Abstract
Purpose: To investigate the effect of head-cooling on resting-state spontaneous brain activity during passive hyperthermia.
Methods: An environmental heat exposure was simulated on 16 healthy men under a normal control condition (NC) at 25 °C and two hot conditions at 50 °C with hyperthermia with head-cooling condition (HHC) and without hyperthermia condition (HOT) keeping the head cool, respectively. Resting-state functional MRI (fMRI) data were acquired under each condition and the values of amplitude low frequency fluctuations (ALFF) and z functional connectivity (zFC) were computed to examine regional activity and functional integration, respectively. Pearson’s correlation analysis between the ALFF value and subjective sensations scores were performed.
Results: Brain regions with significant ALFF differences among the three conditions were found primarily in the right medial prefrontal cortex/anterior cingulate cortex (MPFC/ACC), bilateral posterior cingulate cortex/precuneus (PCC/PCu), and right fusiform gyrus. Compared to the NC or HOT condition, the HHC condition exhibited significantly increased ALFF in the bilateral PCC/PCu and decreased ALFF in the right fusiform gyrus. However, ALFF of the right MPFC/ACC showed no significant difference between the NC and HHC conditions. Positive FC between the right MPFC/ACC and bilateral PCC/PCu was significantly increased in HHC condition with respect to HOT condition. Negative FC between the right fusiform gyrus and the right MPFC/ACC, bilateral PCC/PCu was observed with a decreasing trend from the HHC condition to the HOT condition. Moreover, head-cooling also improved thermal comfort during passive hyperthermia.
Conclusions: Head-cooling could substantially reduce the negative effect of hyperthermia on human brain activity as well as thermal sensation.
Introduction
Hyperthermia is a potential risk factor in many work places (e.g., manufacturing workshops, coal mines, military operation and outdoor sports). Hyperthermia can alter complex cognitive functions including memory [Citation1–4], recognition [Citation5], attention [Citation6,Citation7], executive function and processing speed [Citation4], resulting in a compromised effectiveness and efficiency of cognitive performance in a hot environment. Hyperthermia can elevate brain temperature, skin temperature, and core temperature [Citation3,Citation4], as well as exacerbating negative feelings [Citation8–10]. Hyperthermia can also impair cardiovascular and endocrine systems [Citation11,Citation12]. Of particularly interest, Hocking et al. [Citation13] reported that increased brain electrical activity of frontal and occipitoparietal regions was associated with an increase in body temperature.
To date, many studies have investigated the impaired cognitive performance of passive hyperthermia, but less attention has been paid to the associated emotion change. Previous studies have examined the interaction between brain activation and thermal stimulation of body surface [Citation8,Citation10,Citation14]. Warm or cold stimulation has affective components such as feeling pleasant or unpleasant, which may play an important role in human cognition [Citation8]. In addition, a review by Rolls [Citation15] indicated that the mechanisms involving the effects of cognition and attention on emotion were top-down biased competition and top-down biased activation. Affective and mood states can in turn influence memory and perception, by backprojected biasing influences. It is obvious that heat-related emotions are very important to understanding brain functions.
In an attempt to search an effective solution to preserve brain function in a hot environment, several investigators have explored a number of methods of cooling to reduce heat strain, such as, water-cooling of face [Citation12], cold-air exposure of torso [Citation16] and cold pack for the head [Citation3,Citation4,Citation11]. Particularly, head-cooling has been suggested as an effective treatment to reduce the physiological response and improve cognitive performance at rest and during exercise in a hot environment. For instance, Gaoua et al. [Citation4] reported that regular application of cold packs on head can prevent the detrimental effect of hyperthermia on short-term memory. Katsuura et al. [Citation17] have concluded that localised cooling of the head improved perceived thermal comfort and reduced thermoregulatory response in a hot environment. Moreover, several studies showed cooling head helped alleviate heat related fatigue and cardiovascular strain [Citation11]. Obviously, the benefits of head-cooling on human cognitive performance and physiological response are well established. However, its potential mechanisms remain unclear, with confounding variables such as altered cerebral perfusion [Citation18], elevated core temperature [Citation19], peripheral dehydration [Citation20], emotions and mood state [Citation10]. Previous studies were hampered by a lack of sensitive techniques to identify the brain functional activity alterations in response to the effect of head-cooling during passive hyperthermia.
Over the past years, functional magnetic resonance imaging (fMRI) has been widely used to investigate the perception, cognition, and emotion in a hot environment [Citation8,Citation21,Citation22]. We previously investigated the effect of passive hyperthermia on cognitive function using visual short-term memory tasks and attention network test, respectively. Our results showed enhanced activity of dorsolateral prefrontal cortex, intra-parietal sulcus, etc. [Citation21,Citation22]. These studies not only provided information about brain activity and connectivity, but also illustrated that neuroimaging can play an important role in investigating impaired cognitive performance during passive hyperthermia.
