Abstract
Transcriptional hypoxia-inducible factor-1α (HIF-1α) plays the fundamental role in adaptive processes in response to hypoxia. Specific HIF-1α target genes are involved in glycolysis, erythropoiesis and angiogenesis to promote survival. In our previous study we have demonstrated that naturally low body temperature of newborn rats protects them against damage due to perinatal hypoxia. Therefore, our experiments aimed at checking the effects of body temperature during simulated perinatal anoxia on subsequent changes of expression of HIF-1α and its specific target genes such as vascular endothelial growth factor (VEGF) and erythropoietin (EPO) in the rat brain. Two-day old Wistar rats were divided into three temperature groups: normothermic −33 °C, hyperthermic −37 °C and extremely hyperthermic −39 °C. The temperature was controlled 15 min before start and continued during 10 min of anoxia as well as for 2 h post-anoxia. HIF-1α was analysed by Western blot and immunofluorescence and mRNA levels of HIF-1α and its downstream genes (VEGF, EPO) were quantified by qRT-PCR. Thermal conditions during neonatal anoxia affected the hippocampal and neocortical level of HIF-1α protein. Physiological body temperature of newborn rats led to prominent accumulation of cerebral HIF-1α protein and significant upregulation of VEGF and EPO mRNA. In contrast, anoxia-induced HIF-1α activation at elevated body temperatures was less pronounced. Since HIF-1α and EPO have recently been regarded as promising therapeutical targets against brain lesions due to hypoxia/ischemia, presented data imply that in order to achieve a full effect of neuroprotection, the thermal conditions during and after the insult should be taken into consideration.
Introduction
Complications after neonatal asphyxia are the most common cause of subsequent neurodevelopmental disability and are also a major contributor to infant mortality [Citation1]. It is well established that neonates are more resistant to hypoxia than adults, although some brain structures such as the basal ganglia, thalamus, hippocampus and cortex show particular susceptibility to such early-life hypoxic episodes in both animals [Citation2–4] and humans [Citation5,Citation6].
Hypoxia during delivery triggers different types of processes to protect the newborn brain against the adverse perinatal complications [Citation3]. Among these endogenous provoked mechanisms, hypoxia-inducible transcription factors (HIFs) play the fundamental role as regulators of oxygen homeostasis under hypoxic conditions [Citation7]. HIF-1 is a heterodimer comprising an oxygen-labile α-subunit (HIFα) and a constitutively expressed β-subunit (HIFβ). Two HIF-1α homologues, HIF-2α and HIF-3α, have been also identified [Citation8]. Although HIF-1α and HIF-2α are generally induced by reduction of oxygen supply, accumulation of these proteins in developing brain is regional and cell-specific [Citation9]. In neonatal brain systemic hypoxia strongly induces accumulation of HIF-1α in cerebral cortex and hippocampus [Citation10]. HIF-3α lacks the transactivation domain and may function as an inhibitor of HIF-1α and HIF-2α [Citation11].
During normoxia, HIF-1α protein is negatively regulated by various mechanisms, among which ubiquitination and rapid proteasomal degradation are the most important regulatory systems [Citation12]. Under hypoxic conditions, general protein translation is immediately arrested in order to decrease energy consumption due to energy starvation [Citation13]. Simultaneously, HIF-1α is induced, stabilised, dimerised with the constitutively expressed HIF-1β subunit and translocated to the nucleus. There is also a number of non-hypoxic stimuli capable of upregulating this transcription factor, including growth factors, cytokines, free radicals, hormones [Citation14], metal ions and iron chelators (e.g., deferoxamine), which induce protein stabilisation and transcriptional activity even under normoxic conditions [Citation15]. However, oxygen tension is regarded as the most critical factor in regulation of the intracellular expression of HIF-1α protein.
The functional HIF-1α plays an essential role in cellular oxygen homeostasis by regulating the expression of genes involved in glycolysis, erythropoiesis and angiogenesis [Citation7,Citation16]. Among these genes, vascular endothelial growth factor (VEGF) and erythropoietin (EPO) may directly protect neurons from hypoxic insults. VEGF is a polypeptide growth factor that is activated by tissue hypoxia [Citation17]. It promotes cell survival by stimulating angiogenesis, involving endothelial cell migration, proliferation and differentiation, as well as extracellular matrix proteolysis [Citation18]. EPO has been originally recognised as a humoral mediator involved in erythropoiesis by stimulating the formation and differentiation of erythroid precursor cells [Citation19]. Recent studies have shown that EPO mRNA and EPO protein are found in the brain of a variety of mammals including humans [Citation12] and provides significant neuroprotection during neonatal hypoxia [Citation12,Citation20].
Among endogenous adaptive mechanisms activated in response to hypoxia, one of the crucial protective responses against low oxygen accessibility is decreasing of body temperature, which has been proven in animal models of hypoxia/ischemia [Citation21–24]. In the pathophysiology of cerebral hypoxia-induced injury, decrease of body temperature is thought to block dysregulation of neurons metabolism, including ATP depletion, glutamate release, Ca2+ mobilisation, anoxic depolarisation, generation of reactive oxygen species (ROS), inflammation, permeability of the blood–brain barrier, necrotic and apoptotic pathways [Citation25,Citation26].
