Abstract
Purpose: This study was designed to evaluate the efficacy and safety of microwave ablation (MWA) in the treatment of intraoperative life-threatening tumour haemorrhage during hepatic surgeries.
Methods: Three cases of MWA application in the emergent control of life-threatening hepatic tumour haemorrhage were analysed and reported.
Results: Satisfactory hemostasis for hepatic tumour rupture was achieved by MWA in all three cases. No major complications, such as post-operative haemorrhage, bile duct injury, liver abscess, colon perforation, skin burns, tumour seeding or renal dysfunction, were identified.
Conclusions: MWA may be a feasible, effective and simple strategy for the emergent control of intraoperative hepatic tumour bleeding. To the best of our knowledge, this study represents the first reported cases of this novel application of MWA.
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer death worldwide. Surgical approaches, including tumour resection and liver transplantation, have been the traditional potentially curative treatments. However, intraoperative haemorrhage is a major concern, as it may cause hemodynamic instability or tumour recurrence [Citation1] and can be life-threatening. Aside from surgical resection, microwave ablation (MWA) has emerged as an effective and safe therapy for small liver tumours over the previous decade [Citation2–4]. However, limited reports are available regarding the use of MWA for hemostasis in hepatic surgeries. Here, we report a case series of MWA for the emergent control of life-threatening tumour haemorrhage in patients undergoing hepatic surgeries.
Case reports
Case #1
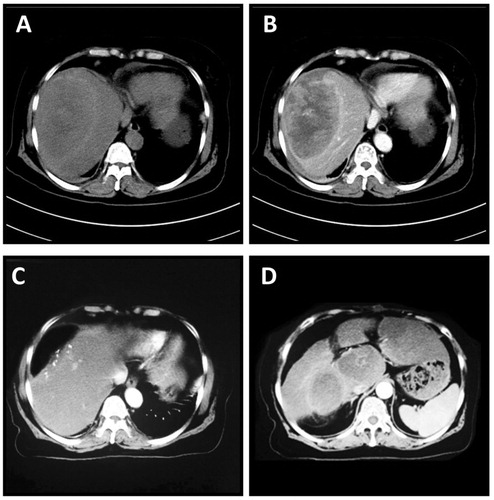
A 68-year-old woman presenting with abdominal pain was admitted to our hospital in July 2014. The patient had persistent dull abdominal pain in the right upper quadrant for over 1 year without receiving medical evaluation or treatment. She had no evidence of hepatitis B or hepatitis C, and her initial vital signs were stable. The alpha-fetoprotein (AFP) was >1210 ng/mL. A liver computed tomography (CT) indicated a large, 11.1 × 7.5 × 9.0 cm hepatic mass in liver segment VIII, which featured external protrusion. It also showed low-density signalling at the centre of the lesion ().
Figure 1. CT scan of the liver in Case #1. A huge 11.1 × 7.5 × 9.0 cm mass was identified in segment VIII. It showed a rich blood supply within the tumour (A and B, plain scan and arterial phase, respectively). Enhanced CT scan was performed at 3 months after surgery. Post-operative image of the liver after partial resection (C). Multiple metastases were identified in the liver (D).
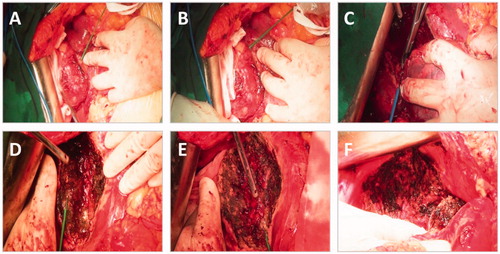
A partial hepatectomy was considered and scheduled after routine preoperative preparation. A reverse “L” shape incision was made for laparotomy. Mild fatty liver-like changes were observed, but no liver cirrhosis was noted. The lesion was exophytic, and the diaphragm was invaded. Continuous haemorrhage occurred during the separation of the peritumor adhesions. The bleeding continued despite the application of multiple conventional hemostasis strategies, such as compression, suture and ligation, electrocoagulation and hepatic portal occlusion. A total volume of 500 ml blood was lost within 5 min, accompanied by hemodynamic compromise. WMA with the ECO system (ECO-100E, Nanjing, China) was subsequently applied to stop the bleeding from the ruptured tumour. After satisfactory hemostasis was achieved, WMA was further used to assist in partial liver resection. The microwave needle was inserted into the peripheral hepatic parenchyma around the tumour base, followed by adequate ablation with 55 W for 10–15 s at each site, as shown in . The hepatic portal triad was subsequently occluded (the Pringle maneuver), and the conventional clamp crushing method was used for tumour removal (). The haemorrhage at the base of the wound was further treated with MWA (). The intermittent clamping strategy was also applied to protect the liver from prolonged ischaemic injury. Finally, areas of the diaphragm invaded by the hepatic tumour were treated by MWA (). The total surgical time was 210 min, and the intraoperative blood loss was ∼600 ml. The patient received 3 units of red blood cells (RBCs) and 320 ml of fresh frozen plasma. The patient recovered well and was discharged on postoperative Day 14.
