Abstract
Purpose: To compare overall local tumour progression (OLTP), defined as the failure of primary ablation or local tumour progression, with single applicator monopolar radiofrequency ablation (RFA), microwave ablation (MWA), cluster-RFA and multi-bipolar radiofrequency (mbpRFA) in the treatment of hepatocellular carcinoma (HCC) ≤ 5 cm abutting large vessels (≥3 mm).
Materials and methods: This multicenter, retrospective, per-nodule study was performed from 2007 to 2015. The study was approved by the ethics review board, and informed consent was waived. A total of 160/914 HCC nodules treated by thermal ablation and abutting large vessels (40 per treatment group) treated by monopolar RFA, MWA, cluster-RFA or mbpRFA were matched for tumour size, alpha-feto-protein level and vessel size. OLTP rates were compared by the log-rank test and the multivariate Cox model after matching.
Results: No differences were observed in tumour size, vessel size or alpha-feto-protein levels among the three groups (p = 1). The cumulative 4-year OLTP rates following monopolar RFA, cluster-RFA, multi-bipolar RFA and MWA were 50.5%, 16.3%, 16.3% and 44.2%, respectively (p = 0.036). On multivariate Cox regression, vessel size ≥10 mm, monopolar RFA and MWA were independent risk factors of OLTP compared to cluster-RFA or mbpRFA.
Conclusion: Multi-applicator RFA provides better local tumour control in HCC abutting large vessels than single-applicator techniques (monopolar RFA or MWA).
Introduction
Percutaneous thermal ablation is extensively used to treat hepatocellular carcinoma (HCC) ≤ 5 cm [Citation1–5]. Single applicator monopolar radiofrequency ablation (RFA) using cooled or multi-tined electrodes is the most extensively studied percutaneous ablation technique [Citation2,Citation4–6]. However, its efficacy is limited in large tumours or in tumours with large neighbouring vessels [Citation4,Citation5,Citation7,Citation8], which may result in primary treatment failure or local tumour progression (LTP). The rate of treatment failure and LTP in HCC abutting large vessels may be >40% [Citation6]. Indeed, all thermal ablation techniques are negatively influenced by the heat-sink effect [Citation9], which results in cooling of ablated area by large vessels. Certain technical solutions have been suggested to improve these results. Several RF electrodes may be clustered simultaneously to obtain larger confluent areas of necrosis [Citation10], which have been shown to increase local efficacy compared to a single electrode with overlapping ablations [Citation11,Citation12]. Multi-bipolar RFA [Citation3] seems to provide a sustained tumour response in perivascular HCC [Citation3,Citation6] by improving energy efficiency to obtain homogeneous necrosis with the same sample power [Citation13]. The temperature is higher with microwave ablation (MWA), and this technique has been shown to be less sensitive to the heat-sink effect in experimental models [Citation14,Citation15]. For the moment, there are no studies evaluating these treatment options in HCC abutting large vessels. Moreover, the theoretical advantages of MWA and multiple applicator techniques have not been clearly shown to improve the outcome of patients with perivascular HCC compared to monopolar RFA [Citation8,Citation16–20].
The aim of this study was to compare overall local tumour progression (OLTP; primary failure + LTP) following single applicator monopolar RFA, cluster monopolar RFA (cluster-RFA), multi-bipolar RFA, and single applicator MWA in patients with HCC ≤5 cm abutting large vessels.
