Abstract
Background: Malnutrition is associated with increased postoperative morbidity in colorectal surgery. This study aimed to determine if preoperative nutritional markers could predict postoperative outcomes for patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) for peritoneal metastasis (PM) of colorectal origin.
Methods: All patients who underwent a complete CRS-HIPEC for colorectal PM between January 2009 and December 2014 were evaluated. Preoperative clinical and biological nutritional factors, including Body Mass Index (BMI), preoperative albumin and prealbumin levels were analysed. Preoperative computed tomography was used to measure the cross-sectional surface of the visceral and subcutaneous adipose tissue, at the third lumbar vertebrae, to assess the abdominal fat composition. Skeletal muscle mass was measured to assess for sarcopenia.
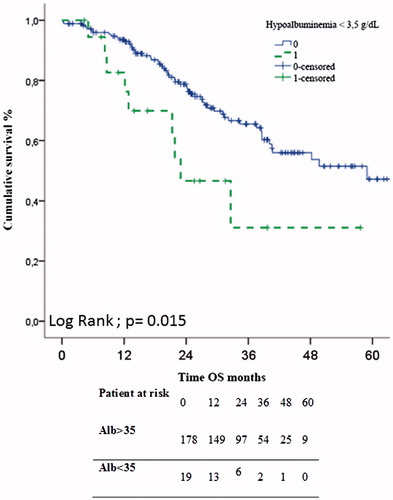
Results: Among 214 patients, 14 (6.5%) had a BMI ≥ 35 kg/m2, 90 (42%) were sarcopenic, 19 (9%) presented albumin <35 g/L and 2 (1%) had pre-albumin <20 mg/dL. Median values for visceral and subcutaneous fat surfaces were 99.2 cm2 and 198 cm2, respectively. Hypoalbuminemia was associated with worse overall survival (23 vs. 59 months, p = 0.015). The other nutritional factors did not impact overall or progression free survival after CRS-HIPEC for colorectal PM. In multivariate analysis, major post-operative complication and hypoalbuminemia were independently associated with decreased overall survival.
Conclusions: Hypoalbuminemia appears as a strong predictive factor for decreased overall survival in patients presenting PM of colorectal origin undergoing CRS-HIPEC.
Introduction
Colorectal cancer (CRC) is the third most common cancer in western countries [Citation1]. In this type of cancer, peritoneal metastasis (PM) is a common form of presentation for distant disease, occurring in 13% of patients [Citation2]. Before the development of efficient systemic chemotherapy and cytoreductive surgery, colorectal cancers with PM were associated with poor prognosis [Citation3]. Recently, survival of patients with PM from CRC has been dramatically improved by complete cytoreductive surgery (CRS) associated with hyperthermic intraperitoneal chemotherapy (HIPEC) for selected patients [Citation4–6].
In this surgical approach, the occurrence of postoperative complications significantly decreases long term survival [Citation7,Citation8]. Preoperative patient selection is a key factor to achieve optimal outcomes. Malnutrition is associated with increased postoperative morbidity in colorectal surgery [Citation9] due to delays in wound healing and an increased rate of postoperative infections, which lead to lengthened hospital stay. Preoperative nutritional assessment aims to identify patients suffering from severe malnutrition in order to improve their nutritional status using oral or parenteral nutritional supplementation. Pre-operative albuminemia, a non-invasive way to evaluate nutritional status, was reported as both an independent predictive factor for severe post-operative complications and as a prognostic factor in ovarian cancer after complete CRS [Citation10]. More recently, other non-invasive nutritional evaluations determined on preoperative CT-scans have raised interest. Sarcopenia, which is defined as low skeletal muscle mass, was associated with increased postoperative morbidity and worse survival after surgery in several cancers. After CRS for colorectal PM, sarcopenia was associated with an increased risk of re-operation and toxicity related to chemotherapy [Citation11,Citation12]. Accumulation of visceral adipose tissue was also associated with a poorer prognosis after oncologic surgery [Citation13–15].
This study aimed to identify, in a large population, the impact of preoperative nutritional markers on post-operative outcomes for patients undergoing CRS ± HIPEC for colorectal PM.