Figure 2. Pictures indicating the multiple steps of the surgery in Case #1. The microwave needle was inserted into the liver parenchyma at different sites around the tumour, and MWA was subsequently conducted (A,B). Tumor resection by the conventional clamp crushing method (C). MWA at the wound base for hemostasis (D,E). MWA of the invaded diaphragm (F).
HCC was identified via pathological analysis. Unfortunately, the patient presented with multiple metastases within the liver at 3 months after surgery (). She subsequently received radiotherapy at another medical centre and died of multiple organ failure at 16 months after surgery.
Case #2
A 40-year-old man presenting with recurrent upper abdominal pain for more than 1 month was admitted to our centre in February 2016. He had been diagnosed with hepatitis B at birth and received anti-viral therapy for more than 10 years. A CT scan showed multiple liver masses with no indication for surgical resection.
The man received liver transplantation from donation-after-circulatory-death donors (DCD). However, during the resection of the recipient’s diseased liver, uncontrollable haemorrhage occurred as a result of the isolation of local adhesions of the tumour located at the liver surface. The bleeding was so vigorous and frustrating that no successful hemostasis was achieved by electrocoagulation or suture and ligation until MMA was applied (55 W at multiple sites for 6 min). The subsequent liver transplantation and postoperative recovery is going well. He is alive and well as of the latest follow-up in November 2016.
Case #3
A 53-year-old woman presented with right upper abdominal pain for 1 day and was admitted in December 2015. Rupture of a hepatic tumour was indicated by a CT scan performed at a local hospital. Hemorrhagic shock was corrected by fluid and blood infusion. The patient was transferred to our centre for further treatment.
An abdominal laparotomy was scheduled, and 1700 ml of dark blood were noted in the peritoneal cavity. A 10-cm-diameter haematoma was identified in liver segment VI, which had previously ruptured and adhered to the lateral abdominal wall. After removal of the haematoma, active bleeding continued and could not be stopped by conventional hemostasis strategies. Subsequent haemorrhagic shock occurred, and enhanced fluid and blood infusion was applied. WMA was then used for hemostasis. After coagulation at multiple sites in the base of the haematoma for 10 min, the bleeding was stopped. The total volume of blood loss was ∼2500 ml; 15.7 units of RBCs and 1000 ml of plasma were infused. Liver haemangioma was identified via postoperative pathological analysis. The patient recovered well, and no liver mass was identified by ultrasound or CT scan during the 1-year follow-up.
Discussion
We have shown that MWA is safe and effective in (i) the control of intraoperative haemorrhage due to liver tumour rupture, (ii) assisting in the resection of large liver tumours and (iii) treating local liver metastases.
HCC is a major health problem worldwide and a leading cause of cancer-related death. Surgical resection or liver transplantation is the gold standard for HCC treatment. However, thermal ablations, such as radiofrequency ablation (RFA), cryoablation and high-intensity focussed ultrasound (HIFU) ablation, are becoming increasingly utilised in the treatment of primary and secondary liver tumours [Citation2]. RFA is the most common type of thermal ablation worldwide. However, as a result of the high tissue perfusion and large blood vessels in the liver [Citation5], also referred to as “heat sinks”, adequate treatment is difficult. The heat sinks may also lead to sub-lethal temperatures and an increased likelihood of local tumour progression [Citation6]. In contrast, MWA does not present this problem, as microwaves propagate readily through charred and desiccated tissues, thus leading to larger ablation zones than RFA. The MWA device we employed is equipped with a water cooling system, which enables a longer ablation time with higher power production [Citation7]. The new generation of multiple microwave antennas enables more efficient heat delivery and larger ablation zones than a single antenna [Citation8]. Although these devices with multiple microwave antennas were not used in our hospital, we placed the single antenna at multiple sites around the tumour to achieve a substantially larger ablation area. These factors and techniques enabled us to achieve adequate ablation zones both in the tumour and peritumor liver parenchyma.
Although many studies have shown the role of MWA in the treatment of solid tumours, limited articles are available on its application in haemorrhage treatment. Thermal ablation was reported to successfully control solid-organ haemorrhage and uterine bleeding in patients who were poor surgical candidates [Citation9,Citation10]. Ophelia et al. [Citation11] reported a case of post-biopsy liver haemorrhage that could not be controlled by transarterial embolisation; however, it was successfully stopped by ultrasound-guided percutaneous MWA. In another case, transcatheter hepatic arterial embolisation followed by WMA was reported as a useful treatment for HCC with portal and biliary tumour thrombi that rupture into the biliary system [Citation12]. Consistent with these findings, satisfactory hemostasis for hepatic tumour rupture was achieved by MWA in all three cases, which indicates that MWA may be a feasible, effective and simple strategy for the emergent control of intraoperative hepatic tumour bleeding.
