Abstract
Objectives: The choice of an optimal administration route for intraperitoneal (IP) chemotherapy and a suitable chemotherapeutic regime in the treatment of ovarian cancer remains a controversy. We investigated survival outcomes according to catheter intraperitoneal chemotherapy (CIPC), normothermic and hyperthermic chemoperfusion (NIPEC and HIPEC) with cytostatic drugs dioxadet and cisplatin in rats with transplantable ascitic ovarian cancer.
Methods: Ascitic liquid containing 1 × 107 tumour cells was inoculated to female Wistar rats and 48 hours after rats received dioxadet and cisplatin at the maximum tolerated doses. Dioxadet at doses 1.5, 30 and 15 mg/kg and cisplatin at doses 4, 40 and 20 mg/kg body weight were administered for CIPC, NIPEC and HIPEC, respectively. Rats in the control groups received physiological saline and CIPC with physiological saline was regarded as the untreated control. The antitumor activity of the drugs was evaluated as an increase in average life expectancy (ALE). Analysis of the data was based primarily on Bayesian statistics and included Kaplan–Meier method, log-rank test and hazard ratio (HR) estimation.
Results: Compared to the untreated control CIPC, NIPEC and HIPEC with dioxadet significantly increased ALE by 101316, 61524 and 1.71735 days, whereas with cisplatin by 61013, 122437 and –13523 days, respectively.
Conclusions: Dioxadet and cisplatin show similar efficacy in the CIPC route. Compared with CIPC IP chemotherapy by chemoperfusions is more effective for both the drugs. Dioxadet in HIPEC showed highest survival benefit whereas largest effect during NIPEC is achieved with cisplatin.
Introduction
Epithelial ovarian cancers (EOCs) rank the fifth place within overall oncological mortality in women and the first place among all gynaecological malignancies [Citation1]. Current standards for the initial EOCs management include optimal debulking and adjuvant intravenous (IV) chemotherapy with platinum-based drugs and taxanes [Citation2]. Despite the fact that large proportion of patients at early disease stages will respond to such treatment, most of the women, which are presented with advanced disease, are less likely to benefit from it with higher rates of recurrences and shorter progression free intervals [Citation3]. Recurrences occurred in a six months period after last chemotherapy cycle are considered potentially resistant to platinum drugs and require second-line therapy, to which only 30% of the patients will respond [Citation4]. As a result, roughly 80% of women are going to develop peritoneal carcinomatosis and ascites-associated complications [Citation5].
The characteristic features of advanced and recurrent EOCs in most cases are the implantation metastases to the parietal and visceral peritoneum, the diaphragm dome, lesser and greater omentum, which prompt that if we are to improve the cure of EOCs, an adequate therapy should also meet the specifics of peritoneal malignancies [Citation5–7]. Intraperitoneal (IP) chemotherapy has been discussed as the most promising strategy to challenge current EOCs treatment standards, though the techniques of its delivery vary [Citation4]. Pharmacokinetic rationale behind IP chemotherapy enables higher doses of anticancer drugs to act locally on the diseased tissues, thus providing less systemic toxicity compared to IV chemotherapy [Citation8]. Generally, three IP chemotherapy routes are described: catheter IP chemotherapy (CIPC) via IP catheter or port, normothermic (36.0–37.0 °C) IP chemoperfusion (NIPEC) with cytostatic solution being instilled and drained via system of pumps, and hyperthermic IP chemoperfusion (HIPEC) via same system under heated conditions (38.5–43.0 °C) [Citation5,Citation7].
