Abstract
Introduction: Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) improve survival in selected patients with peritoneal metastases. However, only some patients who are potentially eligible for the procedure are considered and referred to the appropriate surgical department. By studying the trends of patients being considered for CRS and HIPEC in our centre, we hope to better understand the demographics of our patient cohort and the attitudes of physicians involved towards CRS and HIPEC.
Methods: Patients who were presented and discussed at our institution’s multidisciplinary tumour board (MDTB) for consideration of CRS and HIPEC, between 5 January 2011 and 16 December 2015, were identified from the institutional database and included in the study. Patient demographics and clinico-pathological data were retrospectively collected from electronic records and clinical charts.
Results: A total of 407 patients were presented at the MDTB for consideration of CRS and HIPEC. Referrals were most commonly from oncology-related departments (65.8%, n = 268). This was followed by referrals from other hospitals (15.0%, n = 61), overseas self-referrals (12.0%, n = 49) and non-oncologic departments within the same institution (7.1%, n = 29). Referrals made by oncology-related departments and overseas self-referrals showed an increasing trend over the years. Of the patients discussed, 197 patients (48.4%) were recommended for CRS and HIPEC, and 134 (68.0%) successfully underwent the procedure.
Conclusions: There is growing acceptance of CRS and HIPEC in patients and oncologic-related departments. However, consideration of this procedure as a treatment option remains low in non-oncologic departments. Dissemination of information and well-defined clinical recommendations may help physicians identify and select potentially eligible patients for consideration of CRS and HIPEC.
Introduction
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are currently indicated for selected patients with isolated peritoneal metastases. The procedure comprises complete surgical resection, which aims to remove all macroscopic tumour, followed by intraperitoneal perfusion of heated chemotherapy which targets the microscopic tumour cells, and has been shown to improve survival in selected patients with appendiceal, colorectal, ovarian, gastric, primary peritoneal and peritoneal mesothelioma malignancies [Citation1–6]. Despite the evidence for CRS and HIPEC, only a small proportion of patients who are potentially eligible for the procedures are considered. In our institution, these patients are either presented for discussion by the primary physician at the institution’s multidisciplinary surgical oncology tumour board (MDTB) or referred to the surgical oncology division for consideration of this procedure [Citation7]. Most physicians cite the morbidity and high costs as reasons against the procedure [Citation8] and remain reluctant to include CRS and HIPEC as a potential treatment option. However, studies have shown that the benefits outweigh the risks in properly selected patients [Citation4]. Furthermore, quality of life (QOL) studies have also demonstrated that patients cope well in the post-operative period [Citation9], with the majority enjoying a better QOL when compared to patients with similar-staged disease undergoing palliative chemotherapy [Citation6]. Thus, to ignore CRS and HIPEC as a viable option may deny these patients’ potential survival benefits and possible improvement in QOL.
By studying the patients that were being considered for CRS and HIPEC at the MDTB, we aim to investigate the trend of patient referrals for the procedure, in order to gain insight into the attitudes of other physicians towards CRS and HIPEC and potentially identify barriers to referral.
Methods
This study was approved by the institutional review board and conducted at a large-volume oncological institution. All patients who were considered for CRS and HIPEC from January 2011 to December 2015 were included in the study.
All patients who are referred for consideration of CRS and HIPEC are discussed at the weekly MDTB, where surgical oncologists, medical oncologists, radiation oncologists, pathologists and oncology radiologists are present. Clinical presentation and radiological images are scrutinised, and the likelihood of achieving complete cytoreduction is evaluated, before a consensus is made about the most appropriate treatment plan for the patient.
MDTB recommendations were recorded in a prospective fashion, and used in this study. Additional clinico-pathological data not previously recorded were retrospectively collected from both electronic records and clinical charts. These included patient demographics, source of referral, disease characteristics including site and histology of tumour, treatment received and outcomes of CRS and HIPEC if performed. In the case of CRS and HIPEC, the surgeon calculates the peritoneal carcinomatosis index (PCI) intraoperatively and also documented the completeness of cytoreduction (CC), where CC-0 was defined as no residual disease, CC-1 as residual disease less than or equal to 2.5 mm, CC-2 as residual disease greater than 2.5 mm but smaller than 2.5 cm, and CC-3 as residual disease greater than 2.5 cm [Citation9]. A CC score of 0 or 1 was considered complete cytoreduction, and for the purposes of this study, recorded as successful surgery.