Previous studies have focussed on several brain regions showing task-dependent activation during a wide range of stimulations. Task-based activities are usually defined as a signal change from a resting-state or control state to a task-state [Citation23]. A task-independent approach is highly desirable to assess regional and network-level brain function in a resting-state. A number of resting-state fMRI techniques have been developed, such as amplitude low frequency fluctuations (ALFF), functional connectivity (FC) [Citation24,Citation25], regional homogeneity (ReHo) [Citation26], and voxel-mirrored homotopic connectivity (VMHC) [Citation2]. The low-frequency (<0.08 Hz) fluctuations (LFFs) of blood oxygen level-dependent (BOLD) signal were believed to reflect spontaneous neural activity in the resting state [Citation27]. Among the aforementioned techniques, ALFF has been reported to provide a more direct description of regional spontaneous neuronal activity [Citation28,Citation29]. FC, on the other hand, focuses on correlations of BOLD signals between anatomically distinct brain regions, and may reflect network-level brain function. Both of these methods have been extensively applied to study various neurological and neuropsychiatric diseases, including Alzheimer disease (AD) [Citation24,Citation30], attention deficit hyperactivity disorder (ADHD) [Citation23,Citation31], major depressive disorder (MDD) [Citation32,Citation33], mild cognitive impairment (MCI) [Citation34].
To our knowledge, no resting-state fMRI study has investigated the effect of head-cooling on brain activity during passive hyperthermia, limiting a full understanding of the protective benefits of head-cooling on brain function. The purpose of present study, therefore, was to extend the work of previous studies in investigating what specific susceptible regions could be preserved in hyperthermia by applying a cold hat to the head. Previous studies reported that exposure to heat impaired brain regions mainly in the prefrontal cortex, medial temporal cortex, the pregenual/posterior cingulate cortex, cuneus and the fusiform gyrus, etc. [Citation2–4,Citation8,Citation25,Citation35], as revealed by alterations in the default mode network (DMN) during resting-state [Citation25]. The DMN exhibits high levels of activity during resting baseline and a decrease in activity during performance of externally driven tasks [Citation36]. Moreover, the alterations in DMN have been found in mental diseases [Citation24,Citation37], eye open/closed states [Citation38], abnormal environment [Citation25], and professional individuals [Citation39]. Thus, we hypothesise that passive hyperthermia would affect the DMN and DMN-related regions, including medial prefrontal cortex (MPFC), anterior cingulate cortex (ACC), the posterior cingulate cortex (PCC), precuneus (PCu), and lateral parietal cortex; and head-cooling could prevent detrimental effects of hyperthermia on brain activity.
Methods
Participants
A total of 18 healthy right handed young male college students (23.6 ± 3.4 years, ranging from 20 to 28 years) participated in the present study. All of the participants were free of any neurological or psychiatric disorders and had not participated in any hyperthermia experiments or fMRI experiments previously. The research protocol was approved by the Jinan Ethical Committee. All participants provided written informed consent, and the investigation complied with the laws and regulations in China.
Procedure
Using a counter-balanced design, each participant must complete three experimental trials under a hyperthermia condition, a hyperthermia with head-cooling condition, and normal control condition (NC), respectively. Experiments on the same participant were performed at the same time on different days, separated by at least one week. All participants wore a thermal-lab suit with a protective layer (between the skin and the heating apparatus) that covered the whole body as well as the head before entering the environmental chamber. The suit was designed with a soft embedded pipeline in which the water would circulate to simulate environmental heat during fMRI scan. All participants had rights to discontinue the experiment. The participants were also instructed to drink water ad libitum to ensure body weight changed less than 1% during the experiments.
Hyperthermia condition (HOT): The first sessions were conducted in an environmental chamber, the temperature was set at 50 °C and the relative humidity (rH) at 50% using two temperature controllers (NTFA-18; Gree) and two humidifiers (LH8809; Weiran), respectively. Participants entered the environment chamber and remained in a sitting posture for 50 min. In the second sessions, the participants were taken to the adjacent MRI room for scanning. During the scan, the pipeline in the suit was connected to a subsidiary water temperature control device (OWK-C; Germany Ouchida Int'l Group Limited, Magdeburg, Germany), with water circulating throughout the pipeline. The water was designated at 50 °C. The scan took about 7 min. After the scan, subjective thermal sensations of the participant were evaluated for approximately 3 min. In total, each participant endured heat exposure for approximately 1 h.
Hyperthermia with head-cooling condition (HHC): The procedure was mostly the same as the hyperthermia trial except that a cold hat was put on the head. The cold hat was modified from a commercially available device (Yibang LLC, China), and the cooling component was filled with cold storage material of 400 g. When the cold hat (the direct 250 mm) frozen at 5 °C was used to cool the head including frontal, parietal, occipital, and temporal region. Particularly, the cold hat was applied with a protective layer to the skin of head, and renewed at approximately15 min intervals during the entire experimental trial.
Normal control condition: The procedure was mostly the same as the hyperthermia trial except that the condition of chamber was set at 25 °C and 40% rH and the circulating water was designated at 25 °C. Participants of the normal condition also wore the special device in order to keep the same environment as that of the HOT condition.
Body temperature and body weight recording
Rectal temperature and net weight of each participant were recorded before and after the experiments using a rectal temperature logger (ZWJ-2; Ounuo) and an electronic scale (EF901; Xiang Shan), respectively under the three conditions.
