnecessary surgery and referred for palliative therapy. The 10 patients who had peritoneal-only disease eventually underwent successful CRS and HIPEC, and all achieved complete cytoreduction. The eight patients who had no peritoneal disease went on to have surgical resection of their primary disease without HIPEC. All patients who had disease shown on PET-CT went on to have either histologically proven disease or progression of disease on follow-up scans.
Of the 58 (63.7%) patients where PET-CT was ‘not beneficial’, 31 (53.4%) showed FDG-avid lesions that coincided with the peritoneal lesions seen on the CT/MRI scans but did not provide any additional information. In 25 (43.1%) patients, the peritoneal disease itself had minimal or no FDG avid uptake and hence, the PET scan similarly provided no additional information. Two patients (3.4%) had no disease on the PET-CT and CT/MRI, but had disease noted intra-operatively. In one of the cases, the patient had laparotomy done as he was symptomatic and had raised tumour markers and in the other case, the patient had a biopsy-proven Sister Mary Joseph nodule and was hence scheduled for laparotomy with a plan to proceed with CRS and HIPEC.
Usefulness of PET-CT based on origin of tumour
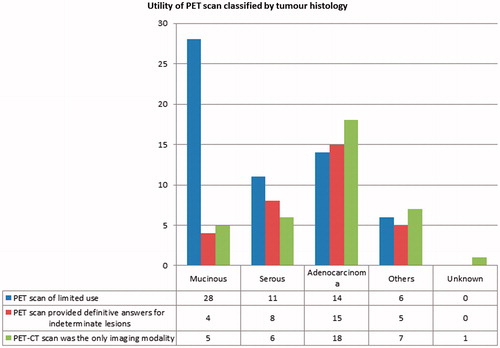
We also looked at the benefit of PET scans based on the origin of tumour (). In appendiceal tumours, PET-CT was not beneficial in 10 patients and beneficial in three patients. For the colorectal tumours, PET-CT was not beneficial in 23 cases but provided definitive answers in 15 cases. It was beneficial in 11 of the ovarian tumours, but did not have additional value in 16 cases. On univariate analysis, tumour origin was not significantly associated with benefit of PET-CT (p = .670).
Table 2. Classification of PET scan utility by site of primary tumour.
Usefulness of PET-CT based on histology
Tumour histology was significantly associated with the usefulness of PET-CT (p = .009). We found that the PET-CT were most useful in adenocarcinomas (15 out of 29, 51.7%) (), followed by serous carcinomas (eight out of 19, 42.1%). However, in the mucinous tumours, PET-CT scans were more often of limited value (28 out of 32, 87.5%) ( and ).
Discussion
CRS and HIPEC have been shown to improve survival for selected patients with PM and are increasingly being utilised in the treatment of patients with PM [Citation1–8]. Achieving complete cytoreduction is paramount to the success of the procedure, making the pre-operative assessment of local and distant disease involvement one of the greatest challenges in selecting patients for CRS-HIPEC.
CT and MRI scans are commonly used but these are anatomic imaging and do not provide information about the presence and activity of viable tumour cells. For example, a lymph node that is not enlarged by size criteria may still harbour tumour cells but would not be picked up on CT or MRI. For this, the PET scan becomes a valuable modality, working on the basis that the deranged cell growth in cancer would result in greater metabolic rate and FDG uptake. Thus, the degree of FDG-avidity can be helpful in differentiating malignant from benign tumours in cases of CT or MRI-indeterminate lesions. To our knowledge, there have only been a few studies evaluating the usefulness of PET-CT in patients undergoing CRS and HIPEC and none in the Asian context [Citation9,Citation22–24].
In our patient cohort, CT and/or MRI provided adequate information for decision-making in 63.7% (58 out of 91) of the cohort with PET-CT scans performed as adjuncts but PET-CT scans resulted in a change in management in 36.2% (33 out of 91) of our patients, mainly due to the avoidance of unnecessary laparotomies in 16.4% (15 out of 91) of the patients. This is similar to its effect when used for evaluating patients who are considered for liver resection of colorectal liver metastases [Citation17,Citation25–27]. Importantly, all patients who had disease shown on PET-CT went on to have either histologically proven disease or progression of disease on follow-up scans, suggesting that PET-CT has a highly positive-predictive value in our cohort though the formal value was not calculated due to the lack of histopathology in a significant proportion of our patients.