Results
Patient demographics
Between January 2011 and December 2015, 407 patients who were considered for CRS and HIPEC were identified from the institutional database. The patient demographics are summarised in . There were more females (65.6%, n = 267) than males (34.4%, n = 140) and the median age was 55 years (13 to 87 years). Chinese patients made up the majority of the patients (74.2%, n = 302), followed by Indians (5.7%, n = 23) and Malays (5.4%, n = 22) which was expected, given the local racial make-up. Local residents (72.5%, n = 295) also made up the bulk of the patients referred, while foreigners made up 27.5% (n = 112) of the cohort. Our centre has the largest known experience with CRS and HIPEC in Southeast Asia, which may explain the foreign patient representation in our cohort.
Table 1. Demographics and clinico-pathological characteristics of patients considered for CRS and HIPEC.
Of the patients who were discussed at the MDTB, 34.2% (n = 139) had synchronous peritoneal metastases, while 63.9% (n = 260) had recurrent disease in the peritoneum. The common sites of origin of the tumours were colorectal (38.1%, n = 155), ovarian (33.4%, n = 136) and appendiceal (18.4%, n = 75). The most frequent histological subtype for colorectal cancer was adenocarcinoma (66.5%, n = 103) followed by mucinous adenocarcinoma (18.1%, n = 28), while papillary serous adenocarcinoma (60.3%, n = 82) was most common in ovarian cancer. Mucinous carcinoma with pseudomyxoma peritonei (PMP) was most common in appendiceal cancer (89.3%, n = 67). The breakdown of histological subtypes is summarised in .
Referral trends
The study period from 2011 to 2015 was chosen because this was the period in which we felt that we had overcome the inherent learning curve. Our centre first attempted CRS and HIPEC in 2001 and the number of procedures completed per year has increased steadily from 2 in 2001 to 14 in 2010, and 30 performed in 2011.
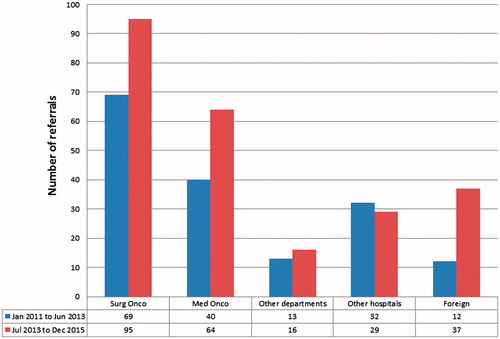
There was also a general increasing trend of referrals over the years with 69 patients considered for CRS and HIPEC in 2011, 60 in 2012, 92 in 2013, 103 in 2014 and 83 in 2015. For ease of comparison, we have categorised our results into 2 groups – from January 2011 to June 2013 and July 2013 to December 2015. The surgical oncology department within the same institution referred the most number of patients (40.3%, n = 164) for consideration for CRS and HIPEC, followed by the medical oncology department within the same institution (25.6%, n = 104). The patients referred by the surgical oncology department were patients with recurrent tumours who were already on follow-up with the same department. Non-oncologic departments within the same institution referred 7.1% (n = 29) of the cohort while other hospitals in the country referred 15.0% (n = 61) of patients. Self-referrals from other countries made up 12.0% (n = 49) of our cohort. This consisted of foreign patients who were not previously seen by any clinician at our institution and had presented for their first visit to either the medical or surgical oncology department. Over the years, there was an increase in the number of cases referred from the surgical and medical oncology departments and self-referrals from foreign patients while the proportion of referrals by non-oncologic departments and other hospitals remained constant. The trend of referrals categorised by source of referral is summarised in .
