Abstract
Aim: The cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has showed promising results for the survival in patients with recurrent ovarian carcinomatosis, however, some of them will recur within the first year. The aim of this study is focussed on identifying the risk factors to develop the recurrence within the first year after an optimal CRS-HIPEC in patients with recurrent ovarian carcinomatosis.
Methods: A total of 100 patients with peritoneal carcinomatosis from recurrent ovarian cancer treated by CRS + HIPEC were selected for analysis. Multivariate logistic regression analysis was performed to evaluate the relationship between the variables and the early recurrence.
Results: The mean follow-up was 42.5 months. The mean age was 56.2 years. Early recurrence was observed in the 36%. The group early recurrence presented a higher rate of optimal cytoreductions CC1 (16.2% vs. 3.5%), lymph nodes (32.5% vs. 15%) and the use of hemoderivates (40.5% vs. 33%). Others parameters as Peritoneal Cancer Index, major morbidity? 3, re-operations rate and time to adjuvant chemotherapy were similar in both groups. The five years OS was 58%, for the non-early recurrence was higher than the early recurrence group (64% vs. 41%). In the multivariate analysis, CC-1 (OR 5.73; 1.16–32.04) and positive lymph nodes (OR 2.26; 1.01–4.32) proved to be independent factors for the early recurrence.
Conclusion: The combination of both (CC1 and positive lymph nodes) makes that the indication of CRS and HIPEC should be individualised. However, the major morbidity, stage IV and the time to the adjuvant treatment were not associated with an early recurrence, so that, a major aggressiveness is recommended to achieve a CC0.
Keywords:
Introduction
Ovarian cancer is the sixth main cause of death in women attributable to cancer, being the most frequent cause of death among gynaecologic malignancies in developed countries. Due to the indolent nature of the disease, approximately 70% of cases are diagnosed at an advanced stage, with peritoneal involvement in most cases and therefore worse prognosis [Citation1,Citation2].
The standard treatment in these patients is maximal effort cytoreductive surgery (CRS) followed by adjuvant, carbo-taxol-based chemotherapy [Citation3]. Despite good initial response to treatment, more than 60% of patients experience recurrence [Citation4], and only 20–25% of patients can be expected to survive long-term [Citation5]. This situation is particularly concerning since therapeutic rescue options usually prove inadequate. Currently, the cytoreductive treatment proposed by Sugarbaker [Citation6,Citation7] with peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC) offers prospects for better survival for recurrent ovarian carcinomatosis [Citation8,Citation9], even though the available evidence originates exclusively in observational studies of primary ovarian carcinomatosis [Citation10].
The interest of our group and the aim of this study focussed on identifying risk factors for new recurrence within the first year after an optimal cytoreductive surgery with peritonectomy procedures (CRS) and HIPEC with paclitaxel in patients with recurrent ovarian carcinomatosis. Identifying these factors could help us select which patients will benefit from this procedure and in which patients this aggressive treatment will be useless.
Patients and methods
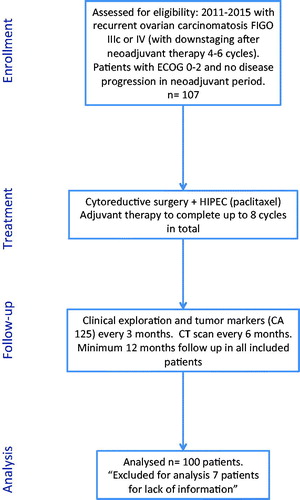
From January 2011 to December 2015, a prospective database was created which was then reviewed retrospectively to identify 107 patients with recurrent peritoneal carcinomatosis from ovarian cancer treated by HIPEC procedures in our department, of whom 100 patients had no loss of information about the multiple factors being analysed ().
Demographic characteristics, the histologic diagnosis, the adjuvant chemotherapy treatment received and the complications derived from it, were collected prospectively. The minimum follow-up period for this patient population was 12 months, including in the analysis those patients who died during follow-up. Postoperative complications were coded based on the Clavien-Dindo Classification.
