Abstract
Background: Temperature increase in tumour tissue during photothermal therapy (PTT) is a significant factor in determining the outcomes of the treatment. Therefore, controlling and optimising temperature distribution in target tissue is crucial for PTT. In this study, we developed a unique ex vivo device to study the temperature distribution during PTT to be used as a guide for the desired photothermal effects for cancer treatment.
Methods: Bovine liver tissue buried inside agarose gel served as a phantom tumour surrounded by normal tissue. A thermostatic incubator was used to simulate tissue environment in live animals. The temperature distributions were measured by thermocouples with needle probes at different locations inside the target tissue, during laser irradiation using an 805-nm laser.
Results: The results obtained using the ex vivo device were verified by comparing the tissue temperature directly measured in animal tumours irradiated under the same conditions. With this model, the spatial distribution of temperature in target tissue can be monitored in real time. A two-dimensional temperature distribution in target tissue allows us to establish the correlations among laser parameters, temperature distribution and tumour size. In addition, the optimal temperature range for tumour destruction and immunological stimulation was determined using metastatic rat mammary tumour model.
Conclusion: The device and method developed in this study can provide guidance for choosing the appropriate treatment parameters for optimal photothermal effects, particularly when combined with immunotherapy, for cancer treatment.
Introduction
Photothermal therapy (PTT) is a relatively new modality for local cancer treatment with minimal invasiveness and limited damage to surrounding normal tissue, due to the sensitivity of tumour cells to temperature elevation [Citation1–3]. Selective photothermal interaction causes a temperature gradient in target tissue to induce different biological responses, ranging from cell stress to cell death that can expose tumour antigens for immunological stimulation [Citation4,Citation5]. However, extremely high temperature elevation can lead to total tissue destruction with denatured proteins, and may additionally damage surrounding healthy tissue and hamper host immune response [Citation6,Citation7]. Therefore, the outcome of PTT depends on the temperature gradient created in the tumour tissue.
Laser immunotherapy (LIT) combines targeted PTT with intratumoural injection of an immunoadjuvant, leading to a synergistic photothermal–immunological effect in the target tumour tissue that is capable of triggering a long-term antitumour immunity. The LIT-induced host antitumour response can eliminate both residual tumour cells at the treatment sites and untreated metastases at distant sites [Citation8–11]. Although still in its developmental stage, LIT has already shown significant clinical success in the treatment of late-stage melanoma and breast cancer patients [Citation12–14]. Previously, non-invasive light delivery in LIT was the main mode of operation, using a laser diffuser over the treatment site [Citation9,Citation12,Citation14]. However non-invasive laser irradiation faces difficulties when treating deeply seated tumours or tumours with heavily pigmented overlying skin, due to limited light penetration in tissue. To circumvent these barriers, we developed interstitial laser immunotherapy (ILIT) to induce a desired photothermal effect [Citation15], while maintaining the advantages of combined immunological stimulation. When laser light is directed into the target tumour tissue through an interstitial fibre, deep tumours can be treated and damage to healthy skin tissue can be avoided. Currently, Visualase and NeuroBlate systems, MRI-guided laser interstitial thermal therapy (LITT), laser interstitial thermal therapy (LITT), have been used for brain tumours [Citation16–18].
In LIT, the objective of laser photothermal interaction is to kill as much tumour cells as possible, while preserving tumour proteins for the immunological stimulations [Citation4,Citation19]. Therefore, accurate determination of tissue temperature distribution during interstitial laser irradiation is crucial to control and improve treatment outcomes.
There are several traditional methods of temperature measurement in biological tissue, such as near infra-red (NIR) thermal imaging, fibre optic temperature sensors, magnetic resonance imaging (MRI) thermometry and computed tomography-thermometry [Citation15,Citation20–23]. NIR imaging can only monitor the surface temperature of biological tissue. Fibre optic temperature sensors monitor the temperature at fixed points inside biological tissue. MRI and computed tomography-thermometry are good techniques for measuring temperature distribution inside tissue with a wide spatial range and a non-invasive detector; however, these methods require special working environment and are not cost effective [Citation20,Citation24].