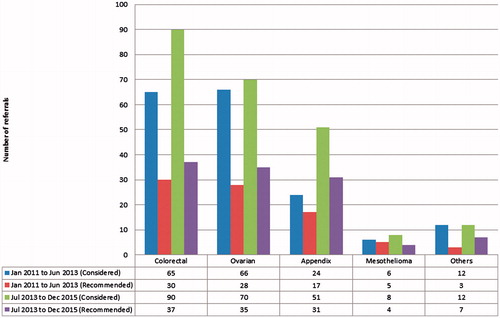
The rate of increase of referrals were seen most in appendiceal cancers with a twofold increase from 24 to 51 referrals, followed by colorectal cancers with a 38% increase in referrals from 65 to 90. The number of referrals for ovarian cancers, peritoneal mesotheliomas and other sites were generally constant. shows the trend of referrals categorised by site of tumour.
MDTB outcomes
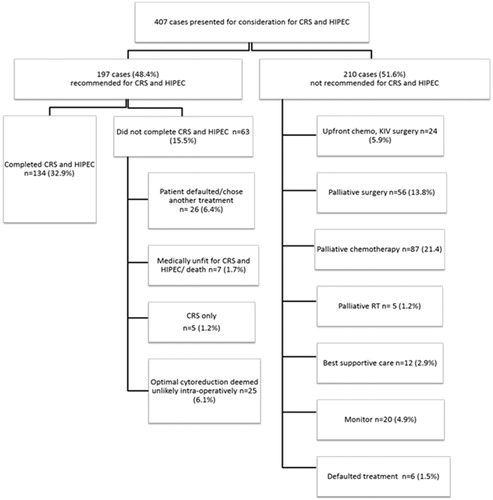
Of the 407 patients considered for CRS and HIPEC, 48.4% patients (n = 197) were recommended at the MDTB to undergo CRS and HIPEC. Of the 197 patients recommended for CRS and HIPEC, 67 (34%) patients had colorectal cancer, 63 (32%) had ovarian cancer, 48 (24%) had appendiceal cancer, 9 (4.6%) had peritoneal mesothelioma and the remaining 10 (5.1%) had peritoneal metastases originating from other sites. Despite the increase in rate of referrals, the number of patients who were eventually recommended for CRS and HIPEC remained largely the same. In colorectal cancer, 46.2% of patients referred between January 2011 and June 2013 were recommended as compared to 41.1% in the subsequent years. The same trend was observed in ovarian cancers with 41.2% of patients recommended in the first half as compared to 50% in the second half of study period and appendiceal cancers with 70.8% of patients recommended in the first half as compared to 60.8% in the second half ().
Of these 197 patients recommended for CRS and HIPEC, 26 patients (13.2%) defaulted or opted for another treatment, while 7 patients (3.6%) were deemed medically unfit to undergo the procedure. The remaining 164 patients (40.3%) underwent elective laparotomy with the plan for CRS and HIPEC. Of these patients, only 134 patients (68.0%) underwent complete CRS and HIPEC successfully ().
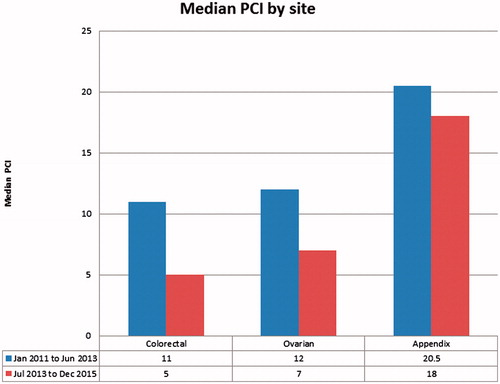
Over the years, the proportion of patients who were considered for and eventually went on to successful CRS and HIPEC decreased. 79.5% (66 out of 83) from January 2011 to June 2013 underwent CRS and HIPEC as compared to 59.6% (68 out of 114) from July 2013 to December 2015. However, there was a decrease over the years in median PCI in patients who underwent successful CRS and HIPEC. The overall median PCI in the second half of study period was 9 (2 to 39) as compared to 14 (2 to 39) in the first half of the study. Similarly, median PCI decreased from 11 (2–27) to 5 (2–24) in colorectal cancers, 12 (2–31) to 7 (2–28) in ovarian cancers and 20.5 (7–39) to 18 (2–39) in appendiceal cancer ().