Materials and methods
Study design and patient selection
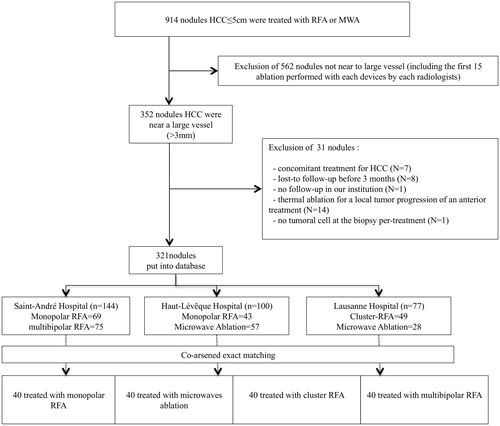
This retrospective study was approved by the institutional ethics review board. Informed consent was not required. All patients underwent contrast-enhanced liver MRI before treatment was decided (or CT-scan if MRI was contraindicated) by the local tumour board. Between 2007 and 2015, we performed a per-nodule analysis of BCLC 0 or A patients with HCC ≤5 cm abutting vessels ≥3 mm [Citation21] treated by percutaneous thermal ablation (). Thermal ablation was not a treatment option for peri-hilar HCC due to the risk of proximal biliary injury. All percutaneous thermal ablation procedures were performed with curative intent and not for preoperative downstaging or liver transplantation. Patients were treated in three interventional radiology units (Saint-André Hospital, Bordeaux, France; Haut-Lévêque Hospital, Pessac, France and Lausanne University Hospital, Switzerland). Patients were identified in pooled prospective databases from the three units. A total of 914 consecutive HCC ≤5 cm were included with 352 that were directly in contact with vessels ≥3 mm (). Additional inclusion criteria were (i) HCC in patients with cirrhosis; (ii) Child-Pugh A or B cirrhosis; and (iii) no concomitant treatment for HCC. Exclusion criteria were: (i) lost-to-follow-up before the first imaging control; (ii) lost-to follow-up before 3 months; (iii) concomitant treatment for HCC (TACE, sorafenib, radiotherapy, etc.); (iv) thermal ablation for LTP or incomplete first ablation; and (v) the first 15 percutaneous thermal ablations performed with each device by each interventional radiologist (learning curve). Patients with previous treatment for other HCC nodules were not excluded. The 352 perivascular HCC were entered into a new database to limit a selection bias, then co-arsened exact matching was performed (see Statistical analysis section).
Figure 1. Flow-chart. Among the 914 HCC treated, 352 were included. After matching, 40 HCC for each treatment group were retained for further analysis (N = 160).
Diagnosis, staging of HCC and follow-up were performed as previously described [Citation6].
Percutaneous thermal ablation
All percutaneous thermal ablation procedures were performed under general anaesthesia in all centres. Real-time ultrasound (US) was chosen as the first-line guidance modality for all treatment sessions. The aim of percutaneous thermal ablation was to ablate at least 0.5 cm of normal hepatic parenchyma surrounding the tumour as a tumour-free margin whenever possible. Six senior interventional radiologists (with at least five years’ experience at the beginning of the study) performed percutaneous thermal ablation using one of the following devices ().
Figure 2. Illustration of the differences between the different ablation devices used to treat HCC abutting large vessel: (A) microwaves ablation; (B) monopolar RFA using a multi-tined electrode; (C) multi-bipolar radiofrequency ablation; and (D) cluster RFA.
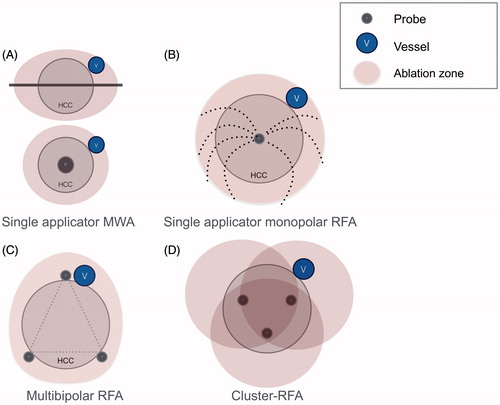
Single applicator monopolar RFA: Multi-tined expandable electrodes, Boston LeVeen™ needles (RF 3000 Boston Scientific Corporate®, Natick, MA, USA) were used with a probe diameter1 cm larger than the tumour diameter. Multiple applications were used in tumours >4 cm after the needle was repositioned. Ablation was performed according to the manufacturer’s instructions. Endpoints were to reach twice the increase in impedance leading to shutdown.
Cluster RFA: Cool-tip™ RFA Cluster Electrode Kit – 2.5 cm active tip (Covidien, Dublin, Ireland) was used. We placed the cluster in the centre of the tumour, with the tip a few millimetres beyond the tumour margin. Endpoints were to reach three times the increase in impedance leading to shutdown. Multiple applications were used in tumours >4 cm after the needle was repositioned.