Patients and methods
Population
The prospective institutional PM database was searched to identify all consecutive patients who underwent a complete CRS for colorectal PM between January 2009 to December 2014 at Lyon Sud hospital. For each of these patients, the following data were collected: age at time of CRS, gender, preoperative body mass index (BMI: kg/m2), preoperative chemotherapy and the number of cycles, preoperative albumin (normal range 35 to 55 g/L) and prealbumin (normal range 16–40 mg/dL) levels measured using the immunoturbidimetric method on an auto analyser (Architect c8000; Abbott Diag., Lake Forest, IL, USA), American Society of Anaesthesiology (ASA) score, Charlson score, extent of PM measured by the peritoneal cancer index (PCI), length of surgery, realisation of HIPEC, major complications occurring within 90 days classified using the NCI (National Cancer Institute) Common Terminology Criteria for Adverse Events classification version 4.0 [Citation16], and the length of hospital stay. The study was performed in accordance with the precepts established by the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Surgical procedure
Patient selection and perioperative management were previously described [Citation17]. Briefly, for all patients deemed fit for CRS, chemotherapy was discontinued at least four weeks before surgery (six weeks for anti-angiogenics). A comprehensive preoperative work-up was performed to evaluate the patient’s physical condition and extent of disease. Before surgery, individual patient treatment strategies were systematically defined in multidisciplinary tumour conferences, based on clinical and radiological data, and according to international guidelines [Citation18]. All patients received 10 days of preoperative oral immunonutrition.
During CRS, after a comprehensive exploration of the abdominal cavity, the peritoneal cancer index (PCI) was determined and a complete CRS was performed when feasible, combining peritonectomies and organ resections. The cytoreduction was considered complete if a completeness of cytoreduction (CC) score of 0 or 1 was achieved (residual nodules <2.5 mm).
Before 2010, all patients received a hyperthermic intraperitoneal chemotherapy (HIPEC) with either Oxaliplatin (360 mg/m2 for 30 min at 43 °C) or Mitomycin C (35 mg/m2 for 90 min at 43 °C) using a closed abdomen technique after a complete CRS. After 2010, all patients were included into the ACCORD 15/PRODIGE7 trial (NCT00769405 clinicaltrial.org), which is a French randomised controlled trial comparing CRS alone versus CRS and HIPEC with oxaliplatin for colorectal PM.
Skeletal muscle mass, visceral and subcutaneous fat content measurements on CT
All patients were scheduled to undergo a CT-scan examination of the abdomen and pelvis for diagnostic purposes within two days before cytoreductive surgery [Citation19]. Standard-dose CT acquisitions of the abdomen and pelvis were performed using a 40-row device (Philips Brilliance, Best, The Netherlands), after intravenous administration of contrast material at the portal venous phase (Xenetix 300; Guerbet, Aulnay, France). The following parameters were used: 40 × 0.625 mm collimation, pitch: 0.874, kV: 120, mAs: auto, standard reconstruction algorithm: 1.5 mm reconstruction thickness, increments: 0.75 mm.
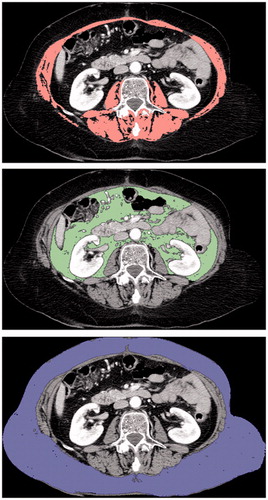
Axial CT slices were exported for further analysis using OsiriX version 5.0 (32-bit; http://www.osirix-viewer.com). Cross-sectional surface measurements of adipose tissue (visceral and subcutaneous) and muscle (psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal oblique, and rectus abdominis), at the third lumbar vertebrae (L3), were performed by a trained radiologist blinded to patient outcomes. Semi-automated specific tissue demarcation was performed using previously described Hounsfield unit (HU) thresholds: −29 to +150 for muscle, −190 to −30 for adipose tissue [Citation20–22]. Manual corrections were performed when other structures, outside the respective compartment, were detected (). The skeletal muscle index, to evaluate sarcopenia, was calculated by dividing the total cross-sectional muscle area by the squared height (cm2/m2). The cut-off values to define sarcopenic patients were 52.4 cm2/m2 for men and 38.5 cm2/m2 for women, as previously reported [Citation21]. Regarding visceral and subcutaneous fat surface values, we tried to determine a threshold using a ROC-curve. However, no cut-off value could be determined, therefore the population was divided using median values.
Figure 1. Measurement of body composition parameters using axial CT slice at the level of the third lumbar vertebra. (a) Delineation of total muscle area (pink), using a threshold of −29 to +150 HU. (b) Delineation of visceral (green) and (c) subcutaneous (blue) fat areas, using a threshold of −190 to −30 HU.
Statistical analysis
The primary endpoint of this study was to evaluate the impact of preoperative nutritional factors on overall survival (OS), defined as the time interval from the CRS-HIPEC to death due to any cause, and progression free survival (PFS), defined as the time interval from CRS-HIPEC to first disease progression. The secondary endpoint was the major complication rate within 90 days after surgery, graded as III, IV or V (death) and based on the NCI common terminology criteria for adverse events (CTCAE) version 4.0 [Citation16].
