Abstract
Introduction: Non-muscle invasive bladder cancer (NMIBC) is a highly recurrent disease with potential progression to muscle invasive disease despite the standard bladder instillations with mitomycin C (MMC) or Bacille Calmette–Guérin immunotherapy. Therefore, alternatives such as radiofrequency-induced chemohyperthermia (RF-CHT) with MMC are being investigated. The mechanism explaining the efficacy of RF-CHT is only partly understood. We examined whether RF-CHT results in higher MMC tissue concentrations as compared to cold MMC instillation.
Patients and methods: Prior to a planned transurethral resection of bladder tumour (TURBT), patients with stage Ta NMIBC were allocated to either (1) cold MMC instillation or (2) RF-CHT. After MMC instillation, three biopsies were taken of both normal and tumour tissue. Biopsies were snap-frozen and MMC tissue concentrations were analysed using ultra-performance liquid chromatography.
Results: Eleven patients were included of which six received RF-CHT. Ten patients had TaG2-LG/HG papillary tumours at pathology. One patient in the RF-CHT group appeared to be free of malignancy and was excluded from the analysis as no tumour biopsies were available. The median MMC concentration in tumour tissue was higher in the RF-CHT group (median 665.00 ng/g vs. 63.75 ng/g, U = 51.0, p = 0.018). Moreover, in both techniques the MMC concentration was lower in normal tissue compared to tumour tissue. Tissue MMC concentration measurements varied substantially within, and between, different patients from the same group.
Conclusion: Intravesical RF-CHT results in higher tumour MMC concentrations vs. cold MMC instillation which contributes to its superior efficacy.
Introduction
Bladder cancer is the 7th most commonly diagnosed cancer in the male population worldwide, and the 11th most commonly diagnosed cancer when both genders are considered [Citation1–3]. It is predominantly seen in men living in developed countries [Citation2,Citation3]. About 75% of patients present with non-muscle invasive bladder cancer (NMIBC) at first diagnosis. NMIBC is a highly recurrent disease with recurrence rates of up to 52% after 5 years. Additionally, patients in the highest risk group have a chance of progression to muscle invasive disease reaching up to 20% [Citation4]. The current EAU (European Association of Urology) and AUA (American Urology Association) guidelines advise a transurethral resection of the bladder tumour (TURBT), followed by adjuvant intravesical chemotherapy with typically mitomycin C (MMC), or immunotherapy with Bacille Calmette–Guérin (BCG) [Citation5,Citation6]. The exact type and scheme of adjuvant instillations depend on the patient’s risk category for recurrence and progression. This risk for recurrence and progression necessitates a high frequency of follow-up and treatments, leading to a significant financial and logistic burden for the patient and the healthcare system [Citation7,Citation8]. Thus, improved treatment options for NMIBC are needed.
A promising and effective alternative treatment appears to be intravesical radiofrequency-induced chemohyperthermia (RF-CHT), typically combining MMC with hyperthermia of the bladder wall [Citation9,Citation10]. It compares favourably to standard MMC instillations, with recurrence rates of 28% vs. 68% [Citation9–11]. In a meta-analysis based on four studies, the overall risk ratio of RF-CHT was 0.41, indicating 59% less recurrences after RF-CHT when compared to cold MMC alone [Citation9]. Moreover, a recent randomised clinical trial showed superiority of RF-CHT over BCG immunotherapy as expressed by the 2-year recurrence free survival rates: 82% vs. 65%, respectively (p = 0.02, Per Protocol analysis) [Citation12].
However, the mechanism explaining the improved RF-CHT efficacy compared to cold MMC is not completely clear [Citation10]. One of the mainstay hypotheses is that hyperthermia enhances tumour penetration of MMC by increasing tumour permeability, while leaving the normal urothelium relatively unaffected. Studies with cold MMC instillations show six-fold higher MMC concentrations in the urothelium and lamina propria interface, and threefold higher MMC concentrations in the lamina propria compared to the muscularis layer [Citation13,Citation14]. However, how tumour or normal tissue drug concentrations relate after cold and warm intravesical application of MMC is unknown.