Multi-bipolar RFA: Multi-polar internally cooled-tip 17G CelonProSurge™ electrodes (CelonLabPower; Olympus-Celon, Teltow, Germany) were used. Two to four needles were placed in the periphery of the tumour, with one needle parallel and adjacent to the vessel when possible [Citation3]. A 3-cm (T30) or 4-cm (T40) active tip was used. Needles were roughly parallel (±30°) 3–4 cm apart. The power used was equal to 1 W per millimetre of active tip (i.e., 90 W for 3 × T30 or 160 W for 4 × T40). Each possible pair of electrodes was then automatically and sequentially activated until the increase in impedance leading to shutdown between each electrode combination was reached.
Single applicator microwave ablation: The French interventional unit used a 2.45 GHz MW ablation platform (HS AMICA, HS Hospital Service, Rome, Italy) [Citation22], with maximum generator output of 140 W and a cooled mini-choked 14 gauge antenna. The Swiss interventional unit also used a 2.45 GHz MW ablation platform (Acculis, AngioDynamics, NY, USA). The MW antenna was placed in the centre of the tumour, with the tip a few millimetres beyond the tumour margin. The power and duration of ablation was chosen according to the manufacturer’s published references and adapted to each case according to the operator’s experience (change of power and duration according to vessel and tumour size). When possible, the tumour was punctured in its longest axis due to the elliptic shape of MWA [Citation22].
Follow-up was performed by contrast-enhanced liver MR imaging at 3 and 6 months and by thoracic CT every 6 months thereafter.
Choice of percutaneous thermal ablation devices
Single monopolar electrode and multi-bipolar RF devices were available in Saint-André Hospital, Bordeaux, France. The latter was chosen to treat patients with one HCC or sub-capsular HCC, which are easier to treat by a no-touch technique [Citation6]), and a monopolar device was chosen in patients with several nodules because ablation time is shorter with this device in our experience. MW antenna was chosen in patients with several nodules or sub-capsular HCC in Haut-Lévêque Hospital, Pessac, France, because in our experience multi-tined expandable electrodes are not suitable for sub-capsular HCC due to the risk of peritoneal seeding). Artificial ascites was performed for HCC near the diaphragm or the right colic angle. In Lausanne University Hospital, Switzerland, the choice of cluster-RFA or MWA was randomised [Citation23] (patients were included in the randomised study number 212/11 registered at “commission d’éthique du canton de Vaud”) [Citation23].
Study endpoints and definitions of terminology
The primary endpoint was to compare the cumulative incidence and hazard ratio of OLTP [Citation6,Citation7] during follow-up among the four groups (RFA, MWA, cluster-RFA and multi-bipolar RFA. OLTP was defined as primary thermal ablation failure or LTP (as described by Ahmed et al. [Citation9])). Primary failure and LTP were grouped together because the heat-sink effect has been shown to be associated with both [Citation24,Citation25].
The secondary endpoint was to compare OLTP between the multi-applicator group (cluster-RFA and multi-bipolar RFA) and the single applicator group (SA-RFA and SA-MWA).
Recurrence (new HCC) in the liver in a location other than the index tumour was not considered to be OLTP unless they developed along the ablation needle tract.
The third endpoint was the comparison of major complications, based on the guidelines of the society of interventional radiology (SIR) [Citation26].
Statistical analysis
Coarsened exact matching (CEM) [Citation6]: We used one-to-one CEM to limit a selection bias and provide an accurate matching of prognostic factors [Citation27]. Matching was performed for the 3 categorised variables, tumour size >/≤30 mm, vessel size ≥/<10 mm and Alfa-Fetoprotein (AFP) level ≥/<10 ng/ml, using a cut-off based on previous studies in our population [Citation2,Citation6].
Analysis. Qualitative variables were expressed as percentages and quantitative variables as medians with the first and third quartiles in brackets unless otherwise specified. They were compared using a Kruskall–Wallis test. Percentages were compared using the chi-square test or Fisher’s exact test.
Patients with liver transplantation were censored at the date of transplantation. To identify factors associated with OLTP, patients with primary failure were censored at the date of the first imaging control. First, we performed a univariate Cox model reporting hazard ratios (HR) and their 95% confidence intervals (95% CI) for several variables: age, gender (male), non-viral hepatitis (vs. viral), Child A score (vs. B), prior treatment for other HCC, multiple tumours treated during the same procedure, platelet count <100 G/L (vs. ≥100 G/L), alpha-fetoprotein >10 ng/ml (vs. ≤ 10 ng/ml), tumour size (mm), vessel size (<10 mm vs. ≥10 mm), and ablation technique. Then, variables with a p values <0.10 were introduced into a multivariate forward regression Cox model. A post hoc power analysis was performed for the non-significant results of multiple Cox regression of OLTP. A p values <0.05 was considered to be significant. Statistical analyses were performed using Stata 13 (College Station, TX, USA).