In order to accurately determine the temperature distribution in tumour tissue for optimising the photothermal effects, we developed an ex vivo simulation model. In this model, a thermostatic incubator was used to provide the temperature environment similar to that in live animals. Bovine liver tissue inserted in phantom gels was used to mimic tumour tissue surrounded by normal tissue. Gel phantom and chicken breast tissue have also been used [Citation15,Citation25], but the temperature increase mode and spatial distribution are different from real tumour tissue in vivo. Using this model, the temperature distribution in target tissue was measured during ILIT, using thermocouple needle probes. Systematic investigation of photothermal interaction using this model will lead to a better understanding of the relationship between temperature distribution and laser parameters, in order to guide future clinical applications of ILIT.
Methods
Ex vivo tumour tissue model for temperature detection
An ex vivo model of ILIT has been developed to study the impact of laser parameters on the temperature distribution in an environment close to in vivo conditions. The schematic of the experimental device is shown in . An incubator was used to provide a constant temperature (35 °C) environment as in live animals. Bovine liver tissue buried inside 10% agarose gel was used to simulate a tumour surrounded by normal tissue.
Figure 1. Schematic of the model for determination of temperature distributions. (A) Schematic diagram of the simulation model for ex vivo temperature measurement during interstitial photothermal therapy. (a) A thermostatic incubator providing constant temperature environment as in live animals. (b) A phantom gel (10%) simulating the normal tissue surrounding the tumour. (c) A bovine liver tissue buried inside the gel simulating tumour tissue. (d) An optical fibre with an interstitial cylindrical diffuser (10 mm) which was directly inserted into the centre of the target tissue for photothermal therapy. (e) Needle probes of thermocouple inserted in the tissue, paralleling the fibre with a separation of 1.28 mm. (B). A sectional view of temperature distribution in target tissue during interstitial laser irradiation. The black circle in the centre of the target tissue represents the active lens of the laser fibre. Channels 1–8 represent eight needle probes of thermocouple that were inserted in the tissue parallelling to the fibre tip. Ring chromaticity diagram of the target tissue indicates the temperature distribution during photothermal therapy. The spatial range for tissue temperature measurement (with eight needle probes) is 1.28–10.24 mm away from the laser fibre tip.
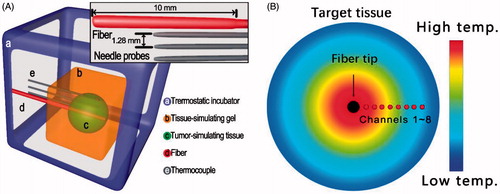
For laser irradiation, a fibre with a 1.0-cm cylindrical diffuse tip was inserted into the centre of the bovine liver tissue. For comparison with rat tumour models, three needle probes were fixed at 1.28, 3.00 and 5.50 mm away from the tip of the fibre tip. For all other experiments, eight thermocouple needle probes were fixed together with a separation distance of 1.28 mm. The needle probes were then inserted into the bovine liver tissue parallel to the laser fibre tip. The position of fibre tip and needle probes is shown in . The sampling rate of the thermocouple detection system was 2 Hz. After the raw temperature data were collected, they were processed using MATLAB R2016b (The MathWorks Inc, Boston, MA).
Tumour models
In this study, metastatic rat mammary tumour, DMBA-4, was used. DMBA-4 cells were cultured with Dulbecco’s Modified Eagle’s medium (DMEM, GIBCO, Gran Island, NY), supplemented with 10% foetal bovine serum (FBS, Sigma, St Louis, MO), penicillin (100 units/ml) and streptomycin (100 µg/ml) (Sigma), in 5% CO2, at 37 °C in a humidified incubator (NuAire, Plymouth, MN).