The aim of our study was to evaluate whether a higher uptake of MMC in tumour tissue after intravesical RF-CHT might explain the superior efficacy as compared to cold MMC instillations.
Patients and methods
Patients
Patients with primary or recurrent tumours suspect for papillary NMIBC on office cystoscopy were included. Patients who had previously received RF-CHT, or who had BCG or MMC bladder instillations 6 months prior to the cystoscopy were excluded from participation. Additionally, patients were ineligible in case of an allergy to MMC, a history of Carcinoma in Situ, an indication for hexaminolevulinaat during TURBT, or an American Society of Anesthesiologists score of >2.
Patients from our onco-urological centre were sequentially included in the study from May 2012 to May 2016. The first half of patients were treated with cold MMC, and subsequent patients with RF-CHT.
Conduction of this study was approved by the Medical Ethical Committee of Nijmegen/Arnhem for human experiments. Informed consent was obtained before participation.
Experimental groups and procedures
Prior to a planned TURBT, patients were allocated to a cold instillation group (MMC 40 mg dissolved in 50 mL of NaCl 0.9% which was left indwelling for 1–2 h, dependent on the patient’s ability to retain the MMC-solution in the bladder), or a warm instillation group using RF-CHT (two times MMC 20 mg in 50 mL of saline for 20–30 min each, total dose of 40 mg in up to 1 h). No randomisation or blinding was performed.
Cold MMC was instilled using simple catheterisation at the patient ward. RF-CHT was performed under full anaesthesia at the operation theatre using the Synergo® device [Citation15]. In short, a 20 Fr catheter with a 915 MHz RF antenna, three luminal thermocouples (TCs) and two urethral TCs was placed. The bladder wall was heated to 42 ± 1 °C and MMC was circulated during two sessions of 20–30 min. In between, the bladder was emptied.
In both groups, the time between end of MMC instillation and TURBT was kept below 15 min. The bladder was rinsed twice with 50 mL of NaCl 0.9% to prevent measurement of luminal MMC sticking to the mucosa. Immediately prior to TURBT, three biopsies were taken of both normal and tumour tissue. Location per biopsy was documented. Biopsies were instantly snap-frozen using liquid nitrogen. TURBT was performed under general anaesthesia according to the standard protocol. Pathologic assessment was performed for standard care.
MMC concentration assessment
Bladder biopsies were homogenised in control human plasma (0.02 g/mL). Sample homogenates were stored at −80 °C until analysis. After thawing, samples were mixed for 15 s and centrifuged (3 min at 3000g). An aliquot of 50 µL was deproteinised with 150 µL of acetonitrile–methanol (1:1, v/v). Samples were shaken (10 min at 1250 rpm) and centrifuged (15 min at 23 100g). The clear supernatants were dried under a gentle stream of nitrogen (−40 °C) and samples were reconstituted in 50 µL of 10 mM ammonium acetate in water. A volume of 2–10 µL was injected onto an Acquity BEH column (50 × 2.1 mm ID, 1.7 µm particle size) using an I Class Acquity UPLC (Ultra Performance Liquid Chromatography) system (Waters, Milford, MA). Eluent A consisted of 5 mM ammonium formate–formic acid (99.9:0.1, v/v) and Eluent B of methanol, and the flow was set to 250 µL/min. Gradient elution was applied and initially (0–1.50 min) 10% B was pumped through the column. From 1.51 to 2.50 min the eluent consisted of 90% B and from 2.60 to 5.00 min the column was equilibrated to the initial conditions. The outlet of the column was connected to a triple quadrupole mass spectrometer (API5500 trap, Sciex, Framingham, MA). For MMC, a transition of m/z 335–242 was optimised and the retention time of the analyte was 2.3 min.