Of the 30 patients who did not undergo HIPEC at the time of laparotomy, 5 underwent CRS alone, as 2 had extensive adhesions and 3 patients were found to have a single site of local recurrence as opposed to peritoneal disease. The remaining 25 underwent palliative debulking surgery only as they were found to have extensive disease, and complete cytoreduction was not possible. The proportion of patients who had aborted CRS and HIPEC was fairly constant. Between January 2011 and June 2013, 10 out of 74 planned for CRS and HIPEC (13.5%) underwent only palliative debulking as compared to 15 out of 90 (16.7%) between July 2013 and December 2015.
Amongst the 210 patients who were not recommended for CRS and HIPEC by the MDTB, 95 (45.2%) patients had extensive peritoneal disease seen on imaging that was deemed not feasible for optimal cytoreduction, 47 (22.4%) patients had distant metastatic disease seen on imaging, 20 (9.5%) patients were medically unfit for surgery, and 16 (7.6%) patients had primary tumour histologies that were not considered suitable for CRS and HIPEC. There were 8 (3.8%) patients with no radiological evidence of peritoneal disease when their computed tomography (CT) or positron emission tomography-CT (PET-CT) scans were reviewed at the MDTB. Five of these patients had an additional PET-CT scan which confirmed no peritoneal disease while the remaining 3 patients had a repeat CT scan within 3 months which did not reveal any peritoneal disease. The remaining 24 patients (11.4%) had indeterminate extra-peritoneal lesions and/or poor primary tumour biology, such as high disease burden, high nodal staging and a short disease-free interval (DFI) of less than 6 months, and were hence offered neoadjuvant chemotherapy first before re-evaluation.
The treatment recommended for these 210 patients were as follows: 24 (11.4%) had chemotherapy and were planned for reassessment for CRS and HIPEC after 3-6 cycles of chemotherapy, 56 (26.7%) underwent palliative surgery, 87 (41.4%) had palliative chemotherapy, 5 (2.4%) had palliative radiotherapy, 12 (5.7%) received best supportive care, 20 (9.5%) were observed further for development of indeterminate extra-peritoneal lesions and 6 (2.9%) had defaulted treatment ().
Of the 24 patients who were offered neoadjuvant chemotherapy, 10 were offered because of indeterminate extra-peritoneal lesions, 4 were deemed to have poor tumour biology by their short DFI, and the remaining 10 were deemed to have peritoneal disease that were too extensive. These patients were offered chemotherapy to allow time to ensure the absence of systemic failure before consideration of CRS and HIPEC, as a better indication of the understanding of their underlying tumour biology was sought. The chemotherapy also simultaneously presented an opportunity for a reduction in disease burden and improvement in the chance for complete resection. These patients were later re-evaluated for eligibility for CRS and HIPEC. Of this cohort, only 4 (16.6%) patients underwent complete CRS and HIPEC post-chemotherapy. The remaining patients underwent palliative debulking surgery or continued palliative chemotherapy.
Discussion
Peritoneal metastases have traditionally been considered a harbinger of dismal prognosis with a fatal outcome. It occurs in up to 20% of patients first diagnosed with colorectal cancer, in more than 25% of those who recur [Citation7,Citation10–12] and even more frequently in ovarian cancer [Citation13]. Palliative treatment with systemic chemotherapy has traditionally been the standard of care for patients with peritoneal metastases but with such treatment, median survival remains poor at 6–12 months [Citation5,Citation14]. CRS and HIPEC were first introduced by Sugarbaker in the 1980s and popularised in the 1990s [Citation15,Citation16] and have since been associated with improved survival for selected patients with colorectal, ovarian, appendiceal and primary peritoneal diseases [Citation1–3,Citation5]. The aim of CRS is to remove all visible disease, after which heated chemotherapeutic agents are perfused intra-peritoneally to target the microscopic disease. The major advantage of HIPEC is the ability to administer the chemotherapeutic agent at a significantly increased dose, while limiting systemic absorption. Studies have also shown that hyperthermia increases the chemotherapeutic penetration [Citation17].
Despite the evidence for CRS and HIPEC, only a minority of patients eligible for this multimodal treatment have been offered the option at present [Citation7]. Recent surveys conducted have also shown that up to 46% of the physicians involved in the care of patients with colorectal peritoneal metastases did not consider CRS and HIPEC as a treatment option for their patients with peritoneal disease and there was significant variance in views across specialties [Citation18,Citation19].