Subjective evaluation
Visual analogue scale (VAS) test was performed to evaluate the subjective replies concerning the temperature sensation and thermal comfort sensation for each condition. The scale ranges, from very cold (0 points) to very hot (10 points), or very uncomfortable (0 points) to very comfortable (10 points). The reference points were given as follows; 0: very cold, 2.5: cold, 5: neutral, 7.5: hot, 10: very hot, 0: very uncomfortable, 2.5: uncomfortable, 5: neutral, 7.5: comfortable, 10: very comfortable.
Data acquisition
The resting-state data were obtained from all participants using a 3 T MRI system (MR 750; General Electric, Milwaukee, WI, USA) with an eight-channel head coil. During the MRI scan, the participants was instructed to lie in a supine position and remain motionless with their eyes closed and without concentrating on anything in particular. The scanning parameters included the following: TR = 2000 ms, TE = 30 ms, flip angle (FA) = 90°, number of slices = 35, matrix = 64 × 64, field of view (FOV) = 240 mm ×240 mm, and slice thickness = 4.0 mm. Additionally, a high resolutionT1-weighted sequence was obtained with the following parameters: TR = 8.2 ms, TE = 3.2 ms, FA = 12°, number of slices = 132, slice thickness = 1.0 mm, FOV = 240 mm ×240 mm.
Data pre-processing
Data pre-processing was performed using SPM8 package (http://fil.ion.ucl.ac.uk/spm) on the MATLAB R2008a platform (MathWorks, Natick, MA, USA). First, the first 10 volumes were discarded to eliminate T1 relaxation effects. All functional images were corrected for intra-volume acquisition time offsets between slices and were then realigned to the first volume image for inter-scan head movement correction. Data of 2 participants in the HOT condition were excluded due to the excessive head motion artefacts (translation or rotation of more than 2 mm or than 2°), resulting in valid data from 16 participants for the further processing. Then, the functional images were normalised to the standard Montreal Neurological Institute (MNI) space (resampled voxel size= 3 × 3 × 3 mm3). Subsequently, the resultant normalised images underwent spatial smoothing with a 4 mm full width at half maximum (FWHM) Gaussian kernel to minimise spatial noise. Finally, Resting-State fMRI Data Analysis Toolkit v1.8 (REST, http://www.restfmri.net/), was used to remove the linear trend and to temporally band-pass filter (0.01–0.08 Hz) the data, leading to reduce low-frequency drift and high-frequency noise.
ALFF analysis
Amplitude low frequency fluctuations analysis was performed using REST software. The calculation procedure was the same as that reported in the previous studies [Citation23,Citation32]. After pre-processing, the filtered time series was first converted to the frequency domain using a Fast Fourier Transform. The ALFF was then generated by calculating the averaged square root of the Fourier coefficient across 0.01–0.08 Hz for voxel. For standardisation purposes, the ALFF of each voxel was divided by the global mean ALFF value within a brain mask. This mask was made from the MNI template to assure matching with the normalisation step.
A repeated measures analysis of variance (ANOVA) using that SPM8 package was performed on the normalised individual ALFF maps in a voxel-by-voxel manner to explore the ALFF differences among the three conditions. We combined threshold of voxel p < 0.05 and a cluster size >85 voxels using an AlphaSim correction offered by REST software to determine significant differences. Then, the regions with significant ALFF differences were chosen as the region of interest (ROI). Mean signal amplitudes (ALFF values) were separately extracted within the ROIs, which were further performed by post hoc analysis to reveal between-condition difference using SPSS version 22 (SPSS Inc., Chicago, IL, USA).
FC analysis
As reported below, significant ALFF differences in three conditions were demonstrated in three brain regions (, ), and these three regions were used as ROIs for FC analysis. The Pearson’s correlation coefficients were evaluated between each pair of ROIs time series for each participant in a ROI-wise manner. Functional connectivity for each participant was obtained and further transformed to a z functional connectivity (zFC) by Fisher’s r-to-z transformation to improve normality. The individual zFC values were extracted using REST software for the following comparisons to reveal between-condition difference by SPSS. Several sources of spurious variance were removed from the data via linear regression: signals from white matter, global mean signal, cerebrospinal fluid.
Figure 1. Result of ALFF analysis under NC, HOT and HHC conditions. The brain region with significant ALFF difference involved in the right MPFC/ACC, bilateral PCC/PCu and right fusiform gyrus. MPFC/ACC: medial prefrontal cortex/anterior cingulate cortex; PCC/PCu: posterior cingulate cortex/precuneus; Fusiform: fusiform gyrus.
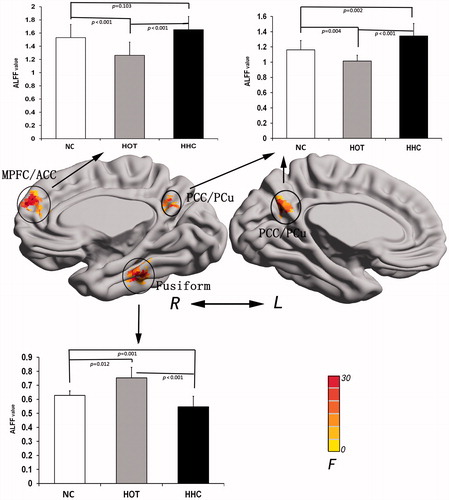
Table 1. Brain regions with significant ALFF differences among the NC, HOT and HHC conditions.