For the quantification, control human bladder homogenates (0.0200 g/mL in control human plasma) were processed until dried extracts were obtained. These samples were reconstituted in aqueous MMC working solutions to obtain calibration standards in the range from 0.5 to 50 ng/mL. MMC area vs. concentration plots were fitted using linear regression with a weighting factor of 1/x2. Average drug concentrations per tissue sample were calculated by dividing the total amount of drug recovered by the total weight of the tissue.
Statistics
For demographics, medians were assessed for continuous variables. Based on the median values per patient and tissue type, the Mann–Whitney U test was used for comparison of unpaired observations (i.e. warm vs. cold MMC), and the Wilcoxon Signed Rank test for paired observations (i.e. normal vs. tumour tissue). All analyses were performed using SPSS software, version 22 (IBM Corp., Armonk, NY, USA), with a two-sided p < 0.05 considered statistically significant.
Results
In total, 11 patients participated in this study. Five were allocated to cold MMC instillations, six patients received MMC-based RF-CHT. The additional 6th patient for the RF-CHT group was included since one patient had benign pathology after TURBT and the associated biopsies were not evaluated for MMC concentration. This patient was excluded from analysis. All other patients (n = 10) had pTaG2, either low grade (LG) or high grade (HG), bladder cancer. Patient characteristics are shown in .
Table 1. Patient characteristics per treatment group.
Normal vs. tumour MMC concentration
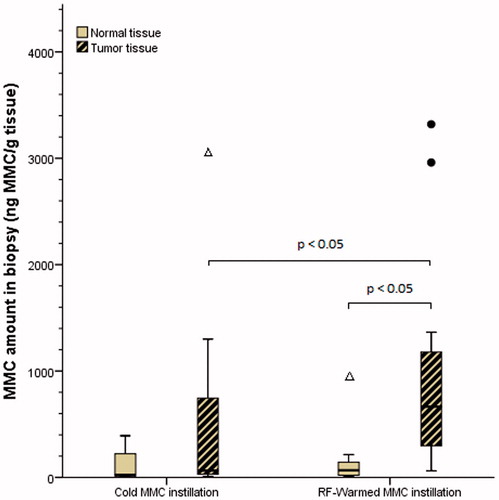
The MMC concentration in tumour tissue was higher than in normal mucosa tissue within the RF-CHT group (sum of ranks 15 vs. 0, z = −2.023, p = 0.043, ). This difference remained after all tumour tissue MMC values were piled together and compared to all normal tissue values regardless of treatment group (sum of ranks 53 vs. 2, z = −2.599, p = 0.009). No significant difference was found between tumour and normal tissue in tissue derived from the cold MMC group (sum of ranks 13 vs. 2, z = −1.483, p = 0.138).
Figure 1. Boxplot showing the concentrations of MMC in both groups for normal and tumour tissue. Median values are depicted by the bold horizontal bars. •: Outlier (i.e. >1.5× interquartile range from 3rd quartile). Δ: Extreme outlier (i.e. >3× interquartile range from 3rd quartile). MMC, mitomycin C; RF, radiofrequency.
Cold vs. RF-CHT MMC instillation
Median MMC concentration in tumour tissue was significantly higher in the RF-CHT group as compared to the cold MMC group (median 665.00 ng/g vs. 63.75 ng/g, U = 51.0, p = 0.018, ). Normal tissue MMC concentration did not differ between both groups (median 67.00 ng/g vs. 24.50 ng/g, U = 100.5, p = 0.619, ).
Inter- and intrapatient variability in tissue drug uptake
Interestingly, tissue concentration measurements varied substantially within both treatment groups and tissue types, even within patients (). The lowest measured tissue concentration was 9.3 ng/g (normal tissue after cold MMC), the highest tissue concentration was 3320 ng/g (tumour tissue after RF-CHT). These differences were not associated with the biopsy location. Indeed, MMC tissue concentration differed strongly: (1) within the same biopsy location, (2) within the same tissue type (i.e. within tumour tissue) and (3) between different patients (a high interpatient variation), especially for the tumour concentrations.
Table 2. MMC concentrations per patient, treatment group, tissue type and location.