In our previous work, we have shown that body temperature of newborn rats in their nest is regulated at the level of 33 °C which represents their normal, physiological body temperature [Citation21]. Rats become fully homeothermic at an age of approximately 20–30 days, when their body temperature reaches adult’s value (37 °C). It is worth noting that body temperature of 32–33 °C of newborn rats [Citation21,Citation27,Citation28] is the same as that considered to be the most effective for preventing brain lesions during therapeutic hypothermia in asphyxiated human neonates [Citation29–31].
Our up-to-date studies also allowed us for concluding that the perinatal anoxia at hyperthermic body temperature: typical of adults or higher enhances the post-anoxic oxidative stress in neonatal rats and depletes cerebral pool of antioxidant enzymes and low molecular antioxidants, whereas physiological body temperature of neonates prevents cerebral lipid peroxidation and assure an optimal antioxidant protection of their brain [Citation32,Citation33].
In this study we proposed that HIF-1α provides a readily available neuroprotective system operating during short-term anoxia episode in the forebrain of newborn rats (area of the brain particularly susceptible to damage caused by oxygen depletion). Considering the role of naturally decreased body temperature of newborn rats as a beneficial adaptation, we hypothesised that an increase in body temperature to the level typical of adults or higher would lead to downregulation of endogenous protective mechanisms such as HIF-1α and its downstream genes activation. Therefore, using a well-established rat model of perinatal asphyxia [Citation34], we decided to examine the effects of body temperature during simulated perinatal anoxia and reoxygenation period on the level of HIF-1α protein, as well as HIF-1α, EPO and VEGF mRNA level in the forebrain of newborn rats.
Material and methods
All animal research was approved by the Local Committee on the Use and Care of Laboratory Animals in Bydgoszcz, Poland (permission no. 28/2012) and was undertaken according to National Ethics Commission For Animal Experimentation (Polish Ministry of Science and Higher Education). The experiments were performed on two days old Wistar rats (n = 96) of both sexes with body weight of 7–8 g. The deliveries were spontaneous. The mothers were housed in individual acrylic cages lined with wood shavings. The cages were located in a well-ventilated thermostatically controlled room (21 ± 1 °C, 12 h light/dark period) and the animals were given a standard rat chow and water ad libitum until the pups were two days old. All efforts were made to minimise animals suffering.
We have chosen 2-day-old animal model because the exposure of rat pups to anoxia by placing them in 100% nitrogen atmosphere ∼30 h after the birth appears to parallel asphyxia event in low-weight premature human infants. The brain maturation stage in rats at this age corresponds to that of humans born prematurely [Citation34].
Experimental groups
The pups were divided into the following six experimental groups: animals maintaining body temperature of 33 °C (which is normal body temperature of newborn rats) exposed to normoxic (33N) (group 1) and anoxic (33AN) (group 2) conditions; animals forced to stabilise their body temperature at 37 °C (which corresponds to body temperature typical of adults) exposed to normoxic (37N) (group 3) and anoxic (37AN) (group 4) conditions; animals forced to maintain their body temperature at 39 °C (reflecting body temperature typical of febrile adults) exposed to normoxic (39N) (group 5) and anoxic (39AN) (group 6) conditions.
Exposure to anoxia
The anoxia procedure we used was described in detail previously [Citation21]. Briefly, rectal temperature of newborn rats was recorded by means of miniature (0.1 mm in diameter) copper-constantan thermocouples (Physitemp Instruments, Clifton, NJ), connected to the recording system (3000-E52 Iso-Thermex Interface & Software Columbus Instruments, Columbus, OH), inserted to a depth of 5 mm into the colon of the rat pups. The pups were placed individually in airtight 200 ml plethysmography chambers which were partially submerged in a water thermostat under controlled thermal conditions (∼31.5 °C, ∼35.5 °C or ∼37.5 °C) in order to maintain their body temperature at 33 °C, 37 °C or 39 °C, respectively. The overall accuracy of the measurements was higher than ±0.05 °C. Gasping movements of the anoxic rats were continuously recorded using the barometric method. When rectal body temperatures had reached the scheduled level, pups were maintained at the above mentioned steady states for 15 min, then they were exposed to 100% nitrogen atmosphere for 10 min (which imposes a critical anoxia upon rat pups at body temperature of 39 °C). At the end of anoxia, the animals were cyanotic and gasping, but the exposure to this degree of anoxia resulted in 100% survival. After anoxia, the animals were allowed to inhale normal atmospheric air under unchanged thermal conditions for 120 min. Normoxic groups were exposed to continuous air flow in the same chambers for the same period of time, i.e. 145 min at the respective temperatures.
Pups from every experimental group were sacrificed under ketamine anaesthesia (100 mg/kg) at three different time points: (1) 2 h after exposure to the above mentioned conditions (n = 72), (2) 72 h after exposure (n = 12) and (3) 7 days after exposure (n = 12). The brains were dissected for proteins immunoassay, histological analysis and quantitative real-time RT-PCR ().
Western blot analysis
To indicate the time courses of the HIF-1α levels at different body temperatures we performed Western blot analysis at mentioned time points: (1) 2 h (six rats per group), (2) 72 h (two rats per group) and (3) 7 days (two rats per group) after anoxia.