One of the strongest criticisms of CRS and HIPEC has been its associated morbidity. The long operative times and prolonged hospital stays commonly result in increased post- operative complications and morbidity [Citation4,Citation20–24]. Morbidity and mortality rates have been reported between 24%-46% and 1.5%-8% respectively [Citation2,Citation24–26]. Our own review of 100 consecutive patients who underwent CRS and HIPEC reported 45% 30-day high-grade (grades 3, 4 and 5) post-operative complications but no mortality [Citation4]. However, the rate of complications has reduced with the overcoming of learning curve. At our institution, we also routinely insert chest tubes in patients who undergo diaphragmatic stripping as many of these patients develop pleural effusions post-operatively. The need for percutaneous drainage of intra-abdominal collections has also reduced significantly since December 2012 when our centre stopped performing early post-operative intra-peritoneal chemotherapy. Since then, the post-operative high-grade morbidity rate has reduced to 13.5% [Citation27].
This paper reports the trend of patients considered for CRS and HIPEC in our centre from 2011 to 2015. We hoped to gain insight with regards to the attitude of various disciplines and departments towards CRS and HIPEC, the potential barriers to referral and the type of patients whom healthcare providers consider candidates for the procedure. With this knowledge, we hope to reach out to the medical community and share our experience with CRS and HIPEC and its potential benefits.
We found a general increase in referral numbers over 5 years. Unsurprisingly, the bulk of the cases presented for discussion at in the multidisciplinary tumour board in our centre came from the medical and surgical oncologists. One of the greatest increases in rate of referrals came from the medical oncologists. This increase could reflect a growing acceptance of the procedure because of the improved patient outcomes demonstrated in international centres [Citation1,Citation2] as well as in our centre [Citation4,Citation5].
An increase was also observed in the foreign self-referrals. Patients who are increasingly educated and internet savvy may have come to know of the treatment options for their disease by seeking information from the internet. This could also be due to our department’s efforts in creating awareness for CRS and HIPEC through publishing papers regarding our centre’s experience [Citation4,Citation5] and outcomes and circulating information booklets on the procedure.
All of the referrals from the surgical oncology team consisted of patients who were already on follow-up with the department from previously resected tumours but suffered intra-peritoneal recurrences. Unsurprisingly, the surgical oncologists made up the majority of the referrals (40.3%; 164 out of 407). However, the proportion of referrals made by the surgical oncology department was generally kept constant (41.5% between January 2011 and June 2013 versus 39.4% between July 2013 and December 2015).
As compared to the medical and surgical oncology departments, lower referral rates were observed from other departments and hospitals. This is expected of non-oncologic departments, as they might not encounter as many patients who might be potentially eligible for the procedure. More notably, the rate of referral remained constant, suggesting that more needs to be done, to engage physicians from non-oncologic departments.
Various groups like Society of Surgical Oncology, European Society of Medical Oncology and the Canadian HIPEC group have come up with guidelines and consensus statements that include CRS and HIPEC as standard of care for selected patients with colorectal peritoneal metastases [Citation28,Citation29]. Thus, in our centre, the bulk of referrals were regarding patients with colorectal peritoneal metastases, followed by ovarian and appendiceal cancers but the greatest increase in rate of referrals was seen in appendiceal cancers followed by colorectal cancers. This is likely because CRS and HIPEC is now accepted to be the gold standard for the treatment of appendiceal cancers, and hence as the knowledge and awareness of this treatment modality increase, so have the referrals. Interestingly, although the incidence of gastric cancers is high in Asia, gastric cancers with peritoneal metastases is generally not considered an indication for CRS and HIPEC in our centre due to the dismal prognosis. In our centre, only 5 patients with gastric cancers were presented for consideration of CRS and HIPEC and 1 went on to undergo the procedures. However, we acknowledge the recent increase in studies showing improvement in survival with CRS and HIPEC in selected gastric cancer patients with limited peritoneal disease, and are continuing to explore and consider this indication for future patients [Citation30,Citation31].
