Abstract
Purpose: To compare the effectiveness and complication between microwave ablation and lobectomy for stage I non-small cell lung cancer.
Materials and Methods: This retrospective study was approved by two institutional ethics committees and all patients were provided with informed consent. From January 2000 to December 2010, 54 and 795 stage I patients who underwent microwave ablation and lobectomy were included in this study. A matched cohort composed of 54 and 108 patients in the microwave ablation and the lobectomy group were selected after adjustment with 1:2 propensity score matching. The overall survival and disease-free survival were evaluated. Survival curves were constructed with the Kaplan–Meier method and compared by using the log-rank test.
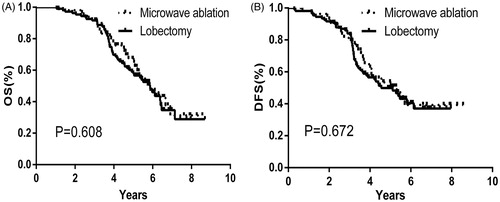
Results: The 1, 3 and 5-year Overall survive were 100, 92.6 and 50.0% for MWA group and 100, 90.7 and 46.3% for lobectomy group. The 1, 3 and 5-year disease free survival was 98.1, 79.6 and 37.0% for MWA group and 98.1, 81.5 and 29.6% for lobectomy group. There was no significant difference between two groups in overall survival (p = 0.608) and disease free survival (p = 0.672). Additionally, local or distant metastasis rate (p = 0.544) were not significantly different between two groups while the complication rate in the MWA group was significantly lower than the lobectomy group (p = 0.008).
Conclusion: Microwave ablation has similar therapeutic effect compared with lobectomy for stage I non-small cell lung cancer, but with fewer complication and less pain.
Introduction
Lung cancer has been identified as one of the leading causes of cancer death [Citation1], while non-small-cell lung cancer (NSCLC) constitutes approximately 80% of primary malignant lung tumours [Citation2]. Lobectomy with sampling or dissection of the mediastinal lymph nodes has been established as the standard therapy for clinical stage I NSCLC, since the results of the Lung Cancer Study Group demonstrated that the rate of recurrence and overall death in lobectomy was significantly lower than sublobar resection for stage I NSCLC patients [Citation3]. Meticulous preoperative assessment of the patient’s overall medical condition, especially the pulmonary reserve and associated comorbidities, is crucial for the evaluation of surgical outcomes [Citation4,Citation5]. Patients with early-stage NSCLC are medically unfit for surgery, and those with insufficient pulmonary reserves might be candidates for local therapies [Citation6]. Recently, the result of a pooled clinical study showed that stereotactic ablative radiotherapy (SABR) achieved a higher overall survival (OS) than lobectomy but had no significant difference in the local, regional, or distant metastasis or recurrence-free survival [Citation7]. A matched-group study also revealed that radiofrequency ablation (RFA) had a similar result compared with lobectomy [Citation8]. These results contribute to the recommendation of SABR and interventional radiology ablation for medically inoperable stage I NSCLC patients in the 2016 NCCN guidelines for NSCLC.
Microwave ablation (MWA) is a new thermal ablative therapy that emerged in the past decade [Citation9]. From a phase III randomised controlled trial, MWA had similar results compared with RFA in hepatocellular carcinoma (HCC) patients, but with fewer sessions, fewer applicator puncture, shorter ablation durations and lower hospitalisation cost [Citation10]. In addition, multiple studies reported that the clinical outcomes of MWA application in the liver and renal tumour were similar to surgery when the tumour was less than 3 cm [Citation11,Citation12]. However, studies on the therapeutic effect of MWA versus lobectomy in stage I NSCLC remain rare. The aim of this study is to compare the effectiveness and complication between MWA and lobectomy in treating stage I NSCLC.
Methods
Ethics statement
Written informed consent for data collection was obtained from all patients. The patents were sufficiently informed of the risks, benefits and alternatives to lobectomy and MWA. The study protocol followed the ethical guidelines of the 1975 Declaration of Helsinki (as revised in Brazil in 2013). This retrospective study was approved by the Ethical Committee of First Affiliated Hospital, Sun Yat-Sen University and Sun Yat-Sen University Cancer Center.