Results
Patient characteristics and follow-up
Nodules were matched according to tumour size (≤ or >30 mm), vessel size (< or ≥10 mm), and AFP serum level (≤ or >10 ng/ml), p = 1 (). Mean vessel size was 6 mm (±2 mm).
Table 1. Baseline characteristics of patients and nodules treated either by single-applicator monopolar, cluster-RF, multi-bipolar radiofrequency, or microwave ablation.
The mean (± standard deviation) and median (1st–3rd quartile) follow-up periods were 28 (±18) months and 25 (15–38) months, respectively.
Twenty (12.5%) patients were transplanted (all for new HCC). This included 6 (15%) in the monopolar RFA group, 4 (10%) in the MWA group, 6 (15%) in the multi-bipolar RFA group, and 4 (10%) in cluster-RFA group, p = 0.860. The median (range) time to transplantation was 20 (9–45), 13 (4–14), 17 (5–57), and 19 (14–22) months in the monopolar RFA, MWA, multi-bipolar RFA, and cluster-RFA groups, respectively.
Comparison and predictive factors of OLTP
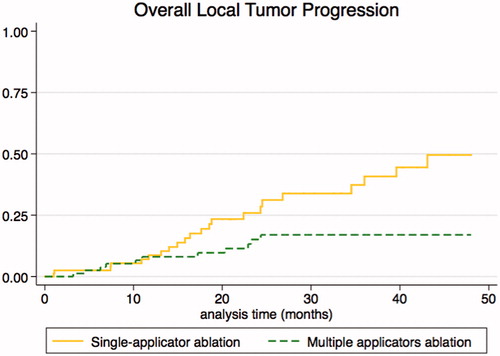
OLTP occurred after a median 17 months (10–24). The 2- and 4-year cumulative OLTP rates were, respectively, 23.7% and 50.5% after monopolar RFA, 14.4% and 16.3% after cluster-RFA, 16.3% and 16.3%, after multi-bipolar RFA and 28% and 44.2% after MWA, p = 0.029 (log-rank test) (). Multivariate Cox regression showed that both single-applicator techniques were associated with a higher risk of OLTP (see ). Post hoc power analysis for comparison between monopolar RFA vs. MWA and cluster-RFA vs. multi-bipolar RFA were 0.068 and 0.026, respectively.
Figure 3. Comparison of overall local tumour progression between monopolar RFA group (N = 40), MWA group (N = 40), Cluster-RFA group (N = 40) and multi-bipolar group (N = 40). Overall local tumour progression includes primary failure and local tumour progression. mbpRFA: multi-bipolar radiofrequency ablation.
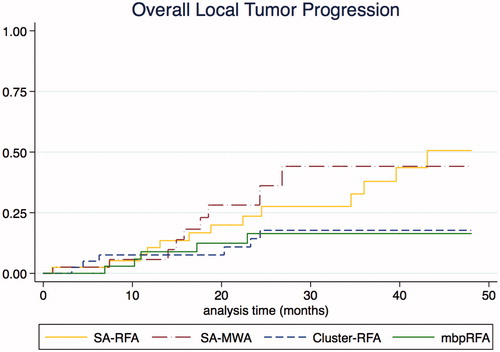
Table 2. Predictive factors of overall local tumour progression (LTP and primary treatment failure).
A comparison of the single applicator (SA-RFA and SA-MWA) and multi-applicator groups (cluster-RFA and multi-bipolar RFA) showed that the latter was associated with a lower OLTP rate (hazard ratio: 0.353 [0.171–0.727], p = 0.005) after adjustment for vessel size. The 2- and 4-year cumulative OLTP rates were respectively, 31.3% and 49.9% with single applicator ablation versus 15.1% and 17% with multi-applicator ablation, p = 0.008 (log-rank test) (). Details on primary percutaneous thermal ablation failure and LTP are provided in Supplementary file E1.