In our patients who underwent laparotomy, 80% (134/164) achieved complete CRS and HIPEC, which can be reassuring for patients and physicians who remain concerned about the risk of an “open-close surgery”. Nevertheless, there was still a significant proportion of patients (32%, 63 out of 197) recommended for CRS and HIPEC who did not undergo the planned procedure for a variety of reasons, the most common of which was having extensive disease prompting abortion of procedure intra-operatively (n = 25). Furthermore, the proportion of patients who were considered for and eventually went on to successful CRS and HIPEC decreased from 79.5% to 59.6% and the rate of our “open-close surgeries” increased slightly from 13.5% to 16.7%. While our rates of open-close surgeries are lower than those reported in other groups (25%-48%) [Citation32,Citation33], this still suggests that more work needs to be done to improve our preoperative prediction of disease burden. Many studies have looked at the role of preoperative imaging [Citation34,Citation35] and routine diagnostic laparoscopy in order to reduce the numbers of unnecessary surgeries [Citation33,Citation36]. However, in our institution, diagnostic laparoscopy is only done for selected patients as many of our patients have undergone multiple previous open surgeries and there is a concern that the adhesions would limit visualisation and compromise accurate assessment of intra-abdominal disease burden.
Lastly, we also found a decreasing trend in the median PCI of colorectal and ovarian cancer patients who had successfully completed CRS and HIPEC. This suggests that patients are being referred earlier for consideration of CRS and HIPEC. Not unexpectedly, the median PCI was not appreciably lower in the appendiceal cancers as PCI does not correlate well with survival in appendiceal peritoneal metastases [Citation37].
Limitations
This paper is a retrospective study that has its inherent biases and caution must be used in interpreting the results. We could only speculate at the reasons behind the evolving trends of referral. Further studies could include qualitative surveys for patients and clinicians, especially in the non-oncologic departments, to look into their attitude and concerns regarding CRS and HIPEC.
Conclusion
In conclusion, there is a growing acceptance of clinicians in oncology-related fields towards CRS and HIPEC. However, adoption remains low in non-oncologic departments. Better awareness and communication across disciplines involved in the treatment of the primary cancers that may present with peritoneal disease and metastases can increase the number of patients being considered for CRS and HIPEC. These specialties could also work together to define the type of patients who should be considered for CRS and HIPEC. Having well-defined clinical recommendations would be valuable not only in standardising optimal care for the patient, but also allow all physicians to identify the patients who are suitable for the procedure, and will potentially encourage greater adoption for the consideration of the procedure.
Disclosure statement
The authors have no disclosures.
References
- Sadeghi B, Arvieux C, Glehen O, et al. (2000). Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88:358–63.
- Verwaal VJ, Bruin S, Boot H, et al. (2008). 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 15:2426–32.
- Razenberg LG, van Gestel YR, Creemers GJ, et al. (2015). Trends in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of synchronous peritoneal carcinomatosis of colorectal origin in the Netherlands. Eur J Surg Oncol 41:466–71.
- Teo MC, Tan GH, Tham CK, et al. (2013). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in Asian patients: 100 consecutive patients in a single institution. Ann Surg Oncol 20:2968–74.
- Wong JF, Tan GH, Wang W, et al. (2015). Repeat cytoreductive surgery and HIPEC for peritoneal surface malignancy and peritoneal carcinomatosis. World J Surg 39:1578–1583.
- Glockzin G, Schlitt HJ, Piso P. (2009). Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 7:5.
- Esquivel J. (2010). Current status of colorectal cancer with peritoneal carcinomatosis. Ann Surg Oncol 17:1968–9.
- Mirnezami R, Mehta AM, Chandrakumaran K, et al. (2014). Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br J Cancer 111:1500–8.
- Tan GH, Cheung M, Chanyaputhipong J, et al. (2013). Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal mesothelioma. Ann Acad Med Singapore 42:291–6.
- Jayne DG, Fook S, Loi C, Seow-Choen F. (2002). Peritoneal carcinomatosis from colorectal cancer. Br J Surg 89:1545–50.
- Quere P, Facy O, Manfredi S, et al. (2015). Epidemiology, management, and survival of peritoneal carcinomatosis from colorectal cancer: a population-based study. Dis Colon Rectum 58:743–52.
- Teo MC, Ching Tan GH, Lim C, et al. (2015). Colorectal peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: the experience of a tertiary Asian center. Asian J Surg/Asian Surg Assoc 38:65–73.