Patients
We queried the NSCLC database of two institutions’ to identify all the patients with stage I NSCLC who underwent MWA and lobectomy from January 2000 to December 2010. Patient selection was from the hospital’s Interventional Radiology and thoracic surgery department. Inclusion criteria for this study were as follows: (1) age between 18 and 75 years; radiographically diagnosed (axial computed tomography [CT] and/or fluorode-oxyglucose positronemission tomography CT images) staging of IA or IB (American Joint Committeeon Cancer Seventh Edition staging); (2) negative mediastinal lymph node metastases after mediastinoscopy for patients with radiographic findings suggesting mediastinal lymph node metastases; (3) biopsy-proven NSCLC and no other malignancies within the previous 2 years. The exclusion criteria were (1) patients with two stage I synchronous NSCLC primary tumours proven to be pathologically distinct; (2) pulmonary metastasis; (3) small cell lung cancer; (4) other adjuvant therapies received before; (5) severe comorbid medical conditions (such as New York Heart Association class IV heart failure, respiratory failure (PaO2 < 60 mmHg or PaCO2 > 50 mmHg in rest state), severe coagulopathy (platelets <50 000/ml, prothrombin time ratio <50%), etc.) who could not tolerate ablation or surgery; and (6) patients who did not fullfil a minimum follow up time.
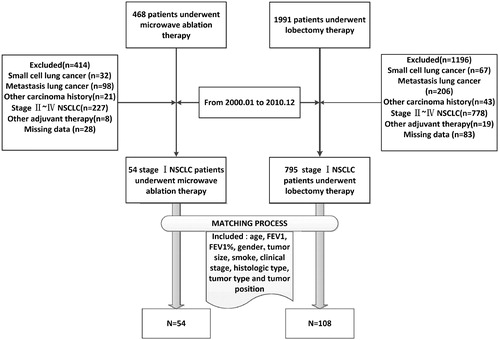
From January 2000 to December 2010, a total of 468 and 1991 patients underwent MWA and lobectomy at two institutions, respectively. Through inclusion and exclusion criteria, there were 54 and 795 staging IA or IB patients who received MWA and lobectomy, respectively (). To minimise the effect of potential confounders on selection bias, propensity score matching analysis was performed to adjust for potential biases by selecting factors related to MWA and lobectomy. The selected variables that were entered into the propensity model included age, Forced expiratory volume in one second (FEV1), FEV1%, gender, tumour size, smoking, clinical stage, histologic type, tumour type and tumour position. One-to-two matching between the groups was accomplished using the nearest-neighbour matching method, with a calliper of 0.25. The adjusted comparisons were done by propensity score and were based on data from 54 patients in the MWA group. After adjustment for these factors, the OS and disease-free survival (DFS) rates were recalculated for the two groups ().
Lobectomy
In this study, the majority of surgeries were performed by Z.Z.H, who has 15 years of experience in open thoracotomy. In our two institutions, lobectomy was performed utilising general anaesthesia with continuous monitoring of the vital signs by an anaesthetist. General anaesthesia was induced by intravenously administering propofol using target control infusion (TCI) (AstraZenaca Pharmaceutical, Shanghai, China), 0.2 mg/kg cisatracurium besylate (Hengrui pharmaceutical company. LTD, Lianyungang, China) and 5 µg/kg sufentanil (IDT Biologika GmbH, Am Pharmapark, Germany). Then, general anaesthesia was continued by intravenously administering propofol with TCI (AstraZenaca pharmaceutical, Shanghai, China) and 0.2 µg/kg/min remifentanil (Humanwell Healthcare company, Wuhan, China). In lobectomy, hilum lymph nodes should be dissected with frozen section sampling performed to guarantee the isolation of negative hilum lymph nodes. The margin of at least 2 cm of healthy lung tissue was required. The margin of the tumour should be negative. Chest catheterisation was routinely performed during close thoracic incision. After the procedure, patients were observed for a minimum of 2 h in the post anaesthesia care unit.