Complications
The rate of major complications according to the SIR classification following monopolar RFA and MWA was 5% (n = 2), 2.5% (n = 1), after cluster-RFA and multi-bipolar RFA (p = 0.692). One patient died in the MWA group due to severe acute liver failure from acute portal thrombosis and one patient required intensive care for sudden cardiac arrest during the procedure.
Discussion
This is the first multicenter study to evaluate the local effectiveness of single-applicator RFA, MWA, cluster-RFA and multi-bipolar RFA for the treatment of HCC abutting large vessels, as well as the predictive factors of OLTP following these treatments.
This multicenter case-matched study showed that both cluster-RFA and multi-bipolar RFA provided better local tumour control than monopolar RFA and MWA for HCC abutting large vessels. Our results also showed that cluster-RFA and multi-bipolar RFA achieved high rates of local tumour control in HCC abutting large vessels.
The rate of LTP (35%) with single-electrode monopolar RFA in our study is similar to reports in the literature with 42–48% in HCC abutting large vessels [Citation6,Citation28]. Our rate of LTP (20%) following single-antenna MWA is slightly higher than that reported in HCC that do not abut large vessels [Citation29]. MWA is usually described as the treatment of choice in tumours abutting vessels, because it heats faster and at higher temperatures than radiofrequency techniques. However, our study did not show any difference in local tumour control between monopolar RFA and MWA. This could be explained by two factors. As reported by Yu et al. [Citation30] although MWA is less affected than RFA [Citation15] by the heat-sink effect, an animal model of healthy livers has shown that a vessel diameter ≥3 mm can result in significant dimpling or invagination in the perivenous coagulation in 50% of the ablation zone and viable cells around the vessel. Also, the higher sensitivity to the heat-sink effect with RFA may be overcome by a longer heating time (as shown in our study) and impedance-monitoring. Indeed, MWA is not monitored by temperature or impedance but is based on the manufacturer’s recommendations of reference values based on the theoretical length, diameter and volume for a specific power and application time. In RFA, impedance monitoring is used to compensate for the heat-sink effect by a longer heating time and an increase in impedance. The absence of monitoring with MWA could explain reports of better primary technical success with monopolar RFA than MWA in tumours abutting vessels [Citation8]. However, our post-hoc power analysis of 0.068 comparing monopolar RFA and MWA may be insufficient to show significant difference.
In our study, the OLTP rate after cluster-RFA (15%) was lower than that in the study by Kang et al. [Citation21], which mainly used single applicator RFA from the same company. The OLTP following multi-bipolar RFA (15%) in our study was similar to that in other recent studies [Citation3,Citation6,Citation7]. We also found that multi-applicator ablation (cluster-RFA or multi-bipolar RFA) was associated with a lower risk of LTP than monopolar RFA or MWA. This suggests that multi-applicator ablation provided better primary and more sustained local tumour control than a single-applicator technique in HCC ≤5 cm abutting large vessels. These results are further supported by multivariate analysis showing that both monopolar RFA and MWA are independent factors of OLTP compared to multi-applicator techniques. Multi-applicator ablation decreased the risk of OLTP by 3 (Hazard Ratio: 0.384 [0.187–0.791], p = 0.009). The better results with multi-applicator RFA may be because more energy is delivered by several radiofrequency electrodes. This results in a larger and more homogeneous area of necrosis than a single electrode with a multiple overlapping ablation technique [Citation10,Citation12,Citation31,Citation32] and a lower OLTP rate (≤15%). Moreover, with multi-applicator ablation one electrode may be placed at the abutting vessels to deliver a maximum amount of energy directly where the heat-sink effect is the highest. There was no difference in OLTP between cluster-RFA and multi-bipolar RFA in our study. We did not assess multi-applicator MWA because none of the three University hospitals used this device during the inclusion period. The use of a multi-applicator MW antenna has several advantages compared to multi-applicator RFA. First, MW can be powered continuously and simultaneously without switching from one electrode to another during electrode activation, so the targeted area is constantly heated. When phased constructively, heating increases in proportion to the square of the number of antennas, allowing more efficient heating and generation of higher temperatures compared to a single antenna [Citation33,Citation34]. This synergetic MWA leads to OLTP ≤17% (close the 15% OLTP rates we found using multi-applicator RFA) in HCC abutting large vessels [Citation35,Citation36]. Recently, Tan et al. [Citation37] also reported high local tumour control with RFA using a multiple-electrode switching system that allows up to three electrodes to be used simultaneously. These results suggest that besides the heating mechanism (radiofrequency or microwave), it is the number of applicators (and thus, the amount of energy) that provides good local tumour control. Therefore, multi-applicator techniques seem to be the best option to treat perivascular HCC. Multi-applicator techniques are usually considered to be more technically demanding than single-applicator techniques. They require the insertion of several roughly parallel needles that may be difficult by US guidance. However, advanced guidance tools are now available that have simplified this, such as fusion imaging, cone beam CT, or CT-scan guidance. One approach is to occlude the vessel abutting the index tumour during ablation with a balloon. However, this results in a longer procedure, a higher dose of radiation for both patients and the radiologist and it can result in permanent vessel occlusion in 30% of cases [Citation38] which is not possible in patients with cirrhosis. Finally, initial results with a non-thermal ablation technique in HCC, i.e., irreversible electroporation [Citation39], may be a promising alternative in HCC abutting vessels, but additional studies are needed.