Early recurrence was defined as a diagnosed recurrence (radiological findings (CT/MRI/PET) with or without an elevated ca 125 level or surgical or percutaneous biopsy) within the first 12 months after CRS + HIPEC treatment. Recurrences were classified as loco-regional (including peritoneal recurrence), lymphatic or systemic.
Patient selection for the HIPEC procedure
All patients included in this study were treated via an institutionally approved protocol and with written informed consent. The HIPEC procedure was indicated for patients with a histologically confirmed diagnosis of peritoneal carcinomatosis of recurrent ovarian origin (stage IIIc FIGO) or stage IV where “down-staging” after neoadjuvant therapy was confirmed. The patients included had a performance status (ECOG) of 0–2, normal renal function (based on preoperative serum creatinine levels and glomerular filtration rate), and adequate haematopoietic and liver functions. Age over 70 years and body mass index (BMI) > 35 kg/m2 were relative contraindications, and these patients were evaluated on an individualised basis.
Neoadjuvant chemotherapy
Most of the patients included received 4–8 cycles of carboplatin + paclitaxel as neoadjuvant treatment, following our protocol for the treatment of advanced ovarian cancer [Citation2,Citation11]. Only two patients were platinum resistant, and they received liposomal doxorubicin-based chemotherapy.
Operative details
The volume and extent of tumour deposits were classified using the Peritoneal Cancer Index (PCI). The principles of CRS and HIPEC, as performed at this centre and by this team, have been reported previously [Citation11,Citation12]. Following optimal cytoreduction (CC0, CC1), HIPEC was administered using the semi-open coliseum technique during 60 min. at a temperature of 41–43 °C with paclitaxel (60 mg/m2) in a 1.5% dextrose solution with a mean flow rate of 1000 ml/min and a global perfusion volume of 4000 ml. In cases with suspected parametrial infiltration, previous pelvic surgery or signs of preoperative hydronephrosis, intraoperative ureteral catheters were placed via cystoscopy.
At discharge, patients were referred to an oncologist for further treatment with systemic chemotherapy completing up to eight cycles in total (neoadjuvant and adjuvant therapy).
Statistical analysis
Qualitative data were recorded in a categorical fashion and quantitative covariates were measured on a continuous, rather than on an interval scale. Qualitative covariate comparison between groups (early recurrence vs. no early recurrence) was performed by chi-square test (Χ2) or Fisher’s exact test according to the sample size. Student t-tests or Mann-Whitney U-tests were used in the case of continuous variables. Univariate logistic regression analysis was performed to evaluate the relationship between the variables and early recurrence. Only the factors that were found to be significant or approaching significance (p < 0.15) by univariate analysis were included in the multivariate analysis. Kaplan-Meier survival analysis and the log-rank test were performed to evaluate survival data. Results are reported as follows: the number of patients (n) and/or the respective percentage (for qualitative covariates), and the median and standard deviation (SD) (for quantitative covariates). Odds ratios (OR) and their 95% confidence intervals (95% CI) were considered for logistic regression. A p value < 0.05 was considered to be significant with statistical analysis performed using SPSS 18.0 (SPSS, Chicago, IL).
Results
In the period of time from January 2011 to December 2015, a total of 107 women with recurrent peritoneal carcinomatosis of ovarian origin underwent surgery in our department, involving CRS + HIPEC procedures in each case (). The median age of our population was 56.2 (25–76) years. The median follow-up was 42.5 months. During this time period, 58% of the patients developed new recurrent disease. During the first year of follow-up, recurrence was observed in 36/100 patients (36%), who were compared to the remaining 64/100 patients (64%) in whom early recurrence did not occur ().
Table 1. Population characteristics and univariate analyses.