Microwave ablation
A FORSEA microwave delivery system (Qinghai Microwave Electronic Institute, Nanjing, China) was used in this retrospective study. This system resembled the one introduced by Kuang et al. [Citation13]. In this study, all the ablations were performed by two authors (L.J.P and H.J.H) with 15–20 years of experience in interventional radiology with a 16-gauge cool-shaft antenna. All ablations were performed under a 16-detector guided CT (LightSpeed 16, GE Medical Systems, Boston, MA), with 1–5 mm collimation. In our two institutions, lung MWA was performed using intravenous conscious sedation with continuous monitoring of the vital signs by a dedicated conscious sedation nurse. Conscious sedation was induced by intravenously administering 10–25 µg of sufentanil citrate (Humanwell Healthcare group, HuBei, China) and 4 0 ∼ 80 mg dynastant (Pfizer, New York). According to the overlapping mathematic mode introduced by Min-hua Chen et al. [Citation14], when the tumour was <3 cm, single-application MWA was performed at 50 W for 10 min through one antenna inserted at the centre of the tumour. When the tumour was between 3 and 5 cm, two antennas were inserted in parallel at the largest tumour surface 2.0 cm apart with the microwave energy- of each at 60 W for 10 min. All ablations were completed in one treatment session. The exudative area encompassing the whole tumour with a 10 mm excess from tumour margin indicated a technically successful ablation. Needle track coagulation was not routinely performed. After the ablation procedure, patients were observed for a minimum of 2 h, with chest radiography performed the second day to assess the pneumothorax.
Follow-up
Follow-up CT imaging was routinely performed with a CT scanner. Unenhanced and contrast material-enhanced CT images of the entire chest were acquired with 1–5 mm collimation. Follow-up three-dimensional PET (Allegro; Philips Medical Systems, Andover) was also performed using a PET scanner when the patient had suspicious tumour recurrence or residual tumour.
In the MWA group, the initial follow-up CT was generally performed monthly for the first 3 months. In both the MWA and lobectomy groups, follow-up CT was performed every 3 months for the rest of the first years and annually thereafter. All patients received intravenous contrast material for all follow-up chest CT examinations, unless the administration of the contrast material was contraindicated. PET was generally performed when local control and/or systemic progression needed to be evaluated. All images were retrospectively compared by consensus of the two authors (G.Y.K and G.F) who had 16 and 13 years of experience, respectively. When the two authors had different opinion on the CT and PET imaging, a third radiologist (H.J.H) with 20 years of experience in diagnostic radiology made the final decision. All the ablation areas that displayed no contrast enhancement were defined as complete ablation. Recurrent disease was defined as the discovery of any new focal enhancement of the soft tissue in CT (more than 15 HU compared with the non-reinforced series) or increased uptake was identified in the PET. If the patients’ physical conditions were tolerable, the biopsy result was needed to confirm recurrence. However, in the MWA group, local recurrence should be recognised carefully in the CT or PET images because a thin symmetric rim of peripheral enhancement in the CT or increased uptake in the PET of less than 5 mm observed up to 6 months after ablation was considered a sign of benign peritumoural enhacement [Citation15]. In this study, patterns of recurrence were classified as local and distant. Local recurrence was defined as a recurrence at the margin of the ablation site or the surgery margin [Citation16]. Distant metastatasis was defined as newly identified lung cancer metastases other than those within the local recurrence. In this study, major complication was defined as complication with an incidence over 5%. Severe complication was defined as life-threatening complication that needed medical intervention.
Statistical methods
In this study, patient death was considered the primary end point of the study, and the main observations were OS and DFS. OS was recorded from the date of treatment to the last contact date (at which point the patients who were still alive were censored). DFS was recorded from the date of treatment to regional recurrence or distant metastasis. The propensity score matching analysis was performed by R software (TIBCO, Silicon Valley, CA). The sample size was estimated using PASS 11.0 software (NCSS, Kaysville, UT). A unpaired t-test was used for the analysis of the quantitative data (included age, tumour size, FEV1, FEV1%) to identify differences between the two groups, while a χ2 test was applied (included smoke, sex, tumour type, tumour position, clinical stage and histologic type) for the qualitative data. The OS, DFS and subgroup survival similarities were estimated by the Kaplan–Meier method. The comparison of the Kaplan–Meier survival estimates were performed using a χ2 statistic with a Log-Rank weighting scheme. Cox proportional hazards regression was used to assess the strength of the association between the OS and potential risk factors and to estimate the hazards ratios (HR) and 95% CI. χ2 was applied to analyse the complication and recurrence rate between the two groups. Then, statistical analyses were performed using SPSS 19.0 software (IBM, Chicago, IL). p < 0.05 was considered a statistically significant difference.