This multicenter study is limited by its retrospective design and the choice of thermal ablation technique, which could create a selection bias because all of the techniques were not available in all the university hospitals. However, we feel that this may be an advantage because all techniques were not available to each operator, decreasing the “competition” among the modalities and limiting bias. Furthermore, to limit this bias we used CEM on variables that were potentially associated with OLTP. We performed a per-nodule analysis because the features of each nodule could result in OLTP, thus it seemed more accurate to match nodules rather than patients. However, we could not assess and compare overall survival since we performed per-nodule analysis. Although our population was not large (160 nodules), it is a result of the close matching of four groups (so each nodule had a pair in the three other groups). Indeed, all matched patients belonging to the same strata of each variable used in the matching model that should limit the influence of confounding factors. We did not evaluate HCC abutting hepatic arteries because of their small size (<3 mm) [Citation21,Citation40], and arteries have been shown to result in a greater heat-sink effect than the hepatic or portal veins [Citation40]. Therefore, a study of this kind is still needed. Finally, there are several MW devices available worldwide that have different local efficacy; therefore, our results with the specific MW devices may not be applicated for all MW devices [Citation41,Citation42].
In conclusion, multi-applicator RFA provide better-sustained local tumour control than the single-applicator approach using either a monopolar electrode or MWA antenna, treating HCC abutting large vessels. Therefore, multi-applicator approach should be considered treating HCC abutting large vessels.
Disclosure statement
Olivier Seror: Activities related to the present article: none to disclose. Activities not related to the present article: has received personal fees and non-financial support from Bayer Healthcare, Angio-dynamics, and Olympus Surgical. Other relationships: none to disclose.
All other authors: None to disclose
References
- European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- Hocquelet A, Balageas P, Laurent C, et al. Radiofrequency ablation versus surgical resection for hepatocellular carcinoma within the Milan criteria: a study of 281 Western patients. Int J Hyperthermia. 2015;31:749–757.
- Seror O, N’Kontchou G, Nault J-C, et al. Hepatocellular carcinoma within Milan criteria: no-touch multibipolar radiofrequency ablation for treatment-long-term results. Radiology. 2016;280:611–621.
- Kim Y, Lim HK, Rhim H, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89–97.
- Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900–909.
- Hocquelet A, Aubé C, Rode A, et al. Comparison of no-touch multibipolar vs. monopolar radiofrequency ablation for small HCC. J Hepatol. 2017;66:67–74.
- Cartier V, Boursier J, Lebigot J, et al. Radiofrequency ablation of hepatocellular carcinoma: mono or multipolar? J Gastroenterol Hepatol 2016;31:654–660.
- van Tilborg AAJM, Scheffer HJ, de Jong MC, et al. MWA versus RFA for perivascular and peribiliary CRLM: a retrospective patient- and lesion-based analysis of two historical cohorts. Cardiovasc Intervent Radiol. 2016;39:1438–1446.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology 2014;273:241–260.
- Laeseke PF, Sampson LA, Haemmerich D, et al. Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: in vivo porcine liver results. Radiology. 2006;241:116–124.
- Goldberg SN, Solbiati L, Hahn PF, et al. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209:371–379.
- Park MJ, Kim Y, Rhim H, et al. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J Vasc Interv Radiol. 2011;22:771–779.