Long-term observations from phase III clinical trials have established that 10-year survival rate for advanced stages of EOCs could be within reach for at least 50% of the patients by the means of IP chemotherapy [Citation4]. However, lack of compelling evidence and conflicting results between major clinical trials have hindered its further clinical translation [Citation9]. Moreover, no direct comparison on the efficacy between the three IP administration routes has been conducted. Neither has even been addressed the question of whether the outcome of a particular IP route could be compromised by the choice of antitumor drug. For instance, IV cisplatin is dose-limited by nephrotoxicity and exhibits neural, and ototoxicity [Citation10]. Research has shown that IP delivery of cisplatin has similar toxicity profile due to its rapid clearance into blood plasma, and also a tendency to cause adhesions in the peritoneum [Citation8,Citation10]. While this safety profile does not necessarily change the outcome of HIPEC route, its administration via catheter or port could be rather hazardous.
In this regard, dioxadet, an alkylating agent from the ethylenimine class, has been proposed as a potential substitute for cisplatin-based chemotherapeutic regimes. Dioxadet has been approved in Russia for systemic and intracavitary (intrapleural and IP) administration in the treatment of various malignancies. This includes ascitic ovarian cancer, locally advanced and disseminated breast cancer and lung cancer. The drug is also used for endovascular and endolymphatic chemotherapy of tumoural lesions of retroperitoneal lymph nodes in patients with ovarian, cervical, uterine cancers and malignant lymphomas. Phase II clinical trial of dioxadet has demonstrated its pronounced antitumor efficacy during IV + IP administration with dose-limiting but reversible myelosuppression in women with advanced EOCs [Citation11]. Its ability to penetrate tumour tissue on direct contact as well as cell-cycle independent activity leads to a renewed interest of dioxadet as a compound for IP regimes [Citation11–14].
Our previous studies on rats with ascitic ovarian cancer have demonstrated that single IP administration of some cytostatics, including dioxadet, cisplatin, mitomycin C, melphalan and paclitaxel statistically significantly increased median survival compared to their IV administration, while the highest median survival was achieved with dioxadet and cisplatin [Citation15]. In our other report on the safety and efficacy of cisplatin and dioxadet on the same rodent model it was confirmed that their administration in NIPEC and HIPEC mode also resulted in better survival compared to IV and IP chemotherapy. However, the toxicities associated with cisplatin were more severe [Citation16]. The goal of the present study was to directly compare the efficacy of CIPC, NIPEC and HIPEC with dioxadet and cisplatin in a rat model of an ascitic ovarian cancer.
Methods and materials
Experimental design
Outbred female Wistar rats were purchased from “Rappolovo” animal nursery (Leningrad Region, Russia) and housed 5–7 in polypropylene cages under standard 24-h light–dark regimen (12L:12D) at 22 ± 2 °C with relative humidity 50–60%. Rats received standard laboratory crop from Laboratorkorm Ltd. (Moscow, Russia) and tap water ad libitum. All animal experiments were performed following the approval of the N.N. Petrov Research Institute of Oncology Ethic Committee within the frame of rules declared by the European Treaty ETS No. 123.
The rat ovarian cancer strain was obtained from N.N. Blokhin Russian Cancer Research Center (Moscow, Russia). Ascitic liquid containing 1 × 107 tumour cells was diluted 1:4 with physiological saline and injected IP to healthy rats. Day of ovarian cancer inoculation was accepted for zero. Two hundred and eighty-eight rats were then randomised into total of nine groups for the three IP chemotherapy regimes with each antitumor drug as well as concomitant control to each regime (physiological saline). A single intervention proceeded 48 h after ovarian cancer inoculation. Dioxadet (Chemconsult, Saint Petersburg, Russia) and cisplatin (Pharmachemie, Haarlem, Netherlands) were administered at the maximum tolerated doses in CIPC via syringe, 1.5 and 4 mg/kg body weight, during NIPEC (36.5–37.5 °C) and HIPEC (40.5–41.5 °C), 30 and 40 as well as 15 and 20 mg/kg body weight, were infused for a continuous circulation using an experimental setting with pumps, respectively. Antitumor effects of the treatments were estimated as an increase in average life expectancy (ALE) compared to the control without treatment (IP saline injection) and to concomitant controls (CIPC, NIPEC and HIPEC with physiological saline). The diagnosis of ovarian cancer was confirmed by cytological examination of ascitic smear stained with haematoxylin and eosin. The methodology has been thoroughly described in our previous reports [Citation12–14]. Nine rats died due to surgical complications and were excluded from the analysis.