Non-normally distributed continuous data were summarised in terms of the median with range and compared with the Mann–Whitney U-test. Categorical data were compared by chi-squared tests. A p values of less than 0.05 was considered statistically significant. Continuous variables were converted into binary categories for comparison purposes. Overall (OS) and progression-free survival (PFS) were calculated using the Kaplan–Meier method and compared using the log-rank test. Cox regression survival analyses with enter method for the covariates were conducted to determine factors associated with overall survival. Only factors with p values of less than 0.1 in the multivariate analyses were reported. Survival was calculated from the date of CRS. Statistical analysis was performed with the SPSS software version 19.0 (SPSS Inc.; IBM, Chicago, IL, USA).
Results
Population
A total of 240 consecutive patients underwent a complete CRS for colorectal PM in the time period studied. Among them, 214 met the criteria for analysis. Twenty patients were excluded because they did not have a preoperative CT-scan and six because of missing weight and/or size data. shows demographics and disease characteristics for all included patients. Fourteen patients had a BMI ≥35 kg/m2, 90 were sarcopenic, and 28 presented a low albumin level (Albumine <35 g/L).
Table 1. Baseline demographic and disease characteristics of the 214 patients.
Survival analysis
Median follow-up was 24 months (range 0–82 months). Median OS and PFS were 58.9 and 13 months, respectively. Three- and five-year OS were 63% and 46%, respectively. Regarding PFS, three- and five-year survival were 17% and 14%, respectively. shows survival after CRS with regard to each nutritional factor. A low albumin level (<35 g/L) before CRS was associated with worse OS (23 vs. 59 months, p = 0.015) (). The other nutritional factors did not impact on OS or PFS.
Table 2. Outcomes after cytoreductive surgery separated by nutritional factors in univariate analysis.
In multivariate analysis, a low albumin level (<35 g/L) and the occurrence of a major postoperative complication () were factors independently associated with overall survival.
Table 3. Univariate and multivariate analyses of overall survival after cytoreductive surgery for colorectal peritoneal metastasis.
Major postoperative complication analysis
shows nutritional factors comparing patients with and without major complications following cytoreductive surgery. Major postoperative complications occurred for 108 patients (50%). No nutritional factors were associated with postoperative morbidity, regardless of the extent of the PM stratified by PCI, using ranges of PCI 0–5; 6–10 and 11–15.
Table 4. Comparison of patients, with or without major complication, after cytoreductive surgery for peritoneal carcinomatosis separated by nutritional factor, n (%).
Discussion
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy is a complex procedure with multiple variables that influence intraoperative, postoperative, and long-term outcomes. Predictive factors are pivotal to improve patient selection and therapeutic strategies. In our study, we found that pre-operative hypoalbuminemia was a strong nutritional marker with statistically significant worse survival outcome without significant predictive value on post-operative morbidity. In contrast, radiological markers of nutritional status did not appear to predict postoperative or survival outcomes.
The albumin level was associated with overall survival in our series, but did not impact morbidity. Moghadamyeghaneh et al. [Citation23] examined the impact of the albumin level on post-operative complications in 108 898 patients who underwent colorectal procedures between 2005 and 2012. They found that 16 962 (15.6%) patients had modest levels of preoperative hypoalbuminemia, defined by serum albumin levels between 30 and 34 g/L. The mortality rate in patients with modest hypoalbuminemia was 6% compared to 1.7% in patients with normal albumin levels. On multivariate analysis, modest hypoalbuminemia was an independent factor of complications [adjusted odds ratio (AOR) = 1.876; 95% CI: 1.51–2.05; p < 0.01]. Additionally, this study showed a linear correlation between the albumin level and postoperative mortality. The increased rate of mortality and morbidity was estimated to be 49% and 24%, respectively, for each 10 g/L decrease in the albumin level (p < 0.05). The hypothesis of the association between pre-operative hypoalbuminemia and the increased risk of post-operative mortality and morbidity is that low serum albumin is a marker of malnutrition, cancer cachexia and chronic inflammatory activity and as such, predicts increased mortality and post-operative complications [Citation24]. The preoperative albumin level appears to be an important nutritional factor when considering surgery for PM of colorectal origin as it impacts long-term survival after CRS-HIPEC.