For Western blotting studies, samples of forebrain (∼50 mg) were isolated and homogenised in extraction buffer containing: 50 mM, Tris–HCl pH 7.5, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DDT, 1 mM PMSF and protease inhibitors cocktail (Roche) and kept on ice for 15 min. The crude protein extracts were centrifuged at 16 000 g at 4 °C for 30 min. The pellet was discarded and the supernatant containing the soluble proteins was used for further experiments. Proteins concentration was determined by the method of Bradford [Citation35] using bovine serum albumin (BSA) as standard. Aliquots of soluble fraction of each supernatant (25 µg) were resolved on a 10% (w/v) SDS-PAGE as described by Laemmli [Citation36]. Resolved proteins on SDS-PAGE were transferred to PVDF membrane by the semi-dry system. After blocking in 3% non-fat dry milk solution, the membrane was incubated overnight at 4 °C with primary rabbit anti-HIF-1α polyclonal antibody (Novus Biologicals, Littleton, CO 1:500 in TBS). After washing, the membrane was incubated for 1 h with secondary horseradish peroxidase conjugated goat anti-mouse IgG (A2554, Sigma-Aldrich, St. Louis, MO) diluted to 1:75 000 in TBS buffer and bands were visualised by chemiluminescence using the Western Bright Sirius system (Advansta, Menlo Park, CA). Protein levels were normalised to β-actin as a loading control. Hypoxia induced and uninduced COS-7 Nuclear Extract (NB800-PC26, Novus Biologicals, Littleton, CO) were used as positive and negative controls for HIF-1α. Relative optical density of protein bands was measured following subtraction of the film background. Densitometric analysis of immunoreactive bands was performed using Quantity One software (BioRad, Hercules, CA). Because HIF-1α protein 72 h post-anoxia was barely detectable and seven days after the insult was not visible in all experimental groups, further analyses were performed only using samples of 2 h after the anoxia. The analyses at this time point were repeated three times (3×2 individuals per experimental group) to assure reproducibility of the results.
Immunofluorescence
For immunofluorescence examination, the brains of 3 animals per each group were used. Whole brains were fixed in 4% paraformaldehyde at 4 °C for 12 h, and embedded in paraffin. Six micrometer coronal sections were cut and then stored until analysis. Two to three slides, with three to four sections per slide, from each brain were used for HIF-1α immunofluorescence staining.
Immunofluorescence assay
For the immunofluorescence assay, sections were deparaffinized in xylene (Avantor Performance Materials, Poland S.A.) and rehydrated through 100–50% graded ethanol to distilled water. After heat-induced antigen retrieval (10 mM Sodium Citrate, 0.05% Tween 20, pH 6.0), washing (PBS/0.3% Triton X-100, pH 7.4) and blocking with normal mouse serum, sections were incubated with the polyclonal rabbit anti-HIF-1α antibody (Novus Biologicals, Littleton, CO, 1:50 in 1% normal mouse serum) overnight at 4 °C. After rinsing with PBS, the sections were treated with Cy3-conjugated sheep anti-rabbit secondary antibody (Sigma Aldrich, St. Louis, MO, 1:200) for 1 h at 37 °C. After washing, sections were subjected to DNA staining with Hoechst (Invitrogen, Carlsbad, CA, 1:5000 in PBS), rinsed and mounted in ProLong Gold antifade reagent (Invitrogen, Carlsbad, CA). For all incubations a humidified chamber was used. To ensure comparable results for quantitative measurements, all sections were processed together at the same time under the same conditions. Simultaneous examinations of negative controls (omission of the primary antibody) confirmed the absence of non-specific immunofluorescent staining.
Semiquantitative evaluation of immunohistochemical staining of HIF-1α in the hippocampus and neocortex was performed using ImageJ software [Citation37]. Images were captured with Leica TCS Sp8 confocal microscope under consistent conditions of acquisition to ensure comparable results. Nine random images from each brain sample were captured within a standard region of interest (ROI), the level of fluorescence was expressed in arbitrary units (as the product of area and mean gray value). Subsequently, the average of the nine measurements was used to represent the immunoreactivity or fluorescence intensity of each sample. Before recording fluorescence intensity, we eliminated the background by adjusting the threshold based on auto fluorescence from negative control.
RNA isolation and qRT-PCR
qRT-PCR was used to analyse the mRNA levels of HIF-1α and its downstream genes (EPO and VEGF) in control and anoxia-induced animals. Forebrain samples were isolated from three animals from each experimental group, homogenised in liquid nitrogen and subjected to RNA isolation via standard Trizol method (Sigma-Aldrich, St. Louis, MO). Briefly, the tissue pellets were homogenised in Trizol, extracted with chloroform and precipitated with isopropanol (1:1 v/v), sodium acetate pH 5.2 (Thermo Fisher Scientific Co., Waltham, MA) and GlycoBlue coprecipitant (Ambion®, Thermo Fisher Scientific Co., Waltham, MA). After DNase treatment with Turbo DNA-free Kit (Ambion®, Thermo Fisher Scientific Co., Waltham, MA), according to manufacturer’s protocol, samples were extracted with acid-phenol:chloroform pH 4.5 (with IAA, 125:24:1; 1:1 v/v; Ambion®, Thermo Fisher Scientific Co., Waltham, MA) and precipitated with ethanol (1:2.5 v/v). Extracted RNA was next subjected to qualitative and quantitative assessment with NanoDrop 2000 (Thermo Fisher Scientific Co., Waltham, MA). For cDNA synthesis, 1 µg of total RNA from each sample was reverse-transcribed with random hexamers and Transcriptor First Strand cDNA Synthesis Kit (Roche, Grenzacherstrasse, Switzerland) according to manufacturer’s instruction. Next, 10 ng of cDNA was amplified with 0.2 µM primers (Sigma-Aldrich, St. Louis, MO) and SYBR Green PCR Master Mix (Roche, Grenzacherstrasse, Switzerland) on CFX ConnectTM Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The gene-specific primers used are shown in . The following PCR cycles were applied: initial denaturation at 95 °C for 10 min, then 40 cycles of 15 s at 95 °C, annealing and extension at 60 °C for 1 min. β-actin was used as an endogenous control and reactions were performed in triplicate. In order to determine the PCR efficiencies, standard curves for both target and control genes were obtained using a series of cDNA dilutions as a template. The relative level of gene expression was calculated according to the Pfaffl [Citation38] method using the REST v2.0.13 2009 software.