The overall characteristics of both groups were similar, as were the proportion of patients who presented initially with stage IV disease, and the number of platinum resistant patients. Except for 2/100 patients (2%), all received neoadjuvant therapy, the majority of them with protocols based on carboplatin and paclitaxel with a mean of six cycles. Good or complete response to neoadjuvant therapy tended to be higher in the non-early recurrence group (44% vs. 32.5%) (p = 0.34) ().
In the perioperative period, the early recurrence group presented higher rates of CC1 cytoreduction (16.2% vs. 3.5%) and positive lymph nodes (32.5% vs. 15%), as well as a higher rate of hemoderivative use (40.5% vs. 33%). The others parameters studied, including PCI, number of intestinal resections, inotropic use, major morbidity ≥3 (postoperative complications are listed in ), re-intervention rate, histologic type, length of hospital stay and the time between surgery and the first cycle of adjuvant chemotherapy were similar in both groups. The pattern of relapse was also similar between the two groups, with lymph node or loco-regional relapse being more frequent than systemic relapse ().
Table 2. Postoperative complications.
Survival analysis
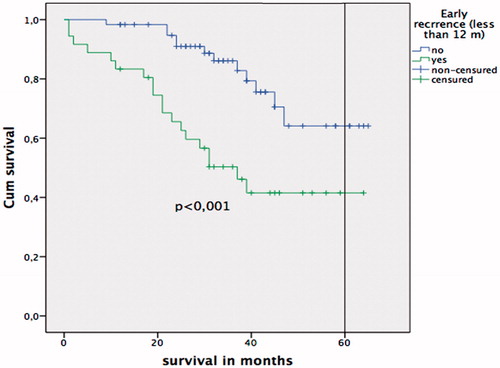
The overall survival of the study population was 48.2 ± 1.9 months, with higher survival in the non-early recurrence group (54.4 ± 2.5 months) than in the early recurrence group (38.6 ± 4 months) (p = 0.0001). The global five-year survival was 58%, with superior five-year survival for the non-early recurrence group (64%) than for the early recurrence group (41%) (p = 0.0001) ().
Figure 2. Kaplan-Meier survival curve. Log-rank test comparing patients with early recurrence vs. non-early recurrence.
A multivariate analysis was performed, and residual lesions smaller than 0.25 cm (CC-1) (OR 5.73; 95% CI: 1.16–32.04; p = 0.021), and the presence of affected lymph nodes in the resected specimens (OR 2.26; 95% CI 1.01–4.32; p = 0.047) proved to be independent predictors of early disease recurrence in patients with recurrent ovarian carcinoma undergoing CRS + HIPEC procedures (). Overall survival was significantly better in the group with negative lymph nodes (N−) than when positive lymph nodes (N+) were found (49.3 ± 1.2 vs. 42.6 ± 4.1 months), and five-year survival was also higher when lymph nodes were negative (58% vs. 46%; p = 0.04) (). When a CC0 cytoreduction was achieved, the overall survival improved from 31.6 ± 6.3 months (CC1) to 49.3 ± 2 months (CC0), and the five-year survival from 35% (CC1) to 59% (CC0) (p = 0.02) ().
Figure 3. Kaplan-Meier survival curve. Log-rank test comparing patients with positive lymph nodes vs. negative lymph nodes.
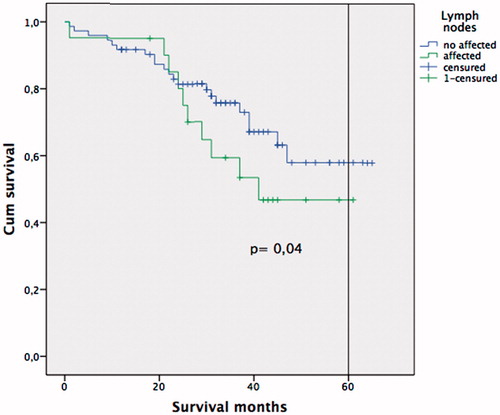
Figure 4. Kaplan-Meier survival curve. Log-rank test comparing patients with complete cytoreduction CC0 vs. CC1.