Results
There were 54 and 795 unmatched patients in the MWA and lobectomy groups, respectively. The median age and tumour size of these patients were 56.3 years (range, 23–78 years) and 3.0 cm (range, 1.0–5.0 cm), respectively. There were no significant differences in the distribution of the baseline characteristics between the patients of the MWA and lobectomy groups, except clinical staging. After propensity-matching, there were 54 and 108 patients in the MWA and lobectomy groups, respectively. No significant difference was identified in all the baseline characteristics between the two groups (). The overall sample size of this study was evaluated through PASS 11.0. The results showed that 162 subjects achieved 93.1% power at a 0.05 significance level to detect an equivalence hazard ratio of 1.70.
Table 1. Base-line characteristic of the patients.
In the MWA group, 51 of the 54 tumours were completely necrotised after the first session of ablation. Another 3 tumours between 3 and 5 cm showed a residual tumour after 1 month of follow-up. After the second session of ablation, these 3 tumours were completely necrotised, as revealed by the CT scan. In the lobectomy group, all 108 patients successfully had the tumour resected with the negative hilum lymph nodes and the surgery margin in frozen section sampling in surgery.
Survival
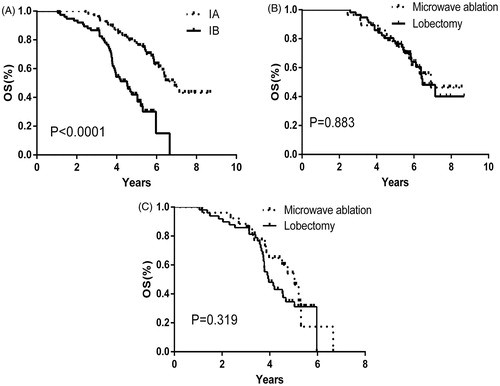
The mean OS was 5.97 ± 0.33 years (95%CI: 5.32, 6.62 years) and 5.81 ± 0.26 years (95%CI: 5.31, 6.32 years) in the MWA and lobectomy groups, respectively. The mean DFS was 5.57 ± 0.39 years (95%CI: 4.81, 6.34 years) and 5.16 ± 0.26 years (95%CI: 4.66, 5.67 years) in the MWA and lobectomy groups, respectively. The 1, 3 and 5-year OS was 100, 92.6 and 50.0% and 100, 90.7 and 46.3% in the MWA and lobectomy group, respectively (). The 1, 3 and 5-year DFS was 98.1, 79.6 and 37.0% and 98.1, 81.5 and 29.6% in the MWA and lobectomy groups, respectively (). There was no significant difference between the two groups in the OS (p = 0.608) and DFS (p = 0.672).
Figure 2. Over-all survival (A) and Disease-free survival (B) cures correspond to patients who were initially enrolled for this study (Microwave ablation, black-dashed line; Lobectomy, black-solid line).
In further analysis, we used cox proportional hazards multivariable models to identify potential influencing factors. In univariate analysis, tumour size (p < 0.0001) and clinical staging (p < 0.001) were significantly influential for OS while other factors were not significant. In multivariate analysis, tumour size (p < 0.0001) and clinical staging (p = 0.005) were eventually demonstrated as independent prognostic factors for OS ().
Table 2. Univariate and multivariate analysis of prognostic factors.