Data analysis
A two-way ANOVA for repeated measures (Time vs. Condition) was performed to assess the main effects of the Condition (NC, HOT or HHC) and Time (before or after the experiment) with regards to changes in the body weight and body temperature. One-way ANOVA for repeated measures was also performed to examine differences in subjective sensation under the three different conditions. Moreover, Pearson’s correlation analyses was performed to explore the relationships between the subjective sensation scores and ALFF values in each brain region. All the reported data were presented as mean ± SD and statistical significance was accepted at p < 0.05. All the statistical analyses were conducted using SPSS.
Results
ALFF comparisons among the three conditions
A repeated measures ANOVA revealed significant differences among the three conditions (p < 0.05, corrected) in the right medial prefrontal cortex/anterior cingulate cortex (MPFC/ACC), bilateral posterior cingulate cortex/precuneus (PCC/PCu), and right fusiform gyrus ().
Compared to the NC condition, the HOT condition exhibited significantly increased ALFF in the right fusiform gyrus and decreased ALFF in right MPFC/ACC, and bilateral PCC/PCu. Compared to the NC and HOT conditions, the HHC condition exhibited significantly increased ALFF in the bilateral PCC/PCu and decreased values in the right fusiform gyrus. In addition, ALFF of the right MPFC/ACC in the HHC condition showed no significant difference compared with the NC condition, but significantly increased compared with the HOT condition. Detailed ALFF results among the three conditions are presented in .
FC comparisons among the three conditions
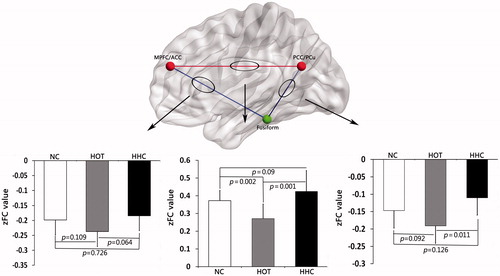
For each condition a positive correlation was observed in the brain region between right MPFC/ACC and bilateral PCC/PCu, while a negative correlation was seen in the brain region between right fusiform and right MPFC/ACC, bilateral PCC/PCu, respectively.
Compared to the NC and HHC conditions, the HOT condition showed significantly decreased zFC values in the brain region between right MPFC/ACC and bilateral PCC/PCu. This connection, however, showed no significant difference between the NC and HHC conditions. The same comparison was performed for the other two negative connections. No significant differences in zFC values were observed among the NC, HOT and HHC conditions, except for zFC values between bilateral PCC/PCu and right fusiform were significantly decreased under the HHC condition with respect to the HOT condition. Detailed FC results among the three conditions are presented in .
Figure 2. Results of FC analysis of each pair brain region under NC, HOT and HHC conditions. The brain region between right MPFC/ACC and bilateral PCC/PCu showed a positive correlation (red line in web/black line in print version). The brain region between right fusiform and right MPFC/ACC, bilateral PCC/PCu showed a negative correlation respectively (blue line in web/grey line in print version).
Body weight and body temperature comparisons among the three conditions
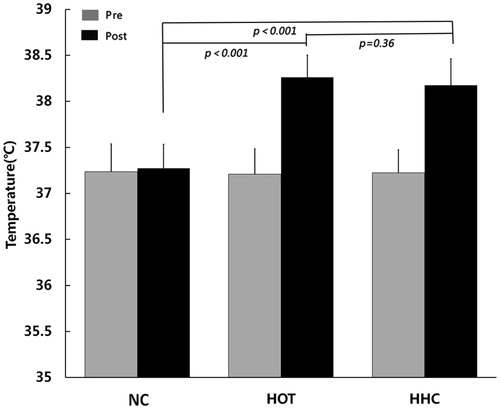
No participant with whose body weight change of more than 1% during the experiment was found, and the condition analysis showed that there were no significant effects of thermal conditions and time (pre- or post-experiment) on body weight (F(2, 45)=1.615, p = 0.210).
For body temperature, the two-way ANOVA for repeated measures showed that the interaction between conditions and time was significant (F(2,45)=49.614, p < 0.001). Post hoc LSD analysis revealed that body temperature did not differ among the three conditions at pre-experiment (NC vs. HOT p = 0.786, NC vs. HHC p = 0.899, HOT vs. HHC p = 0.884), respectively. At post-experiment, there were no significant difference between HOT and HHC conditions (p = 0.36), but HOT and HHC conditions resulted in significantly higher body temperature than NC condition (p < 0.001). These data demonstrated the efficacy of passive hyperthermia in this study ().
Subjective thermal sensations comparisons among the three conditions
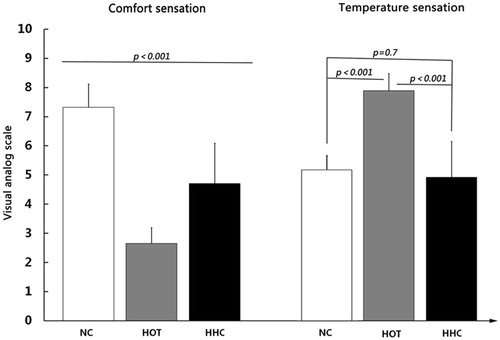
The comfort sensation within repeated measures ANOVA performed on the three conditions (F(2,14)=181.469, p < 0.001) followed by post hoc LSD test showing that there were significant differences in the NC, HOT, and HHC conditions (p < 0.001 in all conditions). It is clear that the NC condition (mean 7.250 ± 0.796) was comfortable, the HOT condition (mean 2.625 ± 0.532) was uncomfortable, and the HHC condition (mean 4.656 ± 1.375) was the neutral. The same pattern was confirmed for the temperature sensation within repeated measures ANOVA performed on the three conditions (F(2,14)=116.128, p < 0.001). Then, post hoc LSD test showed that the HOT condition (mean 7.969 ± 0.591), which was hot, was significantly different from other two conditions (p < 0.001, respectively). However, there were no significant differences between the NC (mean 5.094 ± 0.491) and the HHC conditions (mean 4.969 ± 1.245) (p = 0.7) which was neutral or slight cold ().