Statistical analysis
In accordance with modern tendency we insist on applying Bayesian approach to statistical data analysis together with orthodox frequentist approach, and even instead of it. There is a lot of corresponding publications by foremost biostatisticians including American Statistical Association (ASA) and U.S. Food and Drug Administration (FDA) [Citation17]. Our approach could be referred to as “harmonizing statistical evidences and predictions” as it combines not only confidence interval (CI) estimates for parameters, for the standardised effect size dC, for the achieved power (1 − β), and planned sample sizes, but also prediction intervals (PIs) for them and for the p values, as well as estimates of posterior probabilities for the null hypothesis P(Ho|D) and Bayes factors BF01. To present point estimates of parameters and CIs we prefer to use the format with subscripts invented by John Tukey and recommended by Louis and Zeger [Citation18]. Its advantage consists in compactness and visibility, for instance, 0.71.21.8 against 1.2 (with 95% CI from 0.7 to 1.8). Information about the used software and their appointments is presented in .
Table 1. Software applied for the statistical analysis.
Monte Carlo and bootstrap resampling procedures were applied preferably. Levene’s test was employed to check the assumption of equal variances followed by Tukey’s post hoc multiple comparison. The data were then further tested for statistical homogeneity using one-way ANOVA. Bonferroni’s correction was used to address the multiple comparison problem. Pairwise comparison was carried out using randomised Student’s t-test. The observed effects were verified with Bayes factor (BF) and tested for practical (respectively clinical) significance using Cohen’s dC. Probability of replication (Psrep) along with PIs were calculated for both non-standardised (D) and standardised (dC) metrics. A priori power analysis was carried out to estimate an adequate sample size to achieve the desired power (1 − β = 0.95) for the chosen level of probability of type I error (α* = 0.05). Survival analysis included Kaplan–Meier’s method and two-sided log-rank test. Hazard ratios (HR) were estimated using Cox’s proportional hazards model. Cumulative hazard plots were analysed for violation of proportional hazards assumption. All statistical analyses () were performed using PAST 3.1 [Citation19], JASP 0.7.5.5 [Citation20], BF Calculators [Citation21], G*Power 3.1.9.2 [Citation22], LePrep 2.1.0 [Citation23] and R 3.4.0 [Citation24].
Results
In all rats implantation-take achieved 100% and lead to ascites and peritoneal carcinomatosis. Death of the animals in control without treatment occurred in 7–14 days after ovarian cancer inoculation. The ALE in the control without treatment accounted for 121519 days. Contrarily, CIPC, NIPEC and HIPEC with dioxadet increased this rate by 13 (p = 1.7 × 10−13), 15 (p = 3.1 × 10−7) and 31 days (p = 1.6 × 10−14) or 85%, 97% and 202%, respectively (). Kaplan–Meier’s plots are shown in . In cisplatin-treated rats this increase accounted for 10 (p = 4.4 × 10−9), 24 (p = 2.6 × 10−11) and 19 days (p = 1.8 × 10−7) or 66%, 160% and 126%, respectively ().
Figure 1. Survival outcomes in control groups and according to treatment with cisplatin vs. dioxadet by the means of catheter intraperitoneal chemotherapy (CIPC), normothermic and hyperthermic chemoperfusion (NIPEC and HIPEC) in rats with ascitic ovarian cancer.
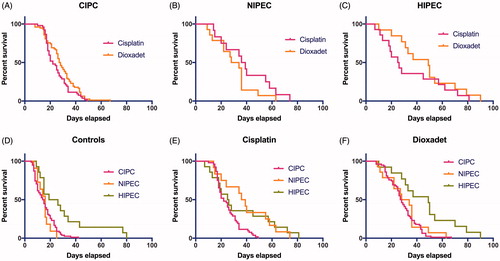
Table 2. The results of harmonised statistical analysis of survival in rats with ascitic ovarian cancer in the course of the various IP chemotherapy routes.