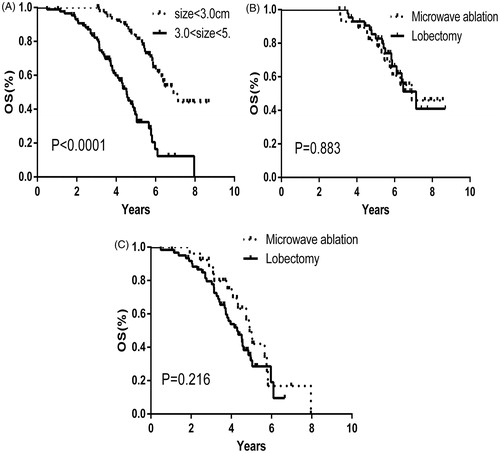
In subgroup analysis, we found that the OS was worse with a tumour that was 3–5 cm (p < 0.0001) and stage IB (p < 0.0001) ( and ). In the sub-group analysis of the tumour size and clinical stage, there was no significant difference between the two groups ().
Figure 3. The negative relationship between over-all survival and tumour size (A) (tumour size <3.0 cm, black-dashed line; 3.0 cm < tumour size <5.0 cm, black-solid line). The over-all survival cures for tumour size <3.0 cm (B) and 3.0 cm < tumour size <5.0 cm (C) (Microwave ablation, black-dashed line; Lobectomy, black-solid line).
Local or distant recurrence
In the MWA group, there were 6 patients (11.1%) with local recurrence and 33 patients (61.1%) with distant recurrence. In the lobectomy group, there were 7 patients (6.4%) with local recurrence and 69 patients (63.8%) with distant recurrence. The recurrence rate in the two groups showed no significant difference (p = 0.544). The median recurrence time was 2.12 ± 0.38 years (95%CI: 1.36, 2.89 years) and 2.32 ± 0.41 years (95%CI: 1.50, 3.17 years) in the MWA and lobectomy group, respectively. The median recurrence time in the two groups showed no significant difference (p = 0.328).
Complication
The major and minor complications are reported in . In the MWA group, 7 patients had pneumothorax, including 3 patients who needed closed thoracic drainage, while all patients had intrathoracic drainage after surgery. In the lobectomy group, 5 patients had chest pain (two need tramadol and others need morphine) while 29 patents had wound pain (Durogesic was applied in these patients). The probability of the pain was significantly different between the two groups (p = 0.01). There were 4 patients in the MWA and 9 patients in the lobectomy group that had postoperative heat absorption. Two other patients in the MWA and 10 patients in the lobectomy group had pulmonary infection as confirmed by X-ray. With the application of antibiotics, all patients recovered in several days. Severe complications after the procedure were observed in 8 patients, with two experiencing bleeding (one in MWA group and one in lobectomy group) and who needed a thoracotomy. The rest all belonged to the lobectomy group, two with heart failure, three with respiratory failure and one with atrial fibrillation. All these severe complications were treated in a timely fashion with no related patient death and recovered in 1 month. The overall mortality rate in the MWA group was lower than in the lobectomy group (p = 0.008), especially with severe complication (p < 0.0001).
Table 3. The major and minor complications between two groups in this study.
Discussion
Lobectomy is the first-line of treatment for early-stage lung cancer [Citation3], but some patients diagnosed with NSCLC were medically unfit for surgery, and some others decline surgery. Meanwhile, with the development of newer diagnostic or treatment technological treatment, the therapeutic effect of ablation therapies has been improved in the past decade [Citation8]. In this case, minimally invasive treatment such as RFA and MWA may be an alternative treatment. Therefore, it is necessary to have a study that compares the effectiveness between MWA and lobectomy for early-stage lung cancer.
Thermal ablation has been used to treat cancer for several decades. Many clinical studies have demonstrated that RFA was an alternative treatment option for tumours <3.0 cm [Citation17]. However, it was limited by ablation size with poor tumour control when the tumour was >3.0 cm [Citation18]. Several studies showed a consistent local rate of 60%, with a 30–40% OS at 3 years and a caner-specific survival of 30–40% at 5 years after RFA, which was better than that seen in untreated patients with clinical stage I NSCLC [Citation19–23]. MWA is a relatively new ablative technique that can create much more ablation lesions, but was less studied as an ablation treatment for lung tumours. Ye et al. [Citation24] applied MWA in 47 stage I NSCLC patients, and the results showed that the OS rate at 1, 3 and 5 years was 89, 43 and 16%, respectively. He et al. [Citation25] conducted an evaluation of MWA in 12 patients with peripheral lung tumours in which no local recurrences were noted in the treated patients and all tumours showed a reduction in size. The clinical evidence makes us believe that MWA may be effective in stage I NSCLC and we look forward to further studies.