Correlation analysis between subjective thermal sensations and ROIs
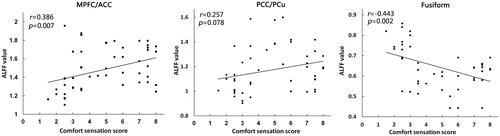
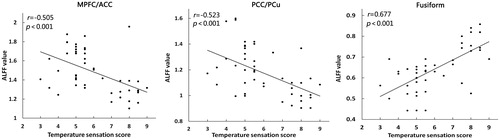
The correlation analysis were divided into two sections. First, the Pearson’s correlation analyses showed that subjective comfort score was positively correlated with ALFF value of the right MPFC/ACC (r = 0.386, p = 0.007), and negatively correlated with ALFF value of the fusiform (r= −0.443, p = 0.002), but it was not significantly correlated with the bilateral PCC/PCu (r = 0.257, p = 0.078) (). Second, the Pearson’s correlation analyses showed that temperature sensation score was negatively correlated with ALFF value of the right MPFC/ACC (r= −0.505, p < 0.001) and bilateral PCC/PCu (r= −0.523, p < 0.001), and positively correlated with ALFF value of the fusiform (r = 0.677, p < 0.001) ().
Discussion
In the present study, resting-state fMRI was applied to investigate alterations of the resting state brain activities in participants with head-cooling during passive hyperthermia. The results of statistical analyses showed that significant difference among brain regions involved in the right MPFC/ACC, bilateral PCC/PCu, and right fusiform gyrus. Compared with the HOT condition, the HHC condition showed increased ALFF in right MPFC/ACC, bilateral PCC/PCu and decreased ALFF in right fusiform gyrus, as well as increased FC within the DMN. Additionally, the results showed that head-cooling improved feelings of thermal comfort and produced a weak cold sensation during passive hyperthermia.
Effect of hot on brain function
The observed brain regions were somewhat consistent with the DMN proposed by Raichle et al. [Citation36], including the MPFC, the PCC and PCu, which constitutes an organised network, exhibiting decreased activity in the resting state during passive hyperthermia. It is well known that the DMN is fundamental for modulating spontaneous cognition and monitoring the internal and external environment [Citation40]. Disruption among the DMN nodes might lead to disability in some cognitive task processing [Citation25]. Some studies reported that activity of the DMN may be attenuated by self-referential aspects of a task such as emotion [Citation41], episodic memory [Citation42], and task-independent thoughts. For example, Berkovich-Ohana et al. [Citation39] found a decreased fluctuations amplitude in the brain regions within DMN and increased fluctuations amplitudes in visual region in the long-term mindfulness meditation practitioners interpreted as a result for long-term reduction of internal, self-related processing leading to increase environmental awareness. In the present study, the participants were exposed to a hot environment, causing discomfort, which disordered the self-referential processing. As a homogenous network, the decreased neuronal activity of DMN may increase the difficulty of cognitive task during passive hyperthermia.
Sun et al. [Citation25] reported that decreased connections between bilateral PCC/PCu and the medial orbitofrontal cortex (mOFC) and ACC during passive hyperthermia may suggest disturbed cognitive function and behaviour. In the present study, we further explored the relationship between each pair of brain regions with significant ALFF differences. Consistent with previous study, the result also showed decreased correlation between the right MPFC/ACC and bilateral PCC/PCu within the DMN during passive hyperthermia, which further supported that passive hyperthermia produced detrimental effect on the DMN, and indicated disorder of cognitive information conveying and processing in a hot environment.
We also observed increased ALFF in the right fusiform gyrus under the HOT condition. Liu et al. [Citation2] used VMHC analyses to examine interhemispheric resting state functional connectivity during passive hyperthermia, showing that the increased information communication of bilateral fusiform gyrus could be an important mechanism in the impairment of face recognition efficiency under heat stress. Although the importance of the fusiform gyrus for visual recognition functions is largely undisputed, it is unclear how functions are impaired by hyperthermia.