- Teo MC. (2014). Update on the management and the role of intraperitoneal chemotherapy for ovarian cancer. Curr Opin Obstet Gynecol 26:3–8.
- Pilati P, Rossi CR, Mocellin S, et al. (2001). Multimodal treatment of peritoneal carcinomatosis and sarcomatosis. Eur J Surg Oncol 27:125–34.
- Sugarbaker PH. (1998). Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol 14:254–61.
- Sugarbaker PH. (1999). Management of peritoneal-surface malignancy: the surgeon’s role. Langenbeck’s Arch Surg/Deutsche Gesellschaft Fur Chirurgie 384:576–87.
- Sugarbaker PH, Mora JT, Carmignani P, et al. (2005). Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. The Oncologist 10:112–22.
- Chan AW, Munene G, Weaver J, et al. (2013). Cytoreductive strategies in the treatment of carcinomatosis of colorectal origin: results of a transdisciplinary national survey. Open Surg Oncol J 4:1–6.
- Braam HJ, Boerma D, Wiezer MJ, van Ramshorst B. (2015). Cytoreductive surgery and HIPEC in treatment of colorectal peritoneal carcinomatosis: experiment or standard care? A survey among oncologic surgeons and medical oncologists. Int J Clin Oncol 20:928–934.
- Roviello F, Pinto E, Corso G, et al. (2010). Safety and potential benefit of hyperthermic intraperitoneal chemotherapy (HIPEC) in peritoneal carcinomatosis from primary or recurrent ovarian cancer. J Surg Oncol 102:663–70.
- Bijelic L, Jonson A, Sugarbaker PH. (2007). Systematic review of cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis in primary and recurrent ovarian cancer. Ann Oncol 18:1943–50.
- Makrin V, Lev-Chelouche D, Even Sapir E, et al. (2005). Intraperitoneal heated chemotherapy affects healing of experimental colonic anastomosis: an animal study. J Surg Oncol 89:18–22.
- Haslinger M, Francescutti V, Attwood K, et al. (2013). A contemporary analysis of morbidity and outcomes in cytoreduction/hyperthermic intraperitoneal chemoperfusion. Cancer Med 2:334–42.
- Glehen O, Osinsky D, Cotte E, et al. (2003). Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol 10:863–9.
- Stephens AD, Alderman R, Chang D, et al. (1999). Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol 6:790–6.
- Hadi R, Saunders V, Utkina O, et al. (2006). Review of patients with peritoneal malignancy treated with peritonectomy and heated intraperitoneal chemotherapy. ANZ J Surg 76:156–61.
- Tan GH, Ong WS, Chia CS, et al. (2016). Does early post-operative intraperitoneal chemotherapy (EPIC) for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) make a difference? Int J Hyperthermia 32:281–8.
- Dube P, Sideris L, Law C, et al. (2015). Guidelines on the use of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal surface malignancy arising from colorectal or appendiceal neoplasms. Curr Oncol (Toronto, ON) 22:e100–12.
- Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. (2014). Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii1–9.
- Chia CS, You B, Decullier E, et al. (2016). Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol 23:1971–9.
- Rudloff U, Langan R, Mullinax JE, et al. (2014). Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 110:275–84.
- van Oudheusden TR, Braam HJ, Luyer MD, et al. (2015). Peritoneal cancer patients not suitable for cytoreductive surgery and HIPEC during explorative surgery: risk factors, treatment options, and prognosis. Ann Surg Oncol 22:1236–42.
- Iversen LH, Rasmussen PC, Laurberg S. (2013). Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br J Surg 100:285–92.
- Turlakow A, Yeung HW, Salmon AS, et al. (2003). Peritoneal carcinomatosis: role of (18)F-FDG PET. J Nuclear Med 44:1407–12.
- Low RN. (2016). Preoperative and surveillance MR imaging of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. J Gastrointestinal Oncol 7:58–71.
- Marmor RA, Kelly KJ, Lowy AM, Baumgartner JM. (2016). Laparoscopy is safe and accurate to evaluate peritoneal surface metastasis prior to cytoreductive surgery. Ann Surg Oncol 23:1461–7.
- Mittal RA-O, Chandramohan A, Moran B. (2017). Pseudomyxoma peritonei: natural history and treatment. Int J Hyperthermia 33:511–19.