Statistics
Data are expressed as mean ± SEM. Statistical significance was determined by one-way ANOVA and two-way ANOVA. Each analysis was followed by multiple comparisons using Tukey post hoc test. The significance level was set at p < 0.05 for all statistical tests. Data analyses were performed with the use of IBM SPSS Statistics 22.0 (IBM SPSS Statistics, Armonk, NY).
Results
Post-anoxic changes in HIF-1α protein accumulation in the forebrain of newborn rat brain
shows the influence of thermal conditions on HIF-1α protein level in the neonatal rat brain at different time points after the insult. During normoxia, in all experimental groups HIF-1α level was low, but detectable. Single bands of 120 kDa, corresponding to HIF-1α protein were detected two and 72 h post-anoxia (). In the forebrains isolated seven days after exposure, no HIF-1α signal was observed (). COS-7 positive and negative controls confirmed the specificity of the used antibody (). Because HIF-1α protein was barely detectable in all anoxic animals 72 h after exposure and was not visible sevendays after the insult, the experimental time point of 2 h post-anoxia was chosen for further analysis.
Figure 1. (A) The time courses of anoxia- induced HIF-1α levels at different body temperatures (33 , 37 and 39 °C) (i) 2 h after exposure to anoxia. Single bands at 120 kDa corresponding to HIF-1α are visible in normoxic and anoxic animals (ii) 72 h after the insult:HIF-1α expression is barely detectable only in anoxic groups (iii) 7 days after exposure to anoxia: there is no signal of HIF-1α in both normoxic and anoxic conditions. (B) Positive and negative control of HIF-1α antibody specificity on COS-7 nuclear exctracts. (C) Effects of body temperature (33 , 37 and 39 °C) during neonatal anoxia on HIF-1α expression (relative changes of HIF-1α) in the forebrain of newborn rats 2 h after the insult. Data are presented as means ± SE (n = 6 per each group) expressed as a percentage of mean values for normoxic animals at physiological body temperature (33N) which was given 100%.
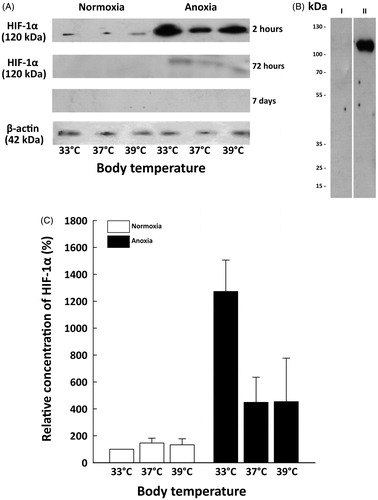
Anoxia led to the significant accumulation of HIF-1α protein in the forebrains of newborn rats 2 h after exposure. show significant HIF-1α protein accumulation in anoxic rats compared to their normoxic counterparts. The highest accumulation of HIF-1α protein was detected in rats maintaining their physiological body temperature of 33 °C (33AN), which was almost three times higher than that in both hyperthermic groups of anoxic animals (37 and 39AN). However, there were no differences in concentrations of HIF-1α protein between 37 and 39AN groups ().
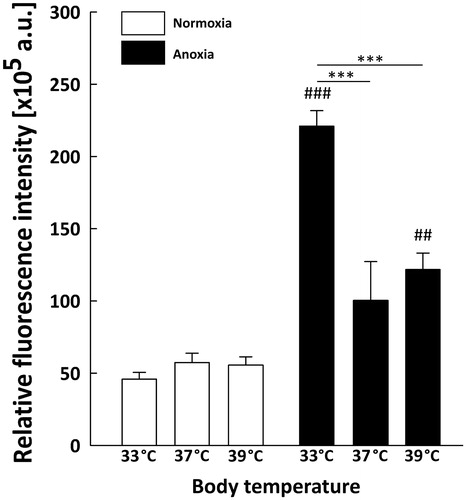
As the structures of neonatal brain most vulnerable to anoxia are hippocampus and neocortex, we assessed spatial HIF-1α localisation in these two regions by immunostaining with anti-HIF-1α antibody. We detected a marked increase in HIF-1α fluorescence level throughout the hippocampus (, ) and neocortex (, ) of anoxic brains compared to normoxic controls. In each group of rats exposed to normoxic conditions the fluorescence was relatively weak ( and ). In the hippocampus ( and ) the anoxic episode triggered a significant increase in HIF-1α staining under all thermal conditions in comparison to their normoxic counterparts (33AN groups, p < 0.001; 37AN groups, p < 0.01 and 39AN groups, p < 0.05; ). The highest increase in the fluorescence level was recorded in normothermic (33 °C) animals exposed to anoxia (33AN) (, ). Furthermore, nuclear accumulation of HIF-1α in anoxic brains was observed exclusively in hippocampus of rats maintaining their physiological body temperature of 33 °C (33AN) (). Under hyperthermic conditions (37 and 39 °C) anoxia-induced increases of HIF-1α level were less pronounced in comparison to that in normothermic conditions (33 °C) (p < 0.001, ). Similar pattern of changes in fluorescence level of HIF-1α was recorded in the cortex (, ). During normoxia, the level of protein was low in all experimental groups (, ). In animals subjected to anoxia the level of fluorescence was higher than that in their normoxic counterparts and culminated in 33AN group (p < 0.001; ). The same concerns animals kept at body temperatures of 39 °C (p < 0.01; ). In animals with body temperature of 37 °C the increase of HIF-1α was non-significant. It must be stressed that post-anoxic level of the protein in animals maintaining their physiological body temperature of 33 °C was significantly higher than those in other groups exposed to anoxia (p < 0.001; ). There were no significant changes in mRNA expression of HIF-1α in all anoxic and control animals ().