In this study, FC was performed to further determine the relationship for each pair brain region, and a negative correlation with the right fusiform was observed in the right MPFC/ACC, bilateral PCC/PCu. According to previously reported patterns of anti-correlated activity between the DMN and various task-positive networks, the anti-correlated and competitive relationships are intrinsically organised during resting and task states [Citation43–45]. In particular, Tian et al. [Citation46] reported that the sensory systems including the visual, auditory, and somatosensory networks are anti-correlated with the DMN individually. In the present study, we observed that when the activity in the right MPFC/ACC and bilateral PCC/PCu within DMN reduced, the activity of the opposing visual networks (e.g., the right fusiform) increased under the HOT condition. Although the post hoc analysis revealed no significant differences in zFC value between HOT condition and NC condition, we still observed an increased tendency of anti-correlation under the HOT condition with respect to the NC condition. Such competitive relationships can significantly impact behavioural performance. For example, Weissman et al. [Citation47] found that spontaneous activity in the attentional network is negatively correlated with the DMN, and momentary lapses in attention are associated with reduced task-induced deactivation of the DMN. It is obvious that the balance between the network reconfiguration of the brain was broken, which may cause deterioration of cognitive performance. Therefore, we hypothesise that increased activity in the fusiform gyrus can be a compensatory for decreased activity in the DMN by enhancing anti-correlation between the DMN and the visual networks during passive hyperthermia.
Effect of head-cooling on subjective thermal sensations
With the wide heat stimulation area (the whole body) in the present study, heat exposure would cause the participants to feel two categories of thermal sensations, “temperature sensation” and “thermal comfort/discomfort” [Citation48]. In order to assess the effect of head-cooling, we performed VAS test to evaluate thermal sensations of participants under the three conditions. The results showed that head-cooling significantly improved feelings of thermal comfort during passive hyperthermia. These findings are consistent with previous studies of head-cooling in the hot environment [Citation11,Citation12,Citation17]. We also analysed the relationship between neuronal activity of resting state and thermal comfort under three conditions. Further analysis revealed subjective thermal comfort score correlated with ALFF of the right MPFC/ACC and fusiform gyrus, but failed to achieve significant correlation in the bilateral PCC/PCu. Previous studies also suggested that activation in the orbitofrontal cortex, anterior cingulated cortex, and ventral striatum were correlated with the comfort/discomfort by warm and cold stimulations [Citation8,Citation10]. The thermal comfort/discomfort is expressed as satisfaction with the surrounding environment. It is important for body temperature regulation that drives an individual to search for a better environment to maintain normal body temperature [Citation16,Citation49].
The other category of thermal sensation, temperature sensation is the perception of a given peripheral stimulus resulting from the stimulation of peripheral and central thermosensor [Citation16,Citation49]. In the current study, all participants reported that the 50 °C gave a strong hot sensation, and keeping the head cooling produced a weak cold sensation, except that two participants still felt slightly hot in the HHC condition. From this perspective, head-cooling could play a part in decreasing hot sensation during passive hyperthermia. Interestingly, we also observed significant correlation between ALFF of the three brain regions and temperature sensation score in participants under the three conditions. Recent neuroimaging studies have showed that thermal signals from skin seem to reach several regions in the cerebral cortex, including the orbitofrontal, cingulate cortices, insula, primary and secondary somatosensory (SI and SII) [Citation9,Citation25,Citation50]. Thus, it is natural that the correlation was reported in the brain regions associated temperature sensation in our study.
Effect of head-cooling on brain function
Using behavioural tests, several investigators reported that the contrasting effect of head-cooling was task dependent during hyperthermia. For example, the regular application of cold packs on the head only prevented the detrimental effect of hyperthermia on short working memory capacity sensitive to disturbances in frontal cortex function. It was ineffective in protecting visual recognition memory sensitive to temporal and medial temporal cortex dysfunctions [Citation4,Citation51]. These studies simply speculated that head-cooling was only effective in improving cognitive functions of brain regions involving the prefrontal cortex, temporal lobe and the parietal cortex [Citation52]. In our study, distinctive and reliable imaging of brain activity could provide a further supplement for behavioural tests, the HHC condition exhibited significantly increased neuronal activity in right MPFC/ACC, bilateral PCC/PCu, and decreased neuronal activity in the fusiform gyrus comparing with the HOT condition, which meant head-cooling could partially eliminate the negative effect of hyperthermia on regional brain activity. In addition, the decreased tendency of anti-correlation between the fusiform gyrus and right MPFC/ACC, bilateral PCC/PCu and increased correlation between the nodes within the DMN in HHC condition with respect to HOT condition, may reflect the ability of head-cooling cope with negative effect of hyperthermia on network-level brain function.
The HHC condition exhibited significantly increased ALFF value in the bilateral PCC/PCu compared with the NC condition, but this difference was not found in the right MPFC/ACC. The result may reflect the differences between these two brain regions in dealing with effect of head-cooling. Although most neuroimaging studies characterise the DMN as a homogenous network [Citation43], it is possible to have functional differentiation within the DMN component. For example, the posterior nodes of the DMN (PCC/PCu) is involved with self-referential cognitive activity [Citation53], episodic memory retrieval [Citation54], the anterior node (MPFC) is associated with the subjective experience of negative affect [Citation55], and decision-making processes [Citation56]. The ACC is involved in emotional processing [Citation57,Citation58], and attentional processing [Citation59]. In particular, Rolls et al. [Citation15,Citation60,Citation61] revealed that the MPFC/ACC can play an important role in representations of the affective value based on warm and cold stimuli. Our data also showed that thermal comfort was positively correlated with activity in the right MPFC/ACC, which may supported the view that the MPFC/ACC is related to emotional functions.