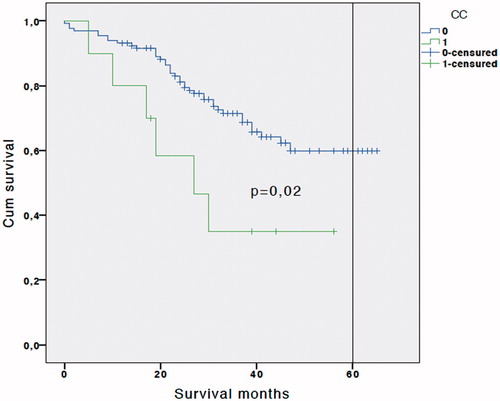
Table 3. Multivariate analysis for early recurrence (less than 12 months).
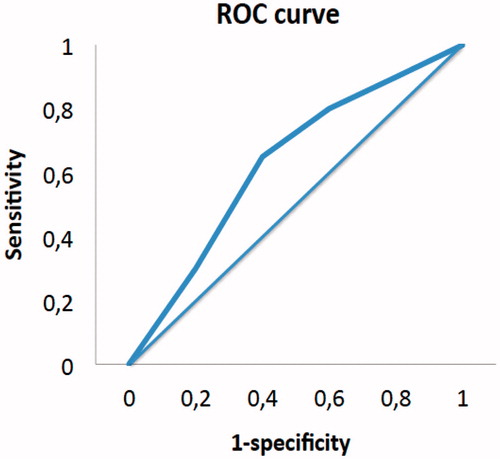
The likelihood of developing early relapse is defined by our major model following the formula: P = 1/1 + e0.96 − 1.73 (CC1) − 0.84 (N+), with the ROC curve revealing an AUC of 72%. The likelihood of early relapse in a patient with both risk factors CC1/N+ would be 83%, 68% for CC1/N− and 47% for CC0/N+. The accuracy of our model to predict the early recurrence has a sensitivity of 75% and a specificity of 52% ().
Discussion
From 1997 to September 2016, 358 patients have been treated in our department with CRS and HIPEC based on paclitaxel for stage IIIc and IV Epithelial Ovarian Cancer (EOC) [Citation12]. We conducted this retrospective analysis to identify the risk factors for developing early relapse (less than 12 months) after optimal CRS with peritonectomy procedures and HIPEC with paclitaxel in recurrent ovarian carcinomatosis. We analysed 100 patients and detected that CC1 cytoreduction (residual tumour less than 0.25 cm) and positive lymph nodes are risk factors for early relapse with an important impact on survival.
The best treatment of advanced primary or recurrent EOC remains an open and critical question. Standard treatment consists of optimal cytoreduction with no residual tumour and adjuvant platinum-based chemotherapy. However, disease will recur in more than half of patients [Citation3]. Since 1996, multiple changes have taken place, including improved survival results with systemic chemotherapy due to the use of platinum associated with taxanes as adjuvant therapy as shown by McGuire et al. (38 months vs. 24 months) [Citation13]. Another interesting and controversial concept has been the definition of optimal cytoreduction. Historically, primary cytoreduction for ovarian carcinoma demonstrated great disparity in surgical success rates, depending on the experience, skill and philosophical approach of the surgeons. In the platinum era, most studies defined optimal cytoreduction as residual tumour less than 2 cm but the Gynaecologic Oncology Group studies evaluating intraperitoneal chemotherapy considered 1 cm to be the cut-off criterion for inclusion. Bristow et al. [Citation14] in 2002 conducted a meta-analysis to resolve this and other controversial questions: the definition of optimal cytoreduction, the impact of residual disease on survival in the platinum era and the effect that the type of department performing the procedure had on patient prognosis. This meta-analysis found maximal cytoreduction to be defined as less than 0.5 cm in only one study, whereas most defined it as less than 2 cm, and the percentage of maximal cytoreduction was 41.9% for all cohorts. While CRS with peritonectomy procedures offered no-residual-disease cytoreduction [Citation15,Citation16], it was not considered in this meta-analysis. The optimal extent of cytoreduction has been studied by different authors and specialties and during different periods. Today, the international clinical guidelines recommend maximal cytoreduction, which includes peritonectomy procedures such as diaphragmatic peritoneum stripping and multivisceral resections [Citation3]. For specialised centres in maximal CRS and peritonectomy like ours, cytoreduction is classified following Sugarbaker’s completeness of cytoreduction (CC) criteria: CC0 (no macroscopic tumour visible), CC1 (largest residual disease <0.25 cm), CC2 (0.25–2.5 cm) and CC3 (>2.5 cm) [Citation17]. Cytoreduction is considered optimal when residual tumour less than 0.25 cm has been achieved (CC1) because adjuvant intraperitoneal chemotherapy can eliminate this residual disease. In contrast, according to the Gynaecologic Oncology Group’s criteria [Citation18] optimal debulking surgery may include residual disease up to 1 cm (RD1), corresponding to a CC2 score under Sugarbaker’s criteria (suboptimal).