Hyperthermic perfusion with physiological solution alone increases ALE by 14 days (p = 3.6 × 10−5) compared to the untreated control (HR 0.182.840.67). Yet HIPEC was the only mode where compared to its concomitant control no statistically significant increase in ALE for neither cisplatin (p = 0.58), nor dioxadet (p = 0.07) has been confirmed. In both cases 95% CIs for mean differences cover the indifferent value D0 = 0 thus indicating the shift towards null hypothesis of no difference in means (). These data are supported by weak BF10 = 0.08 and BF10 = 0.44, respectively. The effect size could also be considered as negligible with dC = 0.2 and dC = 0.72 ().
Figure 2. Peritoneal carcinomatosis after intraperitoneal transplantation of ovarian cancer in rat. The autopsy of the rats reveals merging peritoneal tumour implants of greater and lesser omentum, mesentery, diaphragm dome, and miliary involvement of visceral and parietal peritoneum.
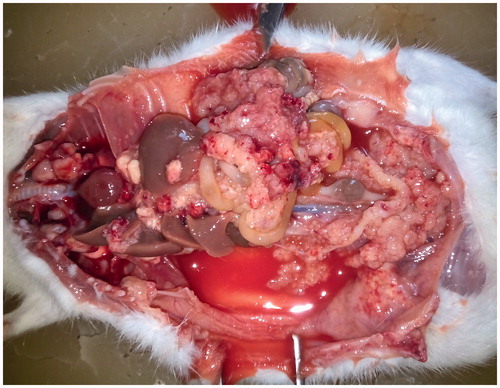
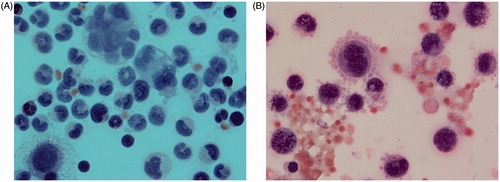
Figure 3. Smear of the ascitic fluid in rat with transplanted ovarian cancer. (A) The image depicts cyst of ovarian adenocarcinoma, cell mitosis and cell necrosis. (B) Haemorrhagic ascetic fluid contains large malignant cells with basophilic cytoplasm, nucleus mitosis, extensive cytoplasm vacuolisation and pseudopods on the external cytoplasmic membrane.
CIPC was associated with favourable survival for either of the applied cytostatics (). Nevertheless, the results of dioxadet-CIPC were slightly more promising (HR = 0.50.721.02): 13 vs. 10 days (p = 1.7 × 10−13 vs. p = 4.4 × 10−9) with dC = 1.3 and dC = 1.1. It has been indicated that the statistical results for CIPC with the both drugs were also the most sensitive with the achieved power 1 − β = 1.00 and 1 − β = 0.99.
Discussion
The autopsy of the rats from the control group revealed carcinomatosis of visceral and parietal peritoneum, greater and lesser omentum, mesentery, metastatic affection of lymph nodes in the abdominal cavity ( and ). The biological pattern of the transplanted ovarian cancer in rats was similar to stages III and IV of ovarian cancer in humans, including redistribution phenomenon of the tumour growth described in progression of peritoneal carcinomatosis. Thus, we believe that our ovarian cancer model in rats can be regarded as a biological equivalent of optimally debulked ovarian cancer in women [Citation14]. In our research we used antitumor agent dioxadet as a promising alternative for various IP routes. Phase II clinical trials of dioxadet have indicated its high antitumor efficacy in malignant tumours of various localisations, however, the most significant effect has been achieved in patients with metastatic ovarian cancer by combining IV and IP treatment modes [Citation11]. The pronounced therapeutic efficacy of the drug is due to intramolecular crosslinking of the tumour cell DNA regardless of the cell cycle; low molecular weight, amphiphilic properties and slow clearance from the peritoneum [Citation12]. According to our data, the largest efficacy of dioxadet could be achieved in HIPEC route. While temperatures above 40 °C only minimally impact the intact cells, in tumour cells it contribute to the DNA replication disorders, changes in the cell membrane structure and denaturation of oncoproteins. On tissue level, changes in blood flow, oxygenation, as well as in metabolic and energetic profile are observed. Chemoperfusion with heated cytostatic solution increases the drug uptake in tumours and hence, cytotoxicity [Citation25]. However, the use of HIPEC is only adequate for patients undergoing cytoreductive surgery and carries all surgery-associated risks, not to mention technical difficulties and toxicological side effects (weight loss, haemorrhages, diarrhoea, gastroptosis) [Citation5,Citation13]. On the other hand, CIPC could be implemented at any stage of the treatment. Though less effective than NIPEC and HIPEC, the magnitude of survival benefit from CIPC is still much favourable compared to IV chemotherapeutic regimes as shown in our previous report [Citation12].