A total of 105 viable tumour cells were injected subcutaneously into the back of female Wistar Furth rats. Laser treatment took place when the tumour reached a diameter of 10–15 mm along its longest principle axis. Before treatment, the animal hair overlying the tumour was removed. Animals were anaesthetised with isoflurane (2%) using a CDS 2000 portable anaesthesia machine with flowmeter (SurgiVet, V700000MRI) and constantly monitored for anaesthetised status using visual signs of respiration. The animal protocol for this study was approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center (OUHSC). All the experiments were conducted in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH) and approved by the Institutional Animal Care and Use Committee (IACUC) of OUHSC.
In vivo temperature measurement during interstitial laser photothermal therapy
Laser treatments were performed using an 805-nm diode laser (Delta-30, AngioDynamics, Queensbury, NY) with continuous-wave irradiation. The laser light was delivered into the target tissue through an optical fibre with an active cylindrical tip of 1.0 cm (pioneer Optics, Windsor Locks, CT).
Before the experiment, the thermocouples were calibrated with InstruNET software (Omega Engineering, Stamford, CT) using freezing and boiling water baths at 0 and 100 °C, respectively. Laser treatments were conducted with a laser power of 3.0 W for 10 min. To acquire the temperature data, three thermocouple needle probes (Omega Engineering, Stamford, CT, HYP1-30) were inserted into the target tissue at 1.28, 3.0 and 5.5 mm away from the centre of the cylindrical active tip. The sampling rate of the thermocouple detection system was set to 2 Hz.
For therapeutic studies of laser immunotherapy on rat tumour, glycated chitosan, an immunoadjuvant that has been applied to the treatment of metastatic breast cancer patients [Citation12], was injected into central of the treated tumour followed by laser irradiation. The animals were observed daily for 100 days.
Cell death analysis and HSP70 release detection
Tumour cells were incubated under different temperature for 20 min and recovered at 37 °C for 3 h. Cells were collected and stained with FITC-conjugated anti-Annexin-V and (fluorochrome) 7AAD (Millipore, Darmstadt, Germany). Fluorescence was then analysed using a Muse cell analyser (Millipore). Supernatants were collected and analysed with HSP70 ELISA kits (R&D systems, Minneapolis, MN).
Results
Testing the ex vivo model with rat tumour model
To verify whether the ex vivo model works as an animal tumour model to study the dynamic temperature distribution during interstitial laser irradiation, the temperature increase in bovine tissue and rat tumour was determined under same treatment conditions. As shown in , temperature increase trend at the same locations is similar in both rat tumour and bovine liver during interstitial laser irradiation at 3 W for 10 min. The elevation of temperature in both tissues ranged from 17 to 53 °C at distances of 5.5–1.28 mm away from the active tip, respectively.
Figure 2. Temperature distributions in rat tumour tissue during in vivo interstitial photothermal therapy, and in bovine liver during ex vivo interstitial laser irradiation, both measured by thermocouple. (A) Temperature increases at different locations in rat tumour and bovine liver, during interstitial laser irradiation (3 W for 10 min). (B) Comparison of difference in measurement of T′’ between rat tumour and bovine liver. (C) Bland–Altman plot of temperature increase in rat tumour and bovine liver at 5.5 mm away fibres. During interstitial laser irradiation, the consistency of temperature increase trend at same locations in rat tumour and bovine liver, indicated that our ex vivo simulation model with bovine liver can predict the temperature distribution in rat tumour tissue during laser therapy.
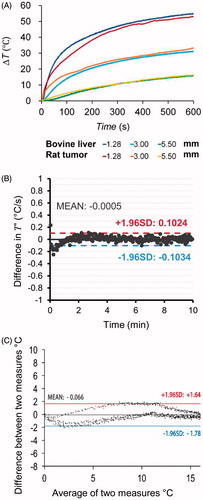
Due to the fact that we are attempting to compare a time–temperature relationship between two measurement modalities, a traditional Bland–Altman plot, where difference in measurement is plotted vs. mean measurement, may not describe the entire measurement procedures and outcomes. The temperature change rate T′ was also considered. From , it can be seen that the measurement of T′ experienced primarily random variations during treatment rather than time-dependent variation. As such, a region of error can be constructed for the differences between the two measurements. The mean difference in measurement of T′ was not statistically different from zero; therefore, the two measurement methods yielded the same time derivative. Thus, the time–temperature–position relationship between the two techniques have the same shape over time. Bland–Altman analysis was also used to compare the measurements using the bovine tumour model and rat DMBA-4 breast cancer tumours (). The results in demonstrated the reliability of our ex vivo model.