Discussion
Treatment of patients with NMIBC with intravesical RF-CHT has been shown to be more effective than treatment with intravesical chemotherapy alone [Citation10]. Thus far, the mechanism explaining this enhanced efficacy has remained elusive. Here, we show that RF-CHT leads to significantly higher drug accumulation in NMIBC compared to cold chemotherapy installation, likely explaining part of its superior effect. Interestingly, drug concentrations in normal urothelium were not affected by RF-CHT, whilst tumour drug concentrations were higher in both groups, suggesting higher drug penetration in tumours, unrelated to heating. Tissue drug uptake differed substantially within – and between – individual patients, regardless of tissue type or biopsy location.
The bladder urothelium is a highly impermeable barrier to luminal molecules, also when compared to pelvic or ureteral urothelium, thus protecting underlying tissue from excreted fluid and molecules [Citation14]. Our results suggest that RF-CHT exerts negligible effects on the normal urothelium barrier function since MMC concentrations are low and similar to concentrations after cold MMC administration. In contrast, drug accumulation in tumours was increased, regardless of the mode of administration. This is most likely the consequence of impaired cell–cell contact, leading to enhanced MMC accumulation. It has been well established that malignant cells lose their original functional properties such as the urothelial barrier function, and cell–cell contact may be impaired as they dedifferentiate [Citation14,Citation16–19].
Application of hyperthermia enhanced MMC accumulation, most likely because it impairs the barrier functions even further. This higher accumulation of MMC in tumour tissue almost certainly explains the improved efficacy of RF-CHT [Citation19]. In addition, drug potentiation by hyperthermia has been suggested, although to the best of our knowledge this has not yet been confirmed. Nevertheless, direct hyperthermic damage to DNA, RNA and proteins, leading to protein synthesis inhibition, failing repair mechanisms due to altered intracellular metabolism, cell death and apoptosis can also contribute to the RF-CHT efficacy [Citation10]. Moreover, hyperthermia might boost the immune system possibly resulting in an increased cellular immune response and autovaccination against tumour cells [Citation20]. Lastly, tumour vasculature is less responsive and thus less efficient in dissipating heat, resulting in a higher local temperature than in non-cancerous cells. This possibly contributes to a higher treatment specificity for tumour tissue, also at the cellular level [Citation10]. Intriguingly, RF might also induce a temperature-independent effect on cell drug permeability and metabolism [Citation21–23].
These proposed modes of action explain why RF-CHT is superior compared to cold MMC instillations [Citation10] and even BCG immunotherapy [Citation12] – the gold standard therapy in intermediate to high risk NMIBC patients [Citation5]. We now demonstrate increased drug accumulation in tumour as one of the main mechanisms of RF-CHT in NMIBC.
The tissue MMC concentrations found in our study are substantially lower than values published by others [Citation13,Citation14,Citation24], which probably is methodology derived. In two pharmacokinetic studies, MMC concentrations in normal human bladder tissue after cold MMC instillation pre-cystectomy were on average 15–40 times higher [Citation13,Citation24]. However, these MMC concentrations were derived from a small subset of patients (7/24) selected based on the highest urine concentrations or swiftly ligated vasculature probably biasing the MMC concentrations toward higher levels. Indeed, the other patients (17/24) had substantially lower bladder wall MMC concentrations of <1 μg/g, and even <0.1 μg/g in the majority of cases (n = 13), approaching our values for normal bladder tissue after cold MMC [Citation13]. Remarkably, in their study the average MMC concentration in tumour-bearing tissues was only about 40% higher than in adjacent normal tissues (n = 6, p = 0.01) [Citation24], whereas we found a 160% higher MMC concentration in tumour compared to normal tissue. No tissue after warm MMC was evaluated in these studies.