In our study, rats treated by CIPEC with both cisplatin and dioxadet had a 66–85% increase in ALE (10 and 13 days). HIPEC with dioxadet had the highest survival compared to the control without treatment (31 days; p = 1.6 × 10−14), whereas no statistical significance was confirmed compared to the concomitant control (HIPEC with physiological saline). This could be explained by the high efficacy of hyperthermia itself with physiological solution (14 days; p = 3.6 × 10−5). Statistically, NIPEC with cisplatin was the most effective drug-to-route combination (HR = 0.20.21.4). To date, cisplatin is the most commonly used drug both in IV and IP chemotherapy [Citation26]. When administered IP, its concentration in the blood corresponds to that of IV chemotherapy. As a result, most of the activated platinum localised on tumour implants enters the tissue via bloodstream, regardless of IV or IP mode of administration, while preserving the same or even higher toxicity [Citation8]. This could mean, that despite theoretically effective combination of cisplatin with hyperthermia, its toxicity hazards the outcome of survival in rats with ascitic ovarian cancer [Citation27]. Perhaps, toxicity of cisplatin during NIPEC might be much tolerable as indicated by our results. We believe that dioxadet could be a better substitute for the current cisplatin-paradigm in the treatment of advanced EOCs.
Conclusions
Our data correspond with the review of the literature and suggest that treatment outcome of peritoneal carcinomatosis in patients with advanced EOCs could be significantly improved by the means of IP chemotherapy. In our opinion, an integrated approach to the treatment of these patients is necessary. Cytoreductive surgery should be supplemented with intraoperative HIPEC and followed by adjuvant IP chemotherapy instead of adjuvant IV treatment courses. Dioxadet has some advantages in IP chemotherapy against the most commonly used today cisplatin, which indicates the prospects of dioxadet for future introduction into clinical practice as a primary drug for HIPEC and CIPC in patients with advanced and recurrent ovarian cancer. We also encourage further research to evaluate the role of cisplatin in NIPEC route.
Acknowledgements
The authors are grateful to Mrs. A.P. Sapozhnikova (N.N. Petrov Research Institute of Oncology) for technical assistance in preparing the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Ervik M, et al. (2013). Estimated cancer incidence, mortality and prevalence worldwide in 2012. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer. Available from: http://globocan.iarc.fr, accessed on day/month/year
- Morgan RJ, Jr., Armstrong DK, Alvarez RD, et al. (2017). Ovarian cancer (Version 1.2016). Clinical practice guidelines in oncology [Internet]. NCCN. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
- Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. (2014). Ovarian cancer. Lancet 384:1376–88.
- Narod S. (2016). Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol 13:255–61.
- Polom K, Roviello G, Generali D, et al. (2016). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for treatment of ovarian cancer. Int J Hyperthermia 32:298–310.
- Grosso G, Rossetti D, Coccolini F, et al. (2014). Intraperitoneal chemotherapy in advanced epithelial ovarian cancer: a survey. Arch Gynecol Obstet 290:425–34.