Characteristics of temperature increase in target tissue during interstitial laser irradiation
The temperature elevations in bovine liver tissue during interstitial laser irradiation were determined using the ex vivo model. As shown in , each curve of temperature increase indicates the dynamic changes of temperature during laser irradiation at different locations away from the fibre tip (1.28 mm separation of each needle). The temperature increased smoothly at each location during laser irradiation and the overall temperature increase depends on the distance away from the laser fibre.
Figure 3. Temperature as functions of locations and time during interstitial laser irradiation with ex vivo simulation model. (A) Temperature increase at different locations in bovine liver during interstitial laser irradiation (3 W for 15 min). Eight needle probes of thermocouple were inserted in the tissue, paralleling the fibre with separation of 1.28 mm. The temperature increase curves indicate the temperature changes at each location detected by eight needle probes of thermocouple (top to bottom of curves vs. near to far of probes). (B) Temperature change rate in the target tissue at different locations. The entire treatment duration is separated into three periods, based on the time derivative of temperature. Period A is a temperature increase phase (about 120 s) with the time derivative of temperature higher than 0.1 °C/s. Period B is a temperature stable phase with the time derivative of temperature less than 0.1 °C/s. Period C is the cooling phase after laser off. (C) Two-dimensional temperature elevation in bovine liver at stable phase during interstitial laser irradiation (3 W for 15 min), based on the characteristic temperature increase in Eq. (2), simulated using the data detected by eight needle probes of thermocouple for a spatial range of 10 mm. (Inset) Correlation of temperature increase to the distance from fibre tip during laser irradiation. The stable temperature during laser irradiation was chosen for the comparison between different treatments.
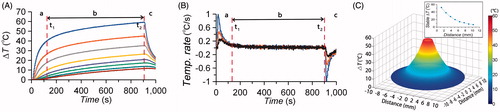
To study the characteristics of the temperature increase at different times during laser irradiation, we utilised the time derivative of temperature.
(1)
where T′ represents the time derivative of temperature and T(t) represents the temperature at time t. Based on the modes of the time derivative of temperature, the treatment duration had been separated into three periods (). Period A is the rapid increase mode (about 120 s) with T′>0.1 °Cs-1. Period B is the stable increase mode with 0≤T′≤0.1 °Cs-1. Period C is the cooling mode that follows laser shutoff with T′<0 °Cs-1.
Furthermore, we define the characteristic temperature increase, ΔT-(p), in order to compare the temperature increase under different conditions,
(2)
where T(p,t) represents the temperature increase at position p and at time t, and time points t1 and t2 refer to the beginning and end of the stable increase mode, respectively. Using this characteristic temperature, the temperature increase at different locations can be analysed using a single metrics. Based on the data detected by the eight needle probes and considering the bovine liver as a radially symmetrical, a two-dimensional (2-D) characteristic temperature increase plots were constructed using MATLAB (). These results not only showed the stability of temperature measurement by thermocouple using the ex vivo model, but also showed a powerful function for detecting spatial distribution of temperature in target tissue in real time that could be used for an in-depth study on photothermal reaction to determine the different relations of temperature distribution and treatment parameters.