In the 58 patients who had PET-CT scans that were not beneficial, 31 of them (53.4%) had no additional information provided when compared to conventional imaging. In the other 43.1% (n = 25), the lesions were only minimally or non-FDG avid and would not be easier to interpret than on CT or MRI. 76% (n = 19) of these lesions were mucinous tumours and this was not surprising as our results found PET scans to be less useful in mucinous tumours (p = .009) probably due to the large amounts of non-viable mucin which would not be metabolically active. This result was similar to previous studies by De Vos et al. and Whiteford et al. who reported lower sensitivity of FDG-PET in mucinous cancers as compared with non-mucinous cancers (58–60% versus 92–96%) [Citation23,Citation24,Citation28]. In the remaining two patients, both conventional imaging and PET scans did not definitively pick up peritoneal disease but they were found to have peritoneal lesions at laparotomy. This raises the question of sensitivity of PET-CT in cases of minimal peritoneal disease. Tanaka et al. reported a sensitivity of 88% in PET as compared with 38% in CT, with the smallest lesion visualised on PET being 15 mm in diameter [Citation29], but these superior results were not observed in other studies by Turlakow et al. and Pfannenberg et al. who reported a sensitivity of 57% and 63%, respectively [Citation30,Citation31]. Pelosi et al. [Citation32] also suggested that PET could give false-negative results in lesions less than 1 cm.
PET-CT was adequate to diagnose disease load in 37 out of 128 patients who had PET-CT as their only imaging modality but in these cases, it is difficult to assess the extent of benefit of PET-CT as compared with conventional imaging.
Lastly, we also looked at the role of origin of tumour in predicting if PET-CT scans would be helpful but found this to be insignificant (p = .670). This result more likely reflects tumour histology, rather than the tumour origin. In appendiceal tumours where all tumours were mucinous, PET-CT scans were of limited value in 76.9% (10 out of 13), while in the case of colorectal cancers where most tumours were non-mucinous, 39.4% (15 out of 38) of the PET-CT scans performed were of benefit.
The limitations of PET-CT have prompted other investigators to suggest the use of diagnostic laparoscopy as a method to assess disease burden [Citation16,Citation33] in patients prior to CRS-HIPEC. Despite a usefulness of more than 80%, the complication rates were reported to be 0–10% [Citation16,Citation33]. Hence diagnostic laparoscopy is still not without risk and non-invasive methods should be considered initially. Furthermore, in our local context, many patients have had previous abdominal surgeries and the adhesions may result in suboptimal visualisation and assessment of the peritoneum. However, in mucinous tumours where the PET-CT is not useful, diagnostic laparoscopy could be considered as an alternative and should be further investigated. Currently, most reports on diagnostic laparoscopy are in patients with gastric and colorectal peritoneal carcinomatosis [Citation17,Citation33].
Limitations
We acknowledge that this paper has its limitations. Given the retrospective nature of this study, there are inherent biases. In addition, we were not able to calculate the sensitivity and specificity of PET-CT in our population, as histopathology is considered as gold standard of reference, but not all patients had undergone surgery and hence we did not have histopathology for all patients. Furthermore, there was heterogeneity in the choice of imaging and imaging technique used. It is not uncommon in our institution for patients to come to us after having done initial investigations at other institutions. Within our own institution, administration of oral contrast for CT, antispasmodic agents for MRI and the sequences of images was left up to the discretion of the radiologist based on the comorbidities and the presentation of the patient and indication of the scan. However, despite the above, we believe that this paper does illustrate the utility of PET-CT in the selection of patients with non-mucinous tumours.
Conclusion
Our study found that pre-operative PET-CT scans affected the management of 36.2% of patients being considered for CRS-HIPEC, avoiding an unnecessary laparotomy in 16.4% (15 out of 91) of patients. PET-CT was not useful for the mucinous tumours, hence should be considered only in patients with non-mucinous tumour histologies, in an attempt to improve patient selection and reduce unnecessary laparotomies.
Disclosures statement
The authors have no disclosures.
References
- Teo MC. (2014). Update on the management and the role of intraperitoneal chemotherapy for ovarian cancer. Curr Opin Obstet Gynecol 26:3–8.
- Teo MC, Ching Tan GH, Lim C, et al. (2015). Colorectal peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: the experience of a tertiary Asian center. Asian J Surg/Asian Surg Assoc 38:65–73.
- Teo MC, Tan GH, Tham CK, et al. (2013). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in Asian patients: 100 consecutive patients in a single institution. Ann Surg Oncol 20:2968–74.
- Sadeghi B, Arvieux C, Glehen O, et al. (2000). Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88:358–63.
- Mirnezami R, Mehta AM, Chandrakumaran K, et al. (2014). Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br J Cancer 111:1500–8.
- Verwaal VJ, Bruin S, Boot H, et al. (2008). 8-Year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 15:2426–32.
- Verwaal VJ, van Ruth S, de Bree E, et al. (2003). Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 21:3737–43.
- Yan TD, Deraco M, Baratti D, et al. (2009). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 27:6237–42.
- Klumpp B, Aschoff P, Schwenzer N, et al. (2013). Correlation of preoperative magnetic resonance imaging of peritoneal carcinomatosis and clinical outcome after peritonectomy and HIPEC after 3 years of follow-up: preliminary results. Cancer Imaging 13:540–7.