Resection of large liver tumours is typically associated with a longer hepatic portal occlusion time and more blood loss. Thus, in the third case, we pre-treated the peritumor margin with MWA for “tumour isolation and devascularisation”, expecting to reduce ischaemic liver injury and intraoperative bleeding. However, further controlled clinical trials are required to confirm the superiority of MWA in the treatment of large liver tumours.
Furthermore, intraoperative bleeding and blood transfusion are associated with an increased likelihood of tumour recurrence after liver surgery for HCC [Citation13,Citation14]. The patient in the first case showed tumour recurrence at 3 months after surgery. However, late-stage HCC, as demonstrated by a large tumour size and diaphragm invasion, was identified during the surgery. Thus, in this single case, whether the intraoperative blood loss was associated with the early HCC recurrence remains uncertain.
MWA has a good safety profile with low rates of complications, such as haemorrhage, bile duct injury, liver abscess, colon perforation, skin burns and tumour seeding [Citation15]. Fever and post-procedural malaise occur after ablation in some cases. Low-grade fever, discomfort and dull abdominal pain in the right upper quadrant occurred in Cases 1 and 3 and improved after symptomatic treatment. No other major complications were identified. The patient in the third case died of tumour metastasis 18 months after surgery. In addition, prolonged administration of microwaves may cause haemoglobinuria or acute kidney failure [Citation16]. In all three cases, 125 ml of NaHCO3 solution was prophylactically used to prevent renal insult during the operation and post-operatively. No haemoglobinuria or renal dysfunction was identified by urine testing and serum renal function examination.
Conclusions
In summary, this study is the first reported case series of MWA application in the emergent control of life-threatening hepatic tumour haemorrhage. However, additional studies are required to further determine its efficiency and safety.
Acknowledgements
The patients were not required to provide informed consent for this study because the study used clinical data that were obtained after the patients agreed to treatment and before the initiation of treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Liu B, Teng F, Fu H, et al. (2015). Excessive intraoperative blood loss independently predicts recurrence of hepatocellular carcinoma after liver transplantation. BMC Gastroenterol 15:138.
- Meloni MF, Chiang J, Laeseke PF, et al. (2016). Microwave ablation in primary and secondary liver tumours: technical and clinical approaches. Int J Hyperthermia 33:15–24.
- Yu J, Liang P. (2016). Status and advancement of microwave ablation in China. Int J Hyperthermia 41:1203–11.
- Eisele RM. (2016). Advances in local ablation of malignant liver lesions. World J Gastroenterol 22:3885–91.
- Chiang J, Hynes K, Brace CL. (2012). Flow-dependent vascular heat transfer during microwave thermal ablation. Conf Proc IEEE Eng Med Biol Soc 2012:5582–5.
- Huang HW. (2013). Influence of blood vessel on the thermal lesion formation during radiofrequency ablation for liver tumors. Med Phys 40:073303.
- Kuang M, Lu MD, Xie XY, et al. (2007). Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna-experimental and clinical studies. Radiology 242:914–24.
- Lubner MG, Brace CL, Hinshaw JL, et al. (2010). Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 21:S192–S203.
- Yeasmin S, Nakayama K, Ishibashi M, et al. (2009). Microwave endometrial ablation as an alternative to hysterectomy for the emergent control of uterine bleeding in patients who are poor surgical candidates. Arch Gynecol Obstet 280:279–82.
- Nakayama K, Rahman MT, Rahman M, et al. (2011). Microwave endometrial ablation is a highly efficacious way to emergently control life-threatening uterine hemorrhage. Arch Gynecol Obstet 283:1065–8.
- Wai OK, Ng LF, Yu PS, et al. (2016). Post biopsy liver hemorrhage successfully controlled by ultrasound-guided percutaneous microwave ablation. J Clin Imaging Sci 6:34.
- Takao Y, Yoshida H, Mamada Y, et al. (2008). Transcatheter hepatic arterial embolization followed by microwave ablation for hemobilia from hepatocellular carcinoma. J Nippon Med Sch 75:284–8.
- Kornberg A, Witt U, Kornberg J, et al. (2016). Prognostic impact of intraoperative blood loss in liver transplant patients with advanced hepatocellular carcinoma. Anticancer Res 36:5355–64.
- Harada N, Shirabe K, Maeda T, et al. (2015). Blood transfusion is associated with recurrence of hepatocellular carcinoma after hepatectomy in Child-Pugh class A patients. World J Surg 39:1044–51.
- Liang P, Wang Y, Yu X, et al. (2009). Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology 251:933–40.
- Ausania F, Iglesias RC, Rivas MC. (2014). Microwave liver ablation and dark urine. Ann R Coll Surg Engl 96:e1–3.