Two-dimensional temperature elevations during interstitial laser irradiation
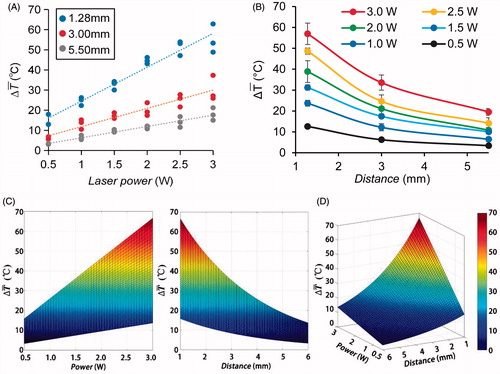
The temperature distribution was correlated to laser power and tumour size. Tumour size of about 12 mm and a treatment time of 10 min was chosen, to be consistent with that used for animal and patient treatment by LIT/ILIT [Citation9,Citation12,Citation14]. The temperature increase at different locations in target tissue during laser irradiation with powers of 0.5–3 W was detected. Based on the raw temperature data, a two-dimensional temperature elevation map in bovine liver at stable phase during interstitial laser irradiation was constructed using MATLAB (). With laser power increase, the spatial distribution of temperature obviously increased indicating more extensive thermal effects in target tissue. A positive linear relation between temperature increase and laser power was established (). The spatial distribution of temperature increase showed a negative, non-linear relation between temperature increase and the distance away from fibre tip (). An exponential curve was chosen to approximate this non-linear relationship. Finally, based on the data, an empirical model of temperature increase during ILIT was developed (). This model shows a clear relationship between spatial temperature elevations and laser power.
Figure 4. Two-dimensional temperature elevations in bovine liver at the stable phase during interstitial irradiation with different laser powers. The X axis and Y axis represent the distance of the locations away from the fibre tip. The Z axis represents the stable temperature at the locations.
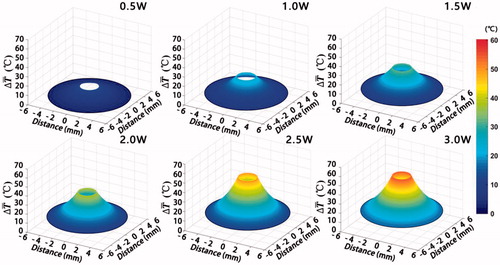
Figure 5. The temperature distribution at different locations in bovine liver during irradiation with different laser powers. (A) Temperature increase during interstitial laser irradiation with different doses, at different locations in bovine liver. Data were chosen from three independent experiments to correlate the temperature increase at different locations to laser powers. (B) The spatial distribution of temperature increase in tissue under the laser irradiation with different powers. Data were averaged from three independent experiments under each laser dose and fitted according to distance. (C) Two-dimensional temperature distribution in bovine liver during irradiation at different laser powers. (D) Three-dimensional simulation graph for the correlation of stable temperature at different locations with different laser powers. The X axis represents the laser power, the Y axis represents the distance of the locations away from the fibre tip and the Z axis represents the stable temperature during treatment.
Immunological effects of temperature increase
HSP70 is sensitive to heat stress and plays an important role in immune response. Therefore, the expression of HSP70 was considered to evaluate the immunogenicity of tumour cells. To further correlate the temperature increase to killing effects and immunological effects, we analysed cell death and HSP70 release of tumour cells incubated under different temperatures. Cell death increased with temperature increase, reached 80% at 18 °C (). The HSP70 release also peaked when the temperature reached 18 °C (). The results clearly showed the optimal temperature range for tumour cell destruction and immunological stimulation.
Figure 6. The relationship between temperature distributions to immunological effects. (A) Cell death and HSP70 release of DMBA4 rat mammary tumour cells stimulated under different temperatures. DMBA4 cells were incubated under different temperature for 20 min and then recovery at 37 °C for another 3 h. The cells were collected for cell death analysis by AnnexinV/7AAD staining and the supernatant were collected for HSP70 release analysis using ELISA (n = 4). (B) Survival rates of tumour-bearing rats under interstitial LIT with different laser irradiation powers.