- Baumgartner JM, Tobin L, Heavey SF, et al. (2015). Predictors of progression in high-grade appendiceal or colorectal peritoneal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 22:1716–21.
- Tan G, Chia C, Kumar M, et al. (2017). 201 Consecutive cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) procedures in a single Asian tertiary centre. Int J Hyperthermia 33:288–94.
- Lin EK, Hsieh MC, Chen CH, et al. (2016). Outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer with peritoneal metastasis. Medicine 95:e5522.
- van Oudheusden TR, Braam HJ, Luyer MD, et al. (2015). Peritoneal cancer patients not suitable for cytoreductive surgery and HIPEC during explorative surgery: risk factors, treatment options, and prognosis. Ann Surg Oncol 22:1236–42.
- Iversen LH, Rasmussen PC, Laurberg S. (2013). Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br J Surg 100:285–92.
- Yong ZZ, Tan GH, Wong JF, et al. (2016). Unresectability during open surgical exploration in planned cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia 32:889–94.
- Jayakrishnan TT, Zacharias AJ, Sharma A, et al. (2014). Role of laparoscopy in patients with peritoneal metastases considered for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). World J Surg Oncol 12:270.
- Wiering B, Krabbe PF, Jager GJ, et al. (2005). The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer 104:2658–70.
- de Bree E, Koops W, Kroger R, et al. (2006). Preoperative computed tomography and selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 32:65–71.
- Tan GH, Kwek JW, Hosseini R, et al. (2016). Proposed radiological criteria for pre-operative determination of resectability in peritoneal-based malignancies. J Med Imaging Radiat Oncol 60:337–43.
- Flicek K, Ashfaq A, Johnson CD, et al. (2016). Correlation of radiologic with surgical peritoneal cancer index scores in patients with pseudomyxoma peritonei and peritoneal carcinomatosis: how well can we predict resectability? J Gastrointestinal Surg 20:307–12.
- Lopez-Lopez V, Cascales-Campos PA, Gil J, et al. (2016). Use of (18)F-FDG PET/CT in the preoperative evaluation of patients diagnosed with peritoneal carcinomatosis of ovarian origin, candidates to cytoreduction and hipec. A pending issue. Eur J Radiol 85:1824–8.
- Pasqual EM, Bertozzi S, Bacchetti S, et al. (2014). Preoperative assessment of peritoneal carcinomatosis in patients undergoing hyperthermic intraperitoneal chemotherapy following cytoreductive surgery. Anticancer Res 34:2363–8.
- De Vos N, Goethals I, Ceelen W. (2014). Clinical value of (18)F-FDG- PET-CT in the preoperative staging of peritoneal carcinomatosis from colorectal origin. Acta Chirurgica Belgica 114:370–5.
- Alessi A, Martinelli F, Padovano B, et al. (2016). FDG-PET/CT to predict optimal primary cytoreductive surgery in patients with advanced ovarian cancer: preliminary results. Tumori 102:103–7.
- Lake ES, Wadhwani S, Subar D, et al. (2014). The influence of FDG PET-CT on the detection of extrahepatic disease in patients being considered for resection of colorectal liver metastasis. Ann R Coll Surgeons England 96:211–5.
- Yip VS, Poston GJ, Fenwick SW, et al. (2014). FDG-PET-CT is effective in selecting patients with poor long term survivals for colorectal liver metastases. Eur J Surg Oncol 40:995–9.
- Briggs RH, Chowdhury FU, Lodge JP, Scarsbrook AF. (2011). Clinical impact of FDG PET-CT in patients with potentially operable metastatic colorectal cancer. Clin Radiol 66:1167–74.
- Whiteford MH, Whiteford HM, Yee LF, et al. (2000). Usefulness of FDG-PET scan in the assessment of suspected metastatic or recurrent adenocarcinoma of the colon and rectum. Dis Colon Rectum 43:759–67. discussion 67–70.
- Tanaka T, Kawai Y, Kanai M, et al. (2002). Usefulness of FDG-positron emission tomography in diagnosing peritoneal recurrence of colorectal cancer. Am J Surg 184:433–6.
- Pfannenberg C, Konigsrainer I, Aschoff P, et al. (2009). 18)F-FDG-PET/CT to select patients with peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 16:1295–303.
- Turlakow A, Yeung HW, Salmon AS, et al. (2003). Peritoneal carcinomatosis: role of (18)F-FDG PET. J Nuclear Med 44:1407–12.
- Pelosi E, Deandreis D. (2007). The role of 18F-fluoro-deoxy-glucose positron emission tomography (FDG-PET) in the management of patients with colorectal cancer. Eur J Surg Oncol 33:1–6.
- Marmor RA, Kelly KJ, Lowy AM, Baumgartner JM. (2016). Laparoscopy is safe and accurate to evaluate peritoneal surface metastasis prior to cytoreductive surgery. Ann Surg Oncol 23:1461–7.