Figure 2. HIF-1α immunolocalization in the hippocampus of neonatal rats at different body temperatures (33 , 37 and 39 °C) 2 h after exposure to normoxic (A–C) or anoxic (D–F) conditions. Confocal images show HIF-1α detection (red). Co-staining for HIF-1α with Hoechst (merge) demonstrates anoxia-induced accumulation of HIF-1α in the hippocampal region. Scale bars – 50 µm.
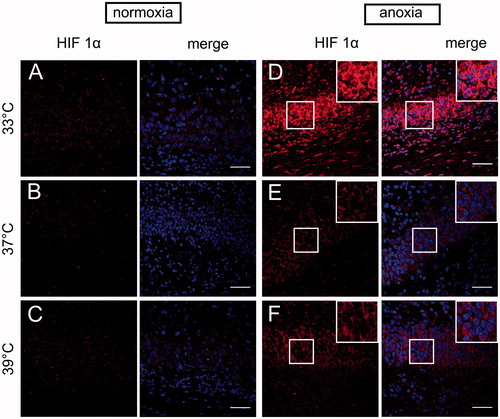
Figure 3. Quantitative analysis of HIF-1α level in the neonatal rats hippocampus (n = 3 per each group) at different body temperatures (33, 37 and 39 °C) 2 h after exposure to normoxic or anoxic conditions. Relative fluorescence intensity is expressed in arbitrary units (a.u). Values are presented as means ± SEM. Statistically significant differences between anoxic animals and their normoxic counterparts with the same body temperatures are denoted: #p < 0.05, ##p < 0.01 and ###p < 0.001; and those between the temperature variants of anoxic animals are denoted: ***p < 0.001.
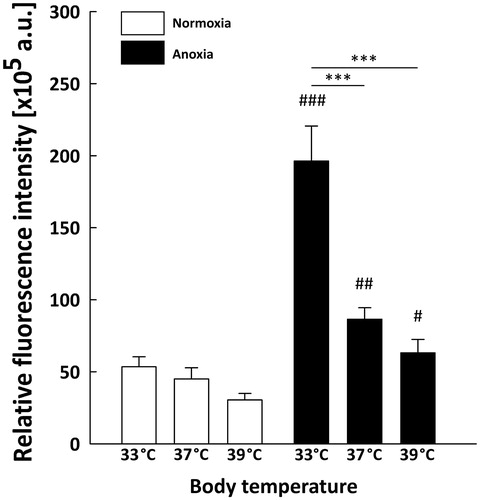
Figure 4. HIF-1α immunolocalization in the neocortex of neonatal rats brain at different body temperatures (33 , 37 and 39 °C) 2 h after exposure to normoxic (A–C) or anoxic (D–F) conditions. Representative images show the HIF-1α immunoreactive cells (red) Co-staining for HIF-1α with Hoechst (merge) demonstrates anoxia-induced accumulation of HIF-1α in neocortex. Scale bars – 50 µm.
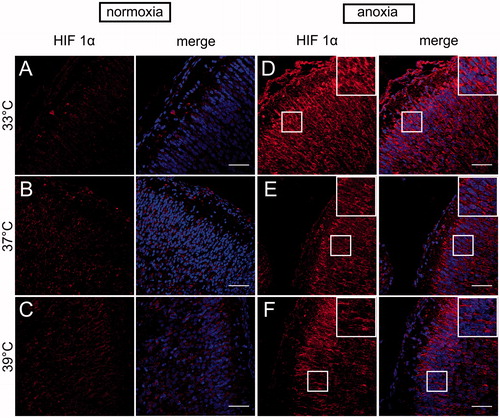
Figure 5. Quantitative analysis of HIF-1α level in the neonatal rats neocortex (n = 3 per each group) at different body temperatures (31 , 33 , 37C and 39 °C) 2 h after exposure to normoxic or anoxic conditions. Relative fluorescence intensity is expressed in arbitrary units (a.u). Data are presented as means ± SEM. Statistically significant differences between anoxic animals and their normoxic counterparts with the same body temperatures are denoted: ##p < 0.01 and ###p < 0.001; and those between the temperature variants of anoxic animals are denoted: ***p < 0.001.
Differential expression of HIF-1-regulated target genes in the anoxic brain
To determine the functional activation of cerebral HIF-1α protein due to anoxia, we analysed mRNA levels of VEGF and EPO, which are HIF-1 target genes, in the forebrains of newborn rats exposed to anoxia. Only in animals exposed to anoxia at physiological body temperature (33 °C, 33AN), cerebral VEGF mRNA levels increased (2.42-fold, p < 0.05) compared to normoxic controls. In anoxic animals with body temperature of 37 and 39 °C, cerebral VEGF mRNA level was not significantly different from that in their normoxic counterparts. Additionally, a strong increase of cerebral EPO mRNA levels was detected in animals exposed to anoxia at physiological body temperature (33 °C) (50.60-fold, p < 0.01) and in rats forced to stabilise their body temperature at 37 °C (11.83-fold, p < 0.05). In anoxic animals maintaining their body temperature at 39 °C the EPO mRNA level was not significantly different from that recorded in normoxic group with the same body temperature ().