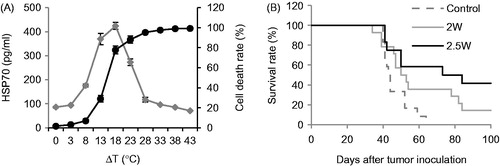
Survival study was performed to determine the therapeutic efficacy of LIT on rat metastatic tumours, with different laser powers. As shown in , with an irradiation time of 20 min, the laser power at 2 W resulted in a 14% animal survival rate, while a laser power at 2.5 W yielded a much higher survival rate (40%). These results showed that the therapeutic effect of LIT was closely related to laser parameters.
Discussion
The purpose of our research is to build a model to determine temperature distribution during interstitial PTT in order to guide its clinical applications to achieve optical treatment outcomes. The thermal effect induced by light energy in tissues is an important indicator for outcomes of phototherapy [Citation1,Citation2]. We developed an ex vivo model to simulate the tumour tissue and determine temperature distribution during interstitial laser irradiation. With this model, we actually monitored the changes of temperature at different locations in target tissue during interstitial laser irradiation. A two-dimensional temperature distribution in target tissue can intuitively reveal the correlation of laser parameters, temperature increase and tumour size.
Because of the non-uniformity of tumour tissue, it is difficult to correlate the temperature distribution to the parameters of treatments for guidance of the application of PTT for cancer treatment [Citation26–28]. Therefore, ex vivo homogeneous tissue models were selected for conducting temperature studies, such as gel phantom, chicken breast tissue and bovine liver tissue [Citation15,Citation25]. However, the photothermal characteristics in these tissue models are different from that of real tumour tissue during laser irradiation because of the influence of tissue absorption, tissue cooling and other factors, particularly, without the constant temperature environment of real tumour tissue in animals. Therefore, we developed an ex vivo system to simulate the real temperature measurement in live animals, as shown in . At temperatures of 37–50 °C, tumours experience increased blood flow and oxygenation [Citation29]. When the temperature rises above 50 °C, tissue experiences coagulation, ablation and vaporisation [Citation29,Citation30]. In ILIT, we used laser to rise the temperature above 50 °C to ablate the tumour tissue. Additionally, this model maximally reduced heat loss during interstitial laser irradiation, due to the constant temperature in the incubator and liver buried in a phantom gel that avoids the thermal exchange with the air. While this system does not take into consideration of the blood flow, the measured temperature distribution in bovine liver tissue was consistent with that in real rat tumour during interstitial laser irradiation, as shown in . Further fine tuning of this system to incorporate air flow and blood flow will render this system more accurate.
Because of the high accuracy and the rapid response of thermocouples with multiple fixed needle probes, the temperature at different locations can be measured precisely in real time to show temporal and spatial distributions of temperature in target tissue during laser irradiation (). The three phases of temperature increase during interstitial laser irradiation () can be used to predict a stable temperature increase for clinical treatment. The correlation between tissue temperature increases and the laser power and location, as shown in and , can be potentially used as a guide to choose the appropriate parameters to achieve the optimal thermal effects. Especially, to maintain the biological viability of treated tumour tissue to increase the immunogenicity, the maximum temperature should be kept below 75 °C [Citation11]. Therefore, the laser power was at relatively low values, which may explain the linearity between temperature and laser power, as seen in .
Irreversible tissue damage due to thermal interaction begins around 41 °C as proteins begin to denature, and it depends on the time at which the tissue is held at a given temperature [Citation31]. About 63% of epidermal tissue would die if it is held at 45 °C for 9 h; however, the same amount of damage would occur in about 1 s at 60 °C [Citation31]. During ILIT, the temperature at the edge of tumour tissue should be increased by 18 °C to ensure the killing effects on target tumours; and the temperature increase inside tumour should be kept below 38 °C to ensure the HSP70 release, based on the data in . With this model, we expect to be able to predict the optimal laser parameters for clinical studies.