In both primary and recurrent EOC treated with CRS and HIPEC, complete cytoreduction remains vital for long-term survival. For CRS alone in recurrent EOC, the DESKTOP OVAR Trial [Citation19] showed that patients with macroscopically complete tumour resection had significantly longer overall survival (45.2 vs. 19.7 months). In a recent meta-analysis [Citation20], the five-year survival rate was 48.4%, 25%, 8.5% and 0% for CC0, CC1, CC2 and CC3, respectively; for optimal resection (CC0/CC1), the five-year OS rate was 41%. These findings highlight the importance of complete cytoreduction in CRS, independent of the use of HIPEC. In our study, the presence of residual tumour less than 0.25 cm (CC1) reduces the five-year survival rate from 59% to 32%. Although CC1 is considered optimal cytoreduction, its negative impact on survival is significant, and every effort should be taken to achieve CC0. Such an attempt could increase morbidity and delay adjuvant chemotherapy, but although these are risk factors for early relapse in other types of carcinomatosis like colorectal [Citation21], in our study, perioperative morbidity and the time to initiation of adjuvant therapy were not risk factors for early relapse. Thus, aggressive efforts in ovarian carcinomatosis to achieve CC0 are recommended. Additionally, treatment-related complications in institutions that routinely perform this procedure are low, with mortality rates ranging from 0% to 1.8% [Citation9]. However, when CC0 is not possible, CC1 cytoreduction is preferred over macroscopic residual disease such as CC2 or more, since this offers an important survival benefit in these patients [Citation9].
Lymph nodes
The presence of positive lymph nodes in the pre-treatment work-up could be a relative contraindication for CRS + HIPEC procedures in recurrent ovarian carcinomatosis, with initiation of neoadjuvant therapy being the preferred approach. Systematic lymphadenectomy during primary CRS does not appear to improve overall survival, but resection of isolated lymph node metastases and recurrences in lymph nodes may be associated with a survival benefit [Citation22]. In our cohort, almost all the patients (98%) received neoadjuvant therapy. We propose CRS and HIPEC as an interval surgery after 4–6 cycles of neoadjuvant therapy, following the results of Vergote et al. [Citation23], because the control of extensive disease or a reduction in tumour burden allows us to achieve a CC0 rate of 93%. Despite neoadjuvance, 21% of patients had positive lymph nodes upon pelvic-periaortic lymphadenectomy. The presence of positive lymph nodes has important consequences, reducing survival from 93 months to 28 months and 5-year OS from 59% to 31% in past reports [Citation11]. In the current study, the presence of positive lymph nodes was associated with early relapse with an independent likelihood of 44% and a combined likelihood of up to 82% if associated with CC1, with a five-year OS rate of 41% in patients with early relapse. These data could raise controversy regarding the performance of maximum-effort surgery in such patients. Thus, when CC1 is anticipated and bulky lymph nodes are present, patient selection should be very cautious and relative. For this purpose, the accuracy of imaging tests such as MRI, CT-scan or PET-CT is essential and should be evaluated in future studies.