The different methodology of these studies plays a role in our different findings. In our study, a biopsy reaching about 1 mm deep was obtained and homogenised without dissection into different layers. Thus, the MMC amount was averaged over both the mucosa and submucosa layers. In contrast, Wientjes et al. dissected samples into a mucosa, submucosa and muscularis layer before defining the concentrations, potentially explaining the lower MMC concentrations measured in our samples. In addition, these studies instilled 1.2–5 times higher dosages of MMC. We evaluated patients undergoing TURBT after flushing the bladder with saline to eradicate luminal MMC from the sample. The above-mentioned studies sampled the full thickness bladder wall without prior flushing. This possibly explains the higher concentrations found by others.
The high inter- and intra-individual variability of MMC accumulation observed in our study is most likely a reflection of a combination of tumour heterogeneity, and different locations, sizes and anatomical depths of our biopsies (i.e. methodology) which influenced MMC exposure as well as the calculated amount of MMC per gram of tissue. Tumour heterogeneity (different tissue and membrane barrier function), and anatomical variations in both tumour and bladder wall orientation (i.e. mucosal folds) will result in variable MMC exposure. Temperature differences over the bladder wall might also play a role but do not fully explain the observed variability since this variability was also present after cold MMC instillation, and was also observed in a study in dogs without bladder cancer [Citation25].
The median intravesical MMC dwell time was substantially longer for cold MMC instillation (94 min) compared to RF-CHT (50 min). This likely resulted in a higher total exposure to MMC in the cold MMC group. Assuming an average urine production of 50 mL/30 min, this implies that after 90 min and a starting dose of 40 mg MMC in 50 mL, the intravesical MMC concentration still is 0.2 mg/mL, being higher than the threshold of 0.12 mg/mL for effective MMC exposure [Citation13]. In comparison, the MMC concentration after 30 min of RF-CHT using the lower dose of 20 mg MMC – as was done in our study – is 0.13 mg/mL [Citation26]. Using hyperthermia, the MMC concentration is known to decrease much faster compared to cold MMC instillation due to additional dilution, absorption and degradation of the drug [Citation26]. Thus, the longer intravesical dwell time for cold MMC merely underlines the observed advantage of RF-CHT over cold MMC in reaching higher MMC accumulation in tumour tissue. Additionally, optimalisation of the intravesical drug instillation by increasing dwell time, reducing drug dilution, inducing urine alkalinisation, and increasing dose to 2 mg MMC/mL is known to enhance efficacy [Citation27].
A drawback of the current study is the limited number of patients studied. Nevertheless, our results strongly suggest that RF-CHT drives higher drug accumulation which substantially contributes to the superiority of intravesical RF-CHT over cold MMC.
Conclusion
Warm application of MMC using intravesical RF-induced hyperthermia results in a higher drug accumulation in papillary NMIBC lesions as compared to a standard cold MMC bladder instillation, partially explaining its improved efficacy. It is credible that multiple factors contribute to the ultimate MMC concentration in tumour tissue explaining the clinical heterogeneity in intravesical treatment response in different patients. Drug concentrations typically were high in tumour tissue, and low in normal tissue, although a high inter- and intrapatient variability in MMC concentration was observed, supporting a multifactorial mechanism for tissue MMC accumulation.
Acknowledgments
We would like to thank Mirjam de Weijert, Experimental Urology Research lab, Radboudumc Nijmegen, for technical support and Hilde Rosing, Slotervaart Hospital, Amsterdam, for her contribution to the UPLC analysis.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure statement
No financial disclosure, no conflict of interest on this study/manuscript.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. (2013). Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49:1374–403.
- Antoni S, Ferlay J, Soerjomataram I, et al. (2017). Bladder cancer incidence and mortality: a global overview and recent rrends. Eur Urol 71:96–108.
- Dy GW, Gore JL, Forouzanfar MH, et al. (2017). Global burden of urologic cancers, 1990–2013. Eur Urol 71:437–46.
- Cambier S, Sylvester RJ, Collette L, et al. (2016). EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta–T1 urothelial bladder cancer patients treated with 1–3 years of maintenance Bacillus Calmette–Guerin. Eur Urol 69:60–9.
- Babjuk M, Bohle A, Burger M, et al. (2017). EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol 71:447–61.