Effect of head-cooling on body temperature
Finally, in the present study, body temperature was significantly higher after heat exposure under the HOT and HHC conditions, but no significant difference between the HOT condition and HHC condition was observed. A number of investigators already established a direct relationship between deep body temperature and the effects of heat stress [Citation51]. It is obvious that a high core temperature is a limiting factor of cognitive performance in a hot environment. Previous studies have reported the protective benefits of head-cooling on both the physiological response and cognitive performance [Citation11,Citation12,Citation52], showing head-cooling could significantly prevent the increase of body temperature [Citation4]. However, the results of the current study showed head-cooling did not significantly attenuate the increase of the body temperature. Such inconsistency could be due to the fact that the freezing point of cool packs (−14 °C) used by Gaoua et al. [Citation4] was lower than that of ours. Each brain region has its own homeostatic temperature [Citation62], suggesting that at the same core temperature thermal load among the different brain regions could be different [Citation4]. Head-cooling was more effective to decrease head temperature than body temperature, and it could play a protective role in brain function. For example, Simmons et al. [Citation11] applied a liquid conditioned balaclava (8°) to head and neck to improve feelings of heat related fatigue and cardiovascular strain. This result is consistent with the current study that the head-cooling improved subjective thermal sensations.
We acknowledge that there are several limitations in the present study. First, the size of participants in each group was relatively small. A study with a larger sample size should be used to further validate the relationship between the brain regions and emotion changes under the HHC condition. Second, we did not record the brain temperature, although previous studies provide the evidence that head-cooling could decrease brain temperature by indirectly using a tympanic thermometer [Citation3,Citation4]. Third, additional cognitive performance tests are also needed to investigate the association between cognitive dysfunction and head-cooling in further studies.
In summary, our findings in this study suggest that head-cooing could substantially reduce the negative effect of hyperthermia on regional and network-level brain functions, as well as thermal sensations. Our study may help contribute to understanding the relationship between brain neuronal activity and subjective thermal sensations.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Deco G, Rolls ET. (2005). Attention, short-term memory, and action selection: a unifying theory. Prog Neurobiol 76:236–56.
- Liu K, Li B, Qian S, et al. (2015). Altered interhemispheric resting state functional connectivity during passive hyperthermia. Int J Hyperthermia 31:840–9.
- Racinais S, Gaoua N, Grantham J. (2008). Hyperthermia impairs short-term memory and peripheral motor drive transmission. J Physiol (Lond) 586:4751–62.
- Gaoua N, Racinais S, Grantham J, El Massioui F. (2011). Alterations in cognitive performance during passive hyperthermia are task dependent. Int J Hyperthermia 27:1–9.
- Sun G, Li M, Yang Z, et al. (2012). Hyperthermia exposure impaired the early stage of face recognition: an ERP study. Int J Hyperthermia 28:605–20.
- Sun G, Yang X, Jiang Q, et al. (2012). Hyperthermia impairs the executive function using the Attention Network Test. Int J Hyperthermia 28:621–6.
- Sun G, Li L, Li M, Jiang Q. (2011). Hyperthermia impaired pre-attentive processing: an auditory MMN study. Neurosci Lett 502:94–8.
- Rolls ET, Grabenhorst F, Parris BA. (2008). Warm pleasant feelings in the brain. Neuroimage 41:1504–13.
- Becerra LR, Breiter HC, Stojanovic M, et al. (1999). Human brain activation under controlled thermal stimulation and fabituation to noxious heat: an fMRI study. Magn Reson Med 41:1044–57.
- Sung EJ, Yoo SS, Yoon HW, et al. (2007). Brain activation related to affective dimension during thermal stimulation in humans: a functional magnetic resonance imaging study. Int J Neurosci 117:1011–27.
- Simmons SE, Saxby BK, McGlone FP, Jones DA. (2008). The effect of passive heating and head cooling on perception, cardiovascular function and cognitive performance in the heat. Eur J Appl Physiol 104:271–80.
- Mundel T, Hooper PL, Bunn SJ, Jones DA. (2006). The effects of face cooling on the prolactin response and subjective comfort during moderate passive heating in humans. Exp Physiol 91:1007–14.
- Hocking C, Silberstein RB, Lau WM, Stough C. (2001). Evaluation of cognitive performance in the heat by functional brain imaging and psychometric testing. Comp Biochem Physiol A Mol Integr Physiol 128:719–34.
- Traceya I, Becerrab L, Changb I, et al. (2000). Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett 288:159–62.
- Rolls ET. (2013). A biased activation theory of the cognitive and attentional modulation of emotion. Front Hum Neurosci 7:74.
- Kanosue K, Sadato N, Okada T, et al. (2002). Brain activation during whole body cooling in humans studied with functional magnetic resonance imaging. Neurosci Lett 329:157–60.
- Katsuura T, Tomioka K, Harada H, et al. (1996). Effects of cooling portions of the head on human thermoregulatory response. Appl Human Sci 15:67–74.
- Ross EZ, Cotter JD, Wilson L, et al. (2012). Cerebrovascular and corticomotor function during progressive passive hyperthermia in humans. J Appl Physiol (1985) 112:748–58.
- González-Alonso J, Teller C, Andersen SL, et al. (1999). Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol (1985) 86:1032–9.