It has been noted that the metallic conductors of the thermocouple highly absorb the radiation emitted by laser, causing a local increase of temperature which entail an overestimation of the actual tissue temperature [Citation26]. Using an 810 nm laser with a cylindrical applicator, Reid et al. recorded a maximum temperature difference of about 10 °C between the values measured by thermocouple and the values measured by the fluoroptic sensor during laser application in the agar-albumin phantom [Citation32]. In our previous study, we compared the temperature measurements of thermocouples and MRI. Under laser irradiation at 1.5 W for 10 min, an increase of 8 °C at 3 mm away from the active tip was measured by MRI, and an increase of 10 °C was measured by the thermocouple [Citation15]. Here, we also showed that the temperature measured by the thermocouple at 1.28 mm away from the fibre tip was affected, while temperatures at 3.00 and 5.5 mm were not significantly affected. While the direct absorption of light by the metal probes of the thermocouple, its contribution to temperature reading decreases with the thickness of the tissue between the optical applicator and the thermocouples [Citation33]. Therefore, the temperature was not significantly affected by the absorption of the metal needles of the thermocouple at 3.00 and 5.5 mm. In spite of this measurement artefact, thermocouples are the most widely used sensors for temperature measurement during laser therapy. Use of thermocouples is reported in several studies dedicated to temperature monitoring during laser therapy in several treatment settings, including: retina, angioplasty, liver and benign prostatic hyperplasia [Citation34,Citation35]. To further address this issue, a correction factor can be developed to compensate the potential overestimate of the temperature by the thermocouples, to improve the accuracy of the prediction of tissue temperature using our ex vivo temperature measurement model.
Conclusions
We demonstrated that our ex vivo simulation model can be used to simulate the temperature distribution of target tissue during ILIT. It can predict temperature elevation for tumour of different sizes with different laser power and duration, in order to optimise laser parameters (power, pulse duration, fibre position and number of fibres). Future experiments will focus on the applicability and generality of the parameter optimisation method to achieve optimum immunological effects using laser immunotherapy under the guidance of this simulating model.
Disclosure statement
The animal protocol for this study was approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center (OUHSC). All the experiments were conducted in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH) and approved by the Institutional Animal Care and Use Committee (IACUC) of OUHSC.
No conflicts of interest, financial or otherwise, are declared by the authors.
Additional information
Funding
References
- Camerin M, Rello S, Villanueva A, et al. (2005). Photothermal sensitisation as a novel therapeutic approach for tumours: studies at the cellular and animal level. Eur J Cancer 41:1203–12.
- Diederich CJ. (2005). Thermal ablation and high-temperature thermal therapy: overview of technology and clinical implementation. Int J Hyperthermia 21:745–53.
- Chu KF, Dupuy DE. (2014). Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 14:199–208.
- den Brok MH, Sutmuller RP, van der Voort R, et al. (2004). In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res 64:4024–9.
- Zhang H-G, Mehta K, Cohen P, et al. (2008). Hyperthermia on immune regulation: a temperature’s story. Cancer Lett 271:191–204.
- Dewhirst MW, Lee C-T, Ashcraft KA. (2016). The future of biology in driving the field of hyperthermia. Int J Hyperthermia 32:4–13.
- van den Tempel N, Horsman MR, Kanaar R. (2016). Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int J Hyperthermia 32:446–54.
- Chen WR, Jeong SW, Lucroy MD, et al. (2003). Induced antitumor immunity against DMBA-4 metastatic mammary tumors in rats using laser immunotherapy. Int J Cancer 107:1053–7.
- Chen WR, Liu H, Ritchey JW, et al. (2002). Effect of different components of laser immunotherapy in treatment of metastatic tumors in rats. Cancer Res 62:4295–9.
- Chen WR, Singhal AK, Liu H, et al. (2001). Antitumor immunity induced by laser immunotherapy and its adoptive transfer. Cancer Res 61:459–61.
- Zhou F, Li X, Naylor MF, et al. (2015). InCVAX – a novel strategy for treatment of late-stage, metastatic cancers through photoimmunotherapy induced tumor-specific immunity. Cancer Lett 359:169–77.