- Lu Z, Wang J, Wientjes MG, Au JL-S. (2010). Intraperitoneal therapy for peritoneal cancer. Future Oncol 6:1625–41.
- Hasovits C, Clarke S. (2012). Pharmacokinetics and pharmacodynamics of intraperitoneal cancer chemotherapeutics. Clin Pharmacokinet 51:203–24.
- Gourley C, Walker JL, Mackay HJ. (2016). Update on intraperitoneal chemotherapy for the treatment of epithelial ovarian cancer. Am Soc Clin Oncol Educ Book 35:143–51.
- Sakuragi N, Nakajima A, Nomura E, et al. (2000). Complications relating to intraperitoneal administration of cisplatin or carboplatin for ovarian carcinoma. Gynecol Oncol 79:420–3.
- Gershanovich ML, Filov VA, Kotova DG, et al. (1998). Multicenter clinical trial of the antitumor drug dioxadet (phase II). Vopr Onkol 44:216–20.
- Bespalov VG, Vyshinskaya EA, Vasil’eva IN, et al. (2017). Comparative study of antitumor efficiency of intraperitoneal and intravenous cytostatics in experimental rats with disseminated ovarian cancer. Bull Exp Biol Med 162:383–6.
- Bespalov VG, Kireeva GS, Belyaeva OA, et al. (2016). Both heat and new chemotherapeutic drug dioxadet in hyperthermic intraperitoneal chemoperfusion improved survival in rat ovarian cancer model. J Surg Oncol 113:438–42.
- Bespalov VG, Kireeva GS, Belyaeva OA, et al. (2016). Experimental study of antitumour activity and effects on leukocyte count of intraperitoneal administration and hyperthermic intraperitoneal chemoperfusion (HIPEC) with dioxadet in a rat model of ovarian cancer. J Chemother 28:203–9.
- Bespalov VG, Vyshinskaya EA, Vasilieva IN, et al. (2015). Intraperitoneal chemotherapy – a method of improving treatment effectiveness in ovarian cancer. Vopr Onkol 61:634–41.
- Bespalov VG, Kireeva GS, Belyaeva OA, et al. (2015). Intraoperative intraperitoneal chemoperfusion treatment with cisplatin and dioxadet on a model of peritoneal carcinomatosis in ovarian cancer: safety and efficacy evaluation. Vopr Onkol 61:647–52.
- Wasserstein RL, Lazar NA. (2016). The ASA’s statement on p-values: context, process, and purpose. Am Stat 70:129–33.
- Louis TA, Zeger SL. (2009). Effective communication of standard errors and confidence intervals. Biostatistics 10:1–2.
- Hammer Ø, Harper DAT, Ryan PD. (2001). Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9–18.
- Love JP, Selker R, Verhagen AJ, et al. (2015). Software to sharpen your stats. APS Obs 28:27–9.
- Rouder JN, Speckman PL, Sun D, et al. (2009). Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev 16:225–37.
- Faul F, Erdfelder E, Buchner A, Lang AG. (2009). Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41:1149–60.
- LePrep – Probabilities of replication. (2017). Available from: http://lmrs.univ-rouen.fr/Persopage/Lecoutre/PAC.htm
- R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Available from: https://www.R-project.org [last accessed 26 Jul 2017].
- Lemoine L, Sugarbaker P, Van der Speeten K. (2017). Drugs, doses, and durations of intraperitoneal chemotherapy: standardizing HIPEC and EPIC for colorectal, appendiceal, gastric, ovarian peritoneal surface malignancies and peritoneal mesothelioma. Int J Hyperth 33:582–92.
- Jaaback K, Johnson N, Lawrie T, (2016). Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev 1:CD005340.
- Cowan RA, O’Cearbhaill RE, Zivanovic O, Chi DS. (2017). Current status and future prospects of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) clinical trials in ovarian cancer. Int J Hyperth 33:548–53.