- Kempton MJ, Ettinger U, Foster R, et al. (2011). Dehydration affects brain structure and function in healthy adolescents. Hum Brain Mapping 32:71–9.
- Jiang Q, Yang X, Liu K, et al. (2013). Hyperthermia impaired human visual short-term memory: an fMRI study. Int J Hyperthermia 29:219–24.
- Liu K, Sun G, Li B, et al. (2013). The impact of passive hyperthermia on human attention networks: an fMRI study. Behav Brain Res 243:220–30.
- Zang YF, He Y, Zhu CZ, et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29:83–91.
- Zhang HY, Wang SJ, Liu B, et al. (2010). Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology 256:598–606.
- Sun G, Qian S, Jiang Q, et al. (2013). Hyperthermia-induced disruption of functional connectivity in the human brain network. PLoS One 8:e61157.
- Zang Y, Jiang T, Lu Y, et al. (2004). Regional homogeneity approach to fMRI data analysis. Neuroimage 22:394–400.
- Logothetis NK, Pauls J, Augath M, et al. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature 412:150–7.
- Yang H, Long XY, Yang Y, et al. (2007). Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage 36:144–52.
- Fox MD, Raichle ME. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–11.
- Greicius MD, Srivastava G, Reiss AL, Menon V. (2004). Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101:4637–42.
- Li F, He N, Li Y, et al. (2014). Intrinsic brain abnormalities in attention defcit hyperactivity disorder: a resting-state functional MR imaging study. Radiology 272:514–23.
- Liu CH, Ma X, Yuan Z, et al. (2017). Decreased resting-state activity in the precuneus is associated with depressive episodes in recurrent depression. J Clin Psychiatry 78:372–82.
- Sheline YI, Price JL, Yan Z, Mintun MA. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA 107:11020–5.
- Han Y, Wang J, Zhao Z, et al. (2011). Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. Neuroimage 55:287–95.
- Qian S, Li M, Li G, et al. (2015). Environmental heat stress enhances mental fatigue during sustained attention task performing: Evidence from an ASL perfusion study. Behav Brain Res 280:6–15.
- Raichle ME, MacLeod AM, Snyder AZ, et al. (2001). A default mode of brain function. Proc Natl Acad Sci USA 98:676–82.
- van Buuren M, Vink M, Kahn RS. (2012). Default-mode network dysfunction and self-referential processing in healthy siblings of schizophrenia patients. Schizophr Res 142:237–43.
- Yan C, Liu D, He Y, et al. (2009). Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS One 4:e5743.
- Berkovich-Ohana A, Harel M, Hahamy A, et al. (2016). Alterations in task-induced activity and resting-state fluctuations in visual and DMN areas revealed in long-term meditators. Neuroimage 135:125–34.
- Buckner RL, Andrews-Hanna JR, Schacter DL. (2008). The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38.
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98:4259–64.
- Shannon BJ, Buckner RL. (2004). Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci 24:10084–92.
- Uddin LQ, Kelly AM, Biswal BB, et al. (2009). Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp 30:625–37.
- Fox MD, Snyder AZ, Vincent JL, et al. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–8.
- Esposito R, Cieri F, Chiacchiaretta P, et al. (2017). Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav [Epub ahead of print]. doi: 10.1007/s11682-017-9686-y
- Tian L, Jiang T, Liang M, et al. (2007). Stabilities of negative correlations between blood oxygen level-dependent signals associated with sensory and motor cortices. Hum Brain Mapp 28:681–90.
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. (2006). The neural bases of momentary lapses in attention. Nat Neurosci 9:971–8.
- Attia M. (1984). Thermal pleasantness and temperature regulation in man. Neurosci Biobehav Rev 8:335–42.
- Flouris AD. (2011). Functional architecture of behavioural thermoregulation. Eur J Appl Physiol 111:1–8.
- Craig AD, Chen K, Bandy D, Reiman EM. (2000). Thermosensory activation of insular cortex. Nat Neurosci 3:184–90.
- Hancock PA, Vasmatzidis I. (2003). Effects of heat stress on cognitive performance: the current state of knowledge. Int J Hyperthermia 19:355–72.
- Lee JK, Koh AC, Koh SX, et al. (2014). Neck cooling and cognitive performance following exercise-induced hyperthermia. Eur J Appl Physiol 114:375–84.
- Fransson P, Marrelec G. (2008). The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage 42:1178–84.
- Ferreira LK, Busatto GF. (2013). Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev 37:384–400.
- Zald DH, Mattson DL, Pardo JV. (2002). Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc Natl Acad Sci USA 99:2450–4.
- Bechara A. (1997). Deciding advantageously before knowing the advantageous strategy. Science 275:1293–5.
- Phan KL, Wager T, Taylor SF, Liberzon I. (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16:331–48.
- Bush G, Luu P, Posner MI. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–22.
- Posner M. (1995). Neuropsychology. Modulation by instruction . Nature 373:198–9.
- Grabenhorst F, Rolls ET, Parris BA. (2008). From affective value to decision-making in the prefrontal cortex. Eur J Neurosci 28:1930–9.
- Rolls ET. Emotion decision (2014). Emotion and decision-making explained: a précis. Cortex 59:185–93.
- Kiyatkin EA. (2005). Brain hyperthermia as physiological and pathological phenomena. Brain Res Rev 50:27–56.