- Li X, Ferrel GL, Guerra MC, et al. (2011). Preliminary safety and efficacy results of laser immunotherapy for the treatment of metastatic breast cancer patients. Photoch Photobio Sci 10:817–21.
- Li X, Naylor MF, Le H, et al. (2010). Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther 10:1081–7.
- Naylor MF, Chen WR, Teague TK, et al. (2006). In situ photoimmunotherapy: a tumour-directed treatment for melanoma. Br J Dermatol 155:1287–92.
- Le K, Li X, Figueroa D, et al. (2011). Assessment of thermal effects of interstitial laser phototherapy on mammary tumors using proton resonance frequency method. J Biomed Opt 16:128001.
- Carpentier A, McNichols RJ, Stafford RJ, et al. (2011). Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med 43:943–50.
- Mohammadi AM, Schroeder JL. (2014). Laser interstitial thermal therapy in treatment of brain tumors – the NeuroBlate System. Expert Rev Med Devices 11:109–19.
- Rahmathulla G, Recinos PF, Kamian K, et al. (2014). MRI-guided laser interstitial thermal therapy in neuro-oncology: a review of its current clinical applications. Oncology 87:67–82.
- Yoon TJ, Kim JY, Kim H, et al. (2008). Anti-tumor immunostimulatory effect of heat-killed tumor cells. Exp Mol Med 40:130–44.
- Fani F, Schena E, Saccomandi P, et al. (2014). CT-based thermometry: an overview. Int J Hyperthermia 30:219–27.
- Quesson B, de Zwart JA, Moonen CT. (2000). Magnetic resonance temperature imaging for guidance of thermotherapy. J Magn Reson Imaging 12:525–33.
- Schena E, Tosi D, Saccomandi P, et al. (2016). Fiber optic sensors for temperature monitoring during thermal treatments: an overview. Sensors 16:1144.
- Winter L, Oberacker E, Paul K, et al. (2016). Magnetic resonance thermometry: methodology, pitfalls and practical solutions. Int J Hyperthermia 32:63–75.
- Chen Y, Gnyawali SC, Wu F, et al. (2008). Magnetic resonance imaging guidance for laser photothermal therapy. J Biomed Opt 13:044033.
- Liu VG, Cowan TM, Jeong S-W, et al. (2002). Selective photothermal interaction using an 805-nm diode laser and indocyanine green in gel phantom and chicken breast tissue. Lasers Med Sci 17:272–9.
- Anvari B, Motamedi M, Torres JH, et al. (1994). Effects of surface irrigation on the thermal response of tissue during laser irradiation. Lasers Surg Med 14:386–95.
- Cox B. (2007). Introduction to laser-tissue interactions. PHAS 4886:1–61.
- Welch AJ, Van Gemert MJ. (2011). Optical-thermal response of laser-irradiated tissue. Vol. 2. The Netherlands: Springer.
- Song C, Park H, Lee C, et al. (2005). Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 21:761–7.
- Roemer RB. (1999). Engineering aspects of hyperthermia therapy. Annu Rev Biomed Eng 1:347–76.
- Anghileri LJ, Robert J. (1986). Hyperthermia in cancer treatment. Vol. 2. Boca Raton, FL: CRC Press.
- Reid AD, Gertner MR, Sherar MD. (2001). Temperature measurement artefacts of thermocouples and fluoroptic probes during laser irradiation at 810 nm. Phys Med Biol 46:N149.
- Schena E, Majocchi L. (2014). Assessment of temperature measurement error and its correction during Nd:YAG laser ablation in porcine pancreas. Int J Hyperthermia 30:328–34.
- Saccomandi P, Schena E, Silvestri S. (2013). Techniques for temperature monitoring during laser-induced thermotherapy: an overview. Int J Hyperthermia 29:609–19.
- Van Nimwegen S, L’Eplattenier H, Rem A, et al. (2008). Nd:YAG surgical laser effects in canine prostate tissue: temperature and damage distribution. Phys Med Biol 54:29.