In our study, the treatment effect was found to negatively correlate with tumour size and clinical stage. Our study showed that MWA had a similar therapeutic effect compared with lobectomy in stage I NSCLC patients. In our study, MWA demonstrated better efficacy compared with the results in previously published references [Citation24–26]. There were many reasons that contributed to this phenomenon. First, this study excluded patients with severe comorbidities (such as heart failure, respiratory failure and so on) in the MWA group, which helped to reduce the non-cancer related death. Second, standardised treatments were strictly executed in our study with all the ablation zones exceeding the tumour diameter by 1.0 cm, which ensured the complete destruction of each tumour and the low follow-up local recurrence rate. In addition, MWA created a much larger lesion than RFA, which also reduced the residual tumour [Citation27].
Local or distant recurrences are generally considered negative factors for NSCLC patients in terms of the OS. Recently, a meta-analysis showed that the 5-year local recurrence rate after open thoracotomy ranged from 2 to 25% and the 5-year distant recurrence rate after open thoracotomy ranged from 9 to 37% [Citation28]. In our study, the local recurrence rate in the lobectomy group was similar to the above reference, but distant recurrence was higher. The small number of patients may be the major reason for this difference. The results in the study by Takao Hiraki et al. [Citation29] showed that the local control rates of stage I NSCLC after RFA were 72% at 1 year, 63% at 2 years and 63% at 3 years. Amini Zheng et al. [Citation30] reported that after MWA, the local progression rates in the tumour with a maximum diameter <3, 3.1–5 and >5 cm were 7.4, 27.4 and 42.3%, respectively. Our data showed the better local control of MWA, which might be ascribed to the much bigger MWA lesion. In our study, the results of the local and distant recurrence rate of MWA were similar to lobectomy. Accurate preoperative assessment and standardised treatment contributed to this outcome. In addition, an adequate margin is considered a vital factor related to local or distant recurrences [Citation31].
In this study, we found the occurrence rate of postoperative pain was significantly lower in the MWA group with no need for general anaesthesia, which reduced the patients’ recovery time and enhanced their tolerance to surgery. In addition, severe complication rates were significantly lower in the MWA group (only one bleeding) and no patient had respiratory or heart failure, which indicated that MWA could be applied for patients with cardiopulmonary problems. These results suggest that MWA would be the optimal choice for patients who cannot tolerate surgery. However, it should be noted that the employment of oversized antennas and multiple punctures could give rise to a higher rate pneumothorax (13.0%). Nevertheless, with decreasing antenna size and increasing acquisition of MWA among physicians, the pneumothorax rate could be expected to decrease in the future.
There were several limitations to this trial. First, the study was retrospective (not prospective), which may reduce the reliability of the data. Second, the patient sample was limited, which was insufficient for precise subgroup analysis. Therefore, a multi-centre prospective randomised controlled trial is expected in the future. Third, since mediastinal lymph node dissection or sampling were unable to be performed in the MWA group, the patient stage in the MWA group may be underestimated, which may influence the result. To reach a more precise patient stage in local therapies, we will perform mediastinal lymph node biopsies by endoscopic ultrasonography when suspicious lymph nodes are detected in the CT or PET-CT over the next few years. In addition, most lobectomy cases were performed by one physician, which would also be an important bias factor for result. Lastly, the strict selection of stage I patients in the MWA group to avoid the influence of non-tumour factors is unlikely in clinical practice, which may lead to selection bias.
Conclusion
Our comparative data show that MWA can be considered as an alternative option because it is a similar therapy, that has fewer complications and less pain for highly selected stage I lung cancer patients. However, this conclusion should be further confirmed by large patient samples and a multi-centre prospective randomised controlled trial.
Disclosure statement
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
- SEER Cancer Statistics Review, 1975–2005. Available from: http://seer.cancer.gov/csr/1975_2005/
- Hoffman PC, Mauer AM, Vokes EE. (2000). Lung cancer. Lancet 355:479–85.