- Chang SS, Bochner BH, Chou R, et al. (2017). Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol 198:552–9.
- Leal J, Luengo-Fernandez R, Sullivan R, Witjes JA. (2016). Economic burden of bladder cancer across the European Union. Eur Urol 69:438–47.
- Sievert KD, Amend B, Nagele U, et al. (2009). Economic aspects of bladder cancer: what are the benefits and costs? World J Urol 27:295–300.
- Lammers RJ, Witjes JA, Inman BA, et al. (2011). The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. Eur Urol 60:81–93.
- van Valenberg H, Colombo R, Witjes F. (2016). Intravesical radiofrequency-induced hyperthermia combined with chemotherapy for non-muscle-invasive bladder cancer. Int J Hyperthermia 32:351–62.
- Liem EI, Crezee H, de la Rosette JJ, de Reijke TM. (2016). Chemohyperthermia in non-muscle-invasive bladder cancer: an overview of the literature and recommendations. Int J Hyperthermia 32:363–73.
- Arends TJ, Nativ O, Maffezzini M, et al. (2016). Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus Bacillus Calmette–Guerin for adjuvant treatment of patients with intermediate- and high-risk non-muscle-invasive bladder cancer. Eur Urol 69:1046–52.
- Wientjes MG, Badalament RA, Wang RC, et al. (1993). Penetration of mitomycin C in human bladder. Cancer Res 53:3314–20.
- Williams NA, Barnard L, Allender CJ, et al. (2016). Evidence of nonuniformity in urothelium barrier function between the upper urinary tract and bladder. J Urol 195:763–70.
- Colombo R, Lev A, Da Pozzo LF, et al. (1995). A new approach using local combined microwave hyperthermia and chemotherapy in superficial transitional bladder carcinoma treatment. J Urol 153:959–63.
- Melicow MM. (1978). The urothelium: a battleground for oncogenesis. J Urol 120:43–7.
- Lewis SA. (2000). Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol 278:F867–74.
- Janssen DA, van Wijk XM, Jansen KC, et al. (2013). The distribution and function of chondroitin sulfate and other sulfated glycosaminoglycans in the human bladder and their contribution to the protective bladder barrier. J Urol 189:336–42.
- van der Heijden AG, Dewhirst MW. (2016). Effects of hyperthermia in neutralising mechanisms of drug resistance in non-muscle-invasive bladder cancer. Int J Hyperthermia 32:434–45.
- Arends TJ, Falke J, Lammers RJ, et al. (2015). Urinary cytokines in patients treated with intravesical mitomycin-C with and without hyperthermia. World J Urol 33:1411–7.
- Curley SA, Palalon F, Lu X, Koshkina NV. (2014). Noninvasive radiofrequency treatment effect on mitochondria in pancreatic cancer cells. Cancer 120:3418–25.
- Curley SA, Palalon F, Sanders KE, Koshkina NV. (2014). The effects of non-invasive radiofrequency treatment and hyperthermia on malignant and nonmalignant cells. Int J Environ Res Public Health 11:9142–53.
- Ware MJ, Tinger S, Colbert KL, et al. (2015). Radiofrequency treatment alters cancer cell phenotype. Sci Rep 5:12083.
- Gao X, Au JL, Badalament RA, Wientjes MG. (1998). Bladder tissue uptake of mitomycin C during intravesical therapy is linear with drug concentration in urine. Clin Cancer Res 4:139–43.
- Wientjes MG, Dalton JT, Badalament RA, et al. (1991). Bladder wall penetration of intravesical mitomycin C in dogs. Cancer Res 51:4347–54.
- Paroni R, Salonia A, Lev A, et al. (2001). Effect of local hyperthermia of the bladder on mitomycin C pharmacokinetics during intravesical chemotherapy for the treatment of superficial transitional cell carcinoma. Br J Clin Pharmacol 52:273–8.
- Au JL, Badalament RA, Wientjes MG, et al. (2001). Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. J Natl Cancer Inst 93:597–604.