Clinicians often question whether a long postoperative time after CRS + HIPEC could affect the Time To Chemotherapy (TTC), delaying the initiation of adjuvant chemotherapy and consequently reducing the benefits of this extensive procedure. For Aletti et al. [Citation24], who retrospectively evaluated data from 218 patients with stage IIIC/IV EOC, the TTC was not a predictor of OS. Similarly, in our study TTC and morbidity were not associated with early relapse or with significant effects on OS. These results suggest that the anticipated TTC should not be used to justify a more conservative surgical approach to advanced EOC. Interestingly, in colorectal carcinomatosis treated with CRS and HIPEC, a delayed start of adjuvant chemotherapy secondary to major morbidity has been associated with early recurrence [Citation21]. One reason for this discrepancy could be the systematic use of neoadjuvant therapy in our patients, particularly since our results showed a trend towards reduced early recurrence in patients with optimal and complete response to neoadjuvant therapy. The use of neoadjuvant therapy for advanced EOC has not shown a clear survival benefit vs. up-front CRS and adjuvant chemotherapy, but the likelihood of achieving CC0 was higher for these patients [Citation23]. When an aggressive CRS is planned, another benefit of neoadjuvance could be that this preoperative systemic chemotherapy (4–6 cycles) helps prevent early recurrence, even if a major complication delays the start of adjuvant treatment.
In a previous meta-analysis, platinum resistance was not a survival factor when CRS and HIPEC were performed [Citation9]. Likewise, in our study, resistance to platinum was not a risk factor for early recurrence, but in our study only two patients had this condition so its influence was not ascertainable.
Postoperative use of hemoderivatives is widely considered to be a risk factor for cancer recurrence [Citation25]. Similarly, we found a nearly significant association between the perioperative use of blood products and early recurrence in univariate analysis. Although this association was not confirmed in the multivariate analysis, we believe it should be considered as a potentially important risk factor for poor outcomes in ovarian carcinomatosis treated with CRS + HIPEC. The possible effect of hemoderivatives on early recurrence indicates that we should improve the general condition of these patients before this procedure, incorporating a multidisciplinary evaluation and optimising recovery after neoadjuvant therapy.
Although this study is the largest single-centre cohort published to date identifying risk factors for early recurrence after CRS and HIPEC in recurrent ovarian carcinomatosis, some limitations are present. This is a retrospective case-control study based on a prospective database and as a result is susceptible to bias. In order to minimise this potential bias, the data were collected by three investigators using strict criteria, and all records were reviewed. The patients discarded for analysis did not have available all the information required; however, the cohort obtained was sufficient for our study.
Conclusions
A residual tumour less than 0.25 cm (CC1) and positive lymph nodes after CRS and HIPEC in recurrent ovarian carcinomatosis have been identified as risk factors for early recurrence. We believe pathologic lymph nodes associated with a large volume of disease in the preoperative imaging tests suggest performing an incomplete cytoreduction rather than neoadjuvant therapy and should require individualisation and restriction of the indications for CRS and HIPEC as well as the consideration of a second line of chemotherapy. However, in the presence of only one of these factors, CRS and HIPEC provide a benefit in overall survival compared to palliative chemotherapy.
Assuming that major morbidity, stage IV disease and time to adjuvant treatment are not risk factors for an early recurrence, maximum-effort cytoreduction with HIPEC would be indicated for patients with recurrent peritoneal carcinomatosis from EOC with previous neoadjuvant therapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jemal A, Siegel R, Ward E, et al. (2007). Cancer statistics, 2007. CA Cancer J Clin 57:43–66.
- Muñoz-Casares FC, Rufián S, Rubio MJ, et al. (2007). Treatment of peritoneal carcinomatosis from ovarian cancer. Present, future directions and proposals. Clin Transl Oncol 9:652–62.