- Ginsberg RJ, Rubinstein LV. (1995). Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thoracic Surg 60:615–22.
- Birim O, Kappetein AP, Goorden T, et al. (2005). Proper treatment selection may improve survival in patients with clinical early-stage nonsmall cell lung cancer. Ann Thorac Surg 80:1021–6.
- Iwasaki A, Shirakusa T, Enatsu S, et al. (2005). Surgical treatment for lung cancer with COPD based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD). Thorac Cardiovasc Surg 53:162–7.
- Donington J, Ferguson M, Mazzone P, et al. (2012). American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest 142:1620–35.
- Chang JY, Senan S, Paul MA, et al. (2015). Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 16:630–7.
- Kim SR, Han HJ, Park SJ, et al. (2012). Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer. Eur J Radiol 81:395–9.
- Murphy KP, Maher MM, O'Connor OJ. (2015). Abdominal ablation techniques. Am J Roentgenol 204:W495–502.
- Yu J, Yu XL, Han ZY, et al. (2017). Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut 66:1172–3.
- Castro A Jr, Jenkins LC, Salas N, et al. (2013). Ablative therapies for small renal tumours. Nat Rev Urol 10:284–91.
- Shi J, Sun Q, Wang Y, et al. (2014). Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. J Gastroenterol Hepatol 29:1500–7.
- Kuang M, Lu MD, Xie XY, et al. (2007). Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna-experimental and clinical studies. Radiology 242:914–24.
- Chen MH, Yang W, Yan K, et al. (2004). Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients-mathematic model, overlapping mode, and electrode placement process. Radiology 232:260–71.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology 273:241–60.
- Ahmed M. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update: supplement to the consensus document. J Vasc Interv Radiol 25:1706–8.
- Lee JM, Jin GY, Goldberg SN, et al. (2004). Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: preliminary report. Radiology 230:125–34.
- Chen MS, Li JQ, Zheng Y, et al. (2006). A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 243:321–8.
- Ambrogi MC, Fanucchi O, Cioni R, et al. (2011). Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thoracic Oncol 6:2044–51.
- Hiraki T, Gobara H, Mimura H, et al. (2011). Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer. J Thoracic Cardiovasc Surg 142:24–30.
- Hsie M, Morbidini-Gaffney S, Kohman LJ, et al. (2009). Definitive treatment of poor-risk patients with stage I lung cancer: a single institution experience. J Thoracic Oncol 4:69–73.
- Lanuti M, Sharma A, Willers H, et al. (2012). Radiofrequency ablation for stage I non-small cell lung cancer: management of locoregional recurrence. Ann Thoracic Surg 93:921–7.
- Pennathur A, Luketich JD, Abbas G, et al. (2007). Radiofrequency ablation for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thoracic Cardiovasc Surg 134:857–64.
- Yang X, Ye X, Zheng A, et al. (2014). Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol 110:758–63.
- He W, Hu XD, Wu DF, et al. (2006). Ultrasonography-guided percutaneous microwave ablation of peripheral lung cancer. Clin Imaging 30:234–41.
- Ambrogi MC, Lucchi M, Dini P, et al. (2006). Percutaneous radiofrequency ablation of lung tumours: results in the mid-term. Eur J Cardio-Thoracic Surg 30:177–83.
- Fan W, Li X, Zhang L, et al. (2012). Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am J Roentgenol 198:W46–50.
- Cai YX, Fu XN, Xu QZ, et al. (2013). Thoracoscopic lobectomy versus open lobectomy in stage I non-small cell lung cancer: a meta-analysis. PloS One 8:e82366.
- Hiraki T, Gobara H, Iishi T, et al. (2007). Percutaneous radiofrequency ablation for clinical stage I non-small cell lung cancer: results in 20 nonsurgical candidates. J Thoracic Cardiovasc Surg 134:1306–12.
- Zheng A, Ye X, Yang X, et al. (2016). Local efficacy and survival after microwave ablation of lung tumors: a retrospective study in 183 patients. J Vasc Interv Radiol 27:1806–14.
- Sawabata N, Ohta M, Matsumura A, et al. (2004). Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 77:415–20.