Discussion
Body temperature during and after an episode of hypoxia/ischemia determines the severity of the insult. It has been shown that even mild hyperthermia significantly enhances brain damage [Citation39] because forcing body temperature of a hypoxic newborn to rise to the hyperthermic value by artificial warming, may put additional metabolic and physiological demands counterproductive to survival [Citation40]. Our up-to-date studies unequivocally suggest that body temperature maintained during and immediately after anoxia is a crucial factor influencing the development of post-anoxic pathological processes in neonatal rat brain [Citation21,Citation41,Citation42,Citation58]. We have demonstrated that elevated body temperature (hyperthermia or fever), as well as exceeding cooling beyond the physiological level of body temperature of newborn rats intensified post-anoxic oxidative stress and depleted the antioxidant pool [Citation33]. Since experimental data concerning effects of overheating on postnatal development are still insufficient, in the present study we evaluated the effects of elevated body temperature during anoxia and reoxygenation period on HIF-1α level at early stages of brain development.
Our data clearly demonstrate that the levels of HIF-1α protein, as well as that of two representative target genes, VEGF and EPO, are directly correlated with body temperature maintained during anoxia and reoxygenation period. Exposure to anoxia of neonatal rats with physiological body temperature of 33 °C triggers the highest increase of HIF-1α protein level whereas in rats with body temperatures elevated to 37 and 39 °C anoxia-induced HIF-1α activation was less pronounced. In the forebrain of newborn rats we have found the detectable basal level of HIF-1α mRNA and protein. This is consistent with other studies confirming that HIF-1α mRNA is expressed under normoxic conditions in rat and mouse brain [Citation43,Citation44]. Consistently, small amounts of HIF-1α protein were also found in normoxic rat brain cortex [Citation45]. Our results strongly support the idea that HIF-1α is a readily available neuroprotective system operating during short-term anoxia episode in the hippocampus and neocortex, areas of the brain particularly susceptible to damage caused by oxygen depletion. The fact that a low but detectable staining of HIF-1α was recorded in normoxic conditions reflects constant availability of HIF-1α for the regulation of physiological oxygen demands. Interestingly, we noted that short-term anoxia followed by 2 h of reoxygenation resulted in HIF-1α protein accumulation but not in the increase of HIF-1α mRNA levels in all experimental groups in forebrain of newborn rats. Other reports also demonstrated that moderate hypoxia in vivo does not affect HIF-1α mRNA levels in the hippocampus at 2 h after exposure [Citation43]. Subsequently, several in vitro studies provided evidence that HIF-1α mRNA is not upregulated under hypoxic conditions [Citation46,Citation47]. However, it has been also reported that HIF-lα mRNA levels, although upregulated during hypoxia, drop rapidly to basal levels following reoxygenation [Citation48,Citation49]. Thus, anoxia-induced transcriptional activation of HIF-1α can occur very rapidly. These studies explain our findings demonstrating the accumulation of HIF-1α protein and no increase in HIF-1α mRNA level 2 h after anoxia. Moreover, our results strongly support the idea that hypoxia induces HIF-1 α gene regulation via protein stabilisation rather than via transcriptional activation [Citation13,Citation14].
In our study we have found that in newborn rats kept at body temperature of 33 °C post-anoxic levels of HIF-1α protein were higher than that detected under hyperthermic conditions. It must be mentioned that only in rats maintaining their physiological body temperature the anoxia-induced HIF-1 stabilisation resulted in nuclear accumulation of this protein in hippocampus and neocortex. This observations are consistent with those of systemic hypoxia that induces nuclear accumulation of HIF-1α protein in cortical neurons and glial cells in developing mouse brain [Citation50]. Maintaining body temperature of an anoxic newborn at hyperthermic level provokes energy-consuming processes counterproductive to survival. The brain is extremely vulnerable to slight changes in temperature. Therefore, even mild hyperthermia can aggravate all aspects of the neurotoxic cascade [Citation51,Citation52]. Indeed, in the present study we found that at body temperatures forced to rise above the physiological value for newborn rats (33 °C) the anoxia-induced accumulation of HIF-1α protein within the hippocampus and neocortex was clearly reduced.
According to the hypothesis that HIF-1α accumulation during anoxia leads to transcriptional activation of HIF-1 target genes, we found an upregulation of VEGF and EPO transcripts that parallels the HIF-1α response. The presented data suggest that normothermic body temperature of newborn rats (33 °C) effectively upregulates HIF-1α target genes level 2 h after anoxia, which would be one more evidence of potential benefit of this physiological decrease in body temperature.