Sarcopenia and fat visceral accumulation were not associated with increased post-operative major complications or poor long-term prognosis in our population. In a population of 206 patients who underwent CRS-HIPEC for PM of CRC, Van Vugt et al. [Citation11] recently reported no significant difference regarding severe complications (33.3 vs. 21.6%; p = 0.058), despite a significantly higher rate of reoperation in sarcopenic patients (25.6 vs. 12.1%; p = 0.012). In our series, several complications, including active bleeding, fistula or abscess, were treated by interventional radiology, which could explain the lower rate of reoperations. Among 94 patients treated by CRS-HIPEC for colorectal PM, Chemama et al. [Citation12] confirmed that sarcopenia was not associated with an increased risk of post-operative complications. However, they reported that sarcopenia could be associated with chemotherapy toxicity before CRS-HIPEC (57 vs. 26%; p = 0.004). Skeletal muscle may reflect a patient’s physiological reserves and sarcopenia could be associated with an increased inflammatory response to surgery [Citation25]. Nevertheless, radiological muscle depletion seems to be a less robust marker than albuminemia to predict post-operative outcome.
This study presents some limitations. First, the selection of patients fit for surgery was performed during preoperative assessment. This might have resulted in the additional exclusion of sarcopenic patients because they were less likely to be fit for surgery. Moreover, all our patients received immunonutrition 10 days before surgery with unknown impact on nutritional and muscular parameters. Second, the database had a small number of missing data regarding biological parameters, leading to the exclusion of several patients. However, this series is one of the largest reported on this subject.
In conclusion, hypoalbuminemia appears to be the main nutritional predictive factor for overall survival in patients with PM from colorectal origin undergoing CRS-HIPEC. However, it was not associated with postoperative morbidity. Radiological sarcopenia and fat accumulation (visceral and subcutaneous) were not associated with outcomes in this population.
Acknowledgements
The authors thank Isabelle Bonnefoy, Peggy Jourdan-Enfer, Martine Serrano for data management, and Laurent Villeneuve for coordinating the project.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–86.
- Franko J, Shi Q, Meyers JP, et al. (2016). Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol 17:1709–19.
- Sadeghi B, Arvieux C, Glehen O, et al. (2000). Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88:358–63.
- Elias D, Gilly F, Boutitie F, et al. (2010). Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 28:63–8.
- Goéré D, Malka D, Tzanis D, et al. (2013). Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg 257:1065–71.
- Elias D, Lefevre JH, Chevalier J, et al. (2009). Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 27:681–5.
- Chua TC, Yan TD, Saxena A, et al. (2009). Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg 249:900–7.
- Baratti D, Kusamura S, Iusco D, et al. (2014). Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum 57:858–68.
- Schwegler I, von Holzen A, Gutzwiller J-P, et al. (2009). Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg 97:92–7.
- Ataseven B, du Bois A, Reinthaller A, et al. (2015). Pre-operative serum albumin is associated with post-operative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol 138:560–5.
- Vugt JLA, van Braam HJ, Oudheusden TR, van, et al. (2015). Skeletal muscle depletion is associated with severe postoperative complications in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 22:3625–31.
- Chemama S, Bayar MA, Lanoy E, et al. (2016). Sarcopenia is associated with chemotherapy toxicity in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. Ann Surg Oncol 23:3891–8.
- Moon H-G, Ju Y-T, Jeong C-Y, et al. (2008). Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol 15:1918–22.
- Okamura A, Watanabe M, Mine S, et al. (2016). Clinical impact of abdominal fat distribution on prognosis after esophagectomy for esophageal squamous cell carcinoma. Ann Surg Oncol 23:1387–94.
- Fujiwara N, Nakagawa H, Kudo Y, et al. (2015). Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 63:131–40.
- CTCAE v4.0 Common Terminology Criteria for Adverse Events (CTCAE) [Internet]; 2010. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- Passot G, Vaudoyer D, Villeneuve L, et al. (2017). A perioperative clinical pathway can dramatically reduce failure-to-rescue rates after cytoreductive surgery for peritoneal carcinomatosis: a retrospective study of 666 consecutive cytoreductions. Ann Surg 265:806–13.
- Passot G, Vaudoyer D, Villeneuve L, et al. (2016). What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: a 25-year experience with 1,125 procedures: a 25-year wxperience of 1,125 HIPEC. J Surg Oncol 113:796–803.
- Mohkam K, Passot G, Cotte E, et al. (2016). Resectability of peritoneal carcinomatosis: learnings from a prospective cohort of 533 consecutive patients selected for cytoreductive surgery. Ann Surg Oncol 23:1261–70.
- Prado CM, Lieffers JR, McCargar LJ, et al. (2008). Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–35.
- Mourtzakis M, Prado CMM, Lieffers JR, et al. (2008). A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006.
- Moghadamyeghaneh Z, Hwang G, Hanna MH, et al. (2015). Even modest hypoalbuminemia affects outcomes of colorectal surgery patients. Am J Surg 210:276–84.
- Garth AK, Newsome CM, Simmance N, et al. (2010). Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet 23:393–401.
- Reisinger KW, Derikx JPM, van Vugt JLA, et al. (2016). Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr 35:924–7.
- Fearon K, Strasser F, Anker SD, et al. (2011). Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–95.