- Ledermann JA, Raja FA, Fotopoulou C, et al. (2013). Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(suppl 6):vi24–32.
- Siegel RL, Miller KD, Jemal A. (2015). Cancer statistics, 2015: Cancer statistics, 2015. CA Cancer J Clin 65:5–29.
- Ozols RF. (2005). Treatment goals in ovarian cancer. Int J Gynecol Cancer 15:3–11.
- Sugarbaker PH. (1995). Peritonectomy procedures. Ann Surg 221:29–42.
- Sugarbaker PH. (1998). Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol 14:254–61.
- Spiliotis J, Halkia E, Lianos E, et al. (2015). Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 22:1570–5.
- Huo YR, Richards A, Liauw W, Morris DL. (2015). Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: a systematic review and meta-analysis. Eur J Surg Oncol 41:1578–89.
- Arjona-Sanchez A, Rufián-Peña S. (2017). Progress in the management of primary and recurrent ovarian carcinomatosis with peritonectomy procedure and HIPEC in a high volume center. Int J Hyperthermia 33:554–61.
- Muñoz-Casares FC, Medina-Fernández FJ, Arjona-Sánchez á, et al. (2016). Peritonectomy procedures and HIPEC in the treatment of peritoneal carcinomatosis from ovarian cancer: long-term outcomes and perspectives from a high-volume center. Eur J Surg Oncol 42:224–33.
- Arjona-Sánchez A, Cadenas-Febres A, Cabrera-Bermon J, et al. (2016). “Assessment of RIFLE and AKIN criteria to define acute renal dysfunction for HIPEC procedures for ovarian and non ovarian peritoneal malignances”. Eur J Surg Oncol 42:869–76.
- McGuire WP, Hoskins WJ, Brady MF, et al. (1996). Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 334:1–6.
- Bristow RE, Tomacruz RS, Armstrong DK, et al. (2002). Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 20:1248–59.
- Rufián S, Muñoz-Casares FC, Briceño J, et al. (2006). Radical surgery-peritonectomy and intraoperative intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis in recurrent or primary ovarian cancer. J Surg Oncol 94:316–24.
- Sugarbaker PH. (1996). Complete parietal and visceral peritonectomy of the pelvis for advanced primary and recurrent ovarian cancer. Cancer Treat Res 81:75–87.
- Jacquet P, Sugarbaker PH. (1996). Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359–74.
- Hoskins WJ, McGuire WP, Brady MF, et al. (1994). The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol 170:974–80.
- Harter P, Bois A, du Hahmann M, et al. (2006). Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR Trial. Ann Surg Oncol 13:1702–10.
- Medina Fernández FJ, Muñoz-Casares FC, Arjona-Sánchez A, et al. (2015). Postoperative time course and utility of inflammatory markers in patients with ovarian peritoneal carcinomatosis treated with neoadjuvant chemotherapy, cytoreductive surgery, and HIPEC. Ann Surg Oncol 22:1332–40.
- Simkens GA, van Oudheusden TR, Luyer MD, et al. (2015). Serious postoperative complications affect early recurrence after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis. Ann Surg Oncol 22:2656–62.
- Berek JS. (2009). Lymph node-positive stage IIIC ovarian cancer: a separate entity? Int J Gynecol Cancer 19(Suppl 2):S18–S20.
- Vergote IB, Van Nieuwenhuysen E, Vanderstichele A. (2016). How to select neoadjuvant chemotherapy or primary debulking surgery in patients with stage IIIC or IV ovarian carcinoma. J Clin Oncol 34:3827–8.
- Aletti GD, Long HJ, Podratz KC, Cliby WA. (2007). Is time to chemotherapy a determinant of prognosis in advanced-stage ovarian cancer? Gynecol Oncol 104:212–16.
- Amato A, Pescatori M. (2006). Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev (1):CD005033. doi: 10.1002/14651858.CD005033.pub2