Experiments using different neonatal rodent models of hypoxia/ischemia also demonstrated upregulation of cerebral VEGF and EPO levels [Citation12,Citation53–55]. Additionally, during hypoxia, HIF-1α plays an important role in expression of VEGF, as was shown both in mouse [Citation50] and rat [Citation54] neonatal brains. We have also found elevated VEGF mRNA level in newborn rats maintaining their physiological body temperature (33 °C) exposed to anoxia. Moreover, accumulating evidence reveals that another target gene of HIF-1α - EPO, is induced in hypoxic brain [Citation12]. Depending on the severity of hypoxia, EPO mRNA levels in the brain can increase between three and 20-fold [Citation52]. Moreover, in the hippocampus of neonatal rats following hypoxia-ischemia EPO expression increased during reoxygenation period [Citation56]. In the present study, we also found that post-anoxic EPO mRNA level was enhanced in the forebrain of newborn rat. In contrast to this data, Yeo et al. [Citation57] have reported that in a mouse model, brain EPO transcription was induced mainly by HIF-2α when compared to HIF-1α under hypoxic conditions. However, there is ample evidence that HIF-1α plays an important role in EPO transcription regulation [Citation44,Citation53–55]. Thus, anoxia-induced activation of genes such as VEGF and EPO in rats with naturally lowered body temperature might be a part of physiological protective processes to prevent neuronal injury. The results presented above highlight that only in thermal condition allowing to maintain physiological body temperature of newborn rats the functional response of the HIF-1α induced pathway can be achieved. On the other hand hyperthermia may prevent the triggering of the endogenous adaptive response such as HIF-1α activation in neonate anoxic brain.
Conclusions
In conclusion, the presented results reveal that activation of HIF-1α represents a rapidly responsive and adaptive system in immature brain especially in areas particularly susceptible to damage caused by oxygen depletion. Additionally, the level of HIF-1α and its target genes expression depends on body temperature maintained during anoxia and reoxygenation period. We find it extremely important since temperature conditions represent only one variety of factors that may affect the severity of injury and efficiency of treatment process (along with the heterogeneity of brain tissue and the regulation and dichotomy of HIF-1α activation), which has not yet been fully elucidated. Further investigation of the spectrum of HIF-1α action would most certainly contribute to minimising the effects of hypoxic-ischemic damage in the immature brain.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Table 1. Number of rats exposed to anoxia or normoxia (in brackets) at different body temperatures (33, 37 and 39 °C) used in various methods.
Table 2. Primers.
Table 3. Effects of body temperature (33, 37 and 39 °C) during neonatal anoxia on expression of HIF-1α and its downstream genes in the forebrain of newborn rats 2 h after the insult.
Additional information
Funding
References
- Dixon BJ, Reis C, Ho WM, et al. (2015). Neuroprotective strategies after neonatal hypoxic ischemic encephalopathy. Int J Mol Sci 16:22368–401.
- Golan MH, Mane R, Molczadzki G, et al. (2009). Impaired migration signaling in the hippocampus following prenatal hypoxia. Neuropharmacology 57:511–22.
- Nyakas C, Buwalda B, Luiten PG. (1996). Hypoxia and brain development. Prog Neurobiol 49:1–51.
- Rodricks CL, Gibbs ME, Castillo-Melendez M, et al. (2010). The effect of hypoxia on the functional and structural development of the chick brain. Int J Dev Neurosci 28:343–50.
- Mañeru C, Serra-Grabulosa JM, Junqué C, et al. (2003). Residual hippocampal atrophy in asphyxiated term neonates. J Neuroimaging 13:68–74.
- Okereafor A, Allsop J, Counsell SJ, et al. (2008). Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 121:906–14.
- Semenza GL. (1999). Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Cell Dev Biol 15:551–78.
- Greer SN, Metcalf JL, Wang Y, et al. (2012). The updated biology of hypoxia-inducible factor. Embo J 31:2448–60.
- Trollmann R, Gassmann M. (2009). The role of hypoxia-inducible transcription factors in the hypoxic neonatal brain. Brain Dev 31:503–9.
- Trollmann R, Strasser K, Keller S, et al. (2008). Placental HIFs as markers of cerebral hypoxic distress in fetal mice. Am J Physiol - Regul Integr Comp Physiol 295:1973–81.
- Zhang P, Yao Q, Lu L, et al. (2014). Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep 6:1110–21.
- Fan X, Heijnen CJ, van der Kooij MA, et al. (2009). The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev 62:99–108.
- Liu L, Cash TP, Jones RG, et al. (2006). Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell 21:521–31.
- Chen W, Ostrowski RP, Obenaus A, et al. (2009). Prodeath or prosurvival: two facets of hypoxia inducible factor-1 in perinatal brain injury. Exp Neurol 216:7–15.
- Fandrey J, Gorr TA, Gassmann M. (2006). Regulating cellular oxygen sensing by hydroxylation. Cardiovasc Res 71:642–51.
- Kiriakidis S, Esteban MA, Maxwell PH. (2007). Genetic insights into the hypoxia-inducible factor (HIF) pathway. Adv Enzyme Regul 47:288–306.
- Semenza GL. (2000). HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol 88:1474–80.
- Lemus-Varela ML, Flores-Soto ME, Cervantes-Munguía R, et al. (2010). Expression of HIF-1α, VEGF and EPO in peripheral blood from patients with two cardiac abnormalities associated with hypoxia. Clin Biochem 43:234–9.
- van der Kooij MA, Groenendaal F, Kavelaars A, et al. (2008). Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev 59:22–33.
- Mu D, Jiang X, Sheldon RA, et al. (2003). Regulation of hypoxia-inducible factor 1α and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Dis 14:524–34.
- Rogalska J, Caputa M. (2005). Spontaneously reduced body temperature and gasping ability as a mechanism of extreme tolerance to asphyxia in neonatal rats. J Therm Biol 30:360–9.
- Laptook AR, Corbett RJT. (2002). The effects of temperature on hypoxic-ischemic brain injury. Clin Perinatol 29:623–49.
- Trescher WH, Ishiwa S, Johnston MV. (1997). Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Dev 19:326–38.
- Maier CM, Sun GH, Cheng D, et al. (2002). Effects of mild hypothermia on superoxide anion production, superoxide dismutase expression, and activity following transient focal cerebral ischemia. Neurobiol Dis 11:28–42.
- Zhao H, Steinberg GK, Sapolsky RM. (2007). General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab 27:1879–94.
- Koda Y, Tsuruta R, Fujita M, et al. (2010). Moderate hypothermia suppresses jugular venous superoxide anion radical, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Brain Res 1311:197–205.
- Bertin R, De Marco F, Mouroux I, et al. (1993). Postnatal development of nonshivering thermogenesis in rats: effects of rearing temperature. J Dev Physiol 19:9–15.
- Gordon CJ, Temperature regulation in laboratory rodents. New York (NY): Cambridge University Press; 1993.
- Wood T, Osredkar D, Puchades M, et al. (2016). Treatment temperature and insult severity influence the neuroprotective effects of therapeutic hypothermia. Sci Rep 6:23430.
- Edwards AD, Brocklehurst P, Gunn AJ, et al. (2010). Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340:c363.
- Azzopardi D, Strohm B, Linsell L, et al. (2012). Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK–analysis of national data. PLoS One 7:e38504.
- Kletkiewicz H, Nowakowska A, Siejka A, et al. (2016). Deferoxamine prevents cerebral glutathione and vitamin E depletions in asphyxiated neonatal rats: role of body temperature. Int J Hyperthermia 32:211–20.
- Kletkiewicz H, Rogalska J, Nowakowska A, et al. (2016). Effects of body temperature on post-anoxic oxidative stress from the perspective of postnatal physiological adaptive processes in rats. J Physiol Pharmacol 67:287–99.
- Semple BD, Blomgren K, Gimlin K, et al. (2013). Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106–107:1–16.
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–54.
- Laemmli UK. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–5.
- Schneider CA, Rasband WS, Eliceiri KW. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–5.
- Pfaffl MW, Horgan GW, Dempfle L. (2002). Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36.
- Minamisawa H, Smith ML, Siesjö BK. (1990). The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of forebrain ischemia. Ann Neurol 28:26–33.
- Belhadj Slimen I, Najar T, Ghram A, et al. (2014). Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. a review. Int J Hyperthermia 30:513–23.
- Caputa M, Rogalska J, Nowakowska A. (2001). Effect of temperature on postanoxic, potentially neurotoxic changes of plasma pH and free iron level in newborn rats. Brain Res Bull 55:281–6.
- Kletkiewicz H, Nowakowska A, Siejka A, et al. (2016). Deferoxamine improves antioxidative protection in the brain of neonatal rats: the role of anoxia and body temperature. Neurosci Lett 628:116–22.
- Heidbreder M, Fröhlich F, Jöhren O, et al. (2003). Hypoxia rapidly activates HIF-3alpha mRNA expression. FASEB J 17:1541–3.
- Stroka DM, Burkhardt T, Desbaillets I, et al. (2001). HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 15:2445–53.
- Chávez JC, Agani F, Pichiule P, et al. (2000). Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol 89:1937–42.
- Wenger RH, Kvietiko I, Rolfs A, et al. (1997). Hypoxia-inducible factor-1 alpha is regulated at the post-mRNA level. Kidney Int 51:560–3.
- Chamboredon S, Ciais D, Desroches-Castan A, et al. (2011). Hypoxia-inducible factor-1α mRNA: a new target for destabilization by tristetraprolin in endothelial cells. Mol Biol Cell 22:3366–78.
- Belaiba RS, Bonello S, Zähringer C, et al. (2007). Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell 18:4691–7.
- Wiener CM, Booth G, Semenza GL. (1996). In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun 225:485–8.
- Trollmann R, Schneider J, Keller S, et al. (2008). HIF-1-regulated vasoactive systems are differentially involved in acute hypoxic stress responses of the developing brain of newborn mice and are not affected by levetiracetam. Brain Res 1199:27–36.
- Manalo DJ, Rowan A, Lavoie T, et al. (2005). Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105:659–69.
- Marti HH, Wenger RH, Rivas LA, et al. (1996). Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci 8:666–76.
- Mu D, Chang YS, Vexler ZS, et al. (2005). Hypoxia-inducible factor 1alpha and erythropoietin upregulation with deferoxamine salvage after neonatal stroke. Exp Neurol 195:407–15.
- Bernaudin M, Tang Y, Reilly M, et al. (2002). Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J Biol Chem 277:39728–38.
- Sharp FR, Ran R, Lu A, et al. (2004). Hypoxic preconditioning protects against ischemic brain injury. NeuroRx 1:26–35.
- Lu J, Jiang L, Zhu H, et al. (2014). Hypoxia-inducible factor-1α and erythropoietin expression in the hippocampus of neonatal rats following hypoxia-ischemia. J Nanosci Nanotechnol 14:5614–19.
- Yeo J, Cho S, Kim S, Park W. (2008). Contribution of HIF-1alpha or HIF-2alpha to erythropoietin expression: in vivo evidence based on chromatin immunoprecipitation. Ann Hematol 87:11–17.
- Rogalska J, Danielisova V, Caputa M. (2006). Effect of neonatal body temperature on postanoxic, potentially neurotoxic iron accumulation in the rat brain. Neurosci Lett 393:249–54.