- Seror O, N’Kontchou G, Van Nhieu JT, et al. Histopathologic comparison of monopolar versus no-touch multipolar radiofrequency ablation to treat hepatocellular carcinoma within Milan criteria. J Vasc Interv Radiol. 2014;25:599–607.
- Dodd GD, Dodd NA, Lanctot AC, et al. Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology. 2013;267:129–136.
- Pillai K, Akhter J, Chua TC, et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore). 2015;94:e580.
- Sun A-X, Cheng Z-L, Wu P-P, et al. Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J Gastroenterol. 2015;21:2997–3004.
- Chinnaratha MA, Chuang MA, Fraser RJL, et al. Percutaneous thermal ablation for primary hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:294–301.
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiogr Rev Publ Radiol Soc N Am Inc. 2005;25(Suppl 1):S69–S83.
- Hinshaw JL, Lubner MG, Ziemlewicz TJ, et al. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation-what should you use and why? RadioGraphics 2014;34:1344–1362.
- Xu Y, Shen Q, Wang N, et al. Microwave ablation is as effective as radiofrequency ablation for very-early-stage hepatocellular carcinoma. Chin J Cancer. 2017;36:14.
- Kang TW, Lim HK, Lee MW, et al. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: retrospective comparison of long-term therapeutic outcomes. Radiology. 2014;270:888–899.
- Amabile C, Ahmed M, Solbiati L, et al. Microwave ablation of primary and secondary liver tumours: ex vivo, in vivo, and clinical characterisation. Int J Hyperthermia. 2017;33:34–42.
- Vietti VN, Duran R, Guiu B, et al. PD-014 microwave ablation and radiofrequency ablation for the treatment of hepatocellular carcinoma: result of the first prospective randomized controlled trial. Ann Oncol. 2017;28 (suppl 3). DOI:10.1093/annonc/mdx263.013
- van Duijnhoven FH, Jansen MC, Junggeburt JMC, et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol. 2006;13:651–658.
- Lu DSK, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954–960.
- Cardella JF, Kundu S, Miller DL, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189–S191.
- Blackwell M, Iacus S, King G, et al. CEM: coarsened exact matching in Stata. Stata J. 2009;9:524–546.
- Lu DSK, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–1274.
- Poulou LS, Botsa E, Thanou I, et al. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054–1063.
- Yu NC, Raman SS, Kim YJ, et al. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19:1087–1092.
- Choi JW, Lee JM, Lee DH, et al. Switching monopolar radiofrequency ablation using a separable cluster electrode in patients with hepatocellular carcinoma: a prospective study. PLoS One. 2016;11:e0161980.
- Lee JM, Han JK, Kim HC, et al. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007;42:676–683.
- Fessenden P, Kapp DS, Lee ER. Noninvasive microwave phased arrays for local hyperthermia: a review. Int J Hyperthermia. 1990;6:715–716.
- Magin RL, Peterson AF. Noninvasive microwave phased arrays for local hyperthermia: a review. Int J Hyperthermia. 1989;5:429–450.
- Dou J-P, Yu J, Yang X-H, et al. Outcomes of microwave ablation for hepatocellular carcinoma adjacent to large vessels: a propensity score analysis. Oncotarget. 2017;8:28758–28768.
- Huang S, Yu J, Liang P, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol. 2014;83:552–558.
- Tan Y, Jiang J, Wang Q, et al. Radiofrequency ablation using a multiple-electrode switching system for hepatocellular carcinoma within the Milan criteria: long-term results. Int J Hyperthermia. 2017;34(3):1–8.
- de Baere T, Bessoud B, Dromain C, et al. Percutaneous radiofrequency ablation of hepatic tumors during temporary venous occlusion. Am J Roentgenol. 2002;178:53–59.
- Sutter O, Calvo J, N’Kontchou G, et al. Safety and efficacy of irreversible electroporation for the treatment of hepatocellular carcinoma not amenable to thermal ablation techniques: a retrospective single-center case series. Radiology. 2017;284:877–886.
- Chiang J, Cristescu M, Lee MH, et al. Effects of microwave ablation on arterial and venous vasculature after treatment of hepatocellular carcinoma. Radiology. 2016;281:617–624.
- Farina L, Nissenbaum Y, Cavagnaro M, et al. Tissue shrinkage in microwave thermal ablation: comparison of three commercial devices. Int J Hyperthermia. 2017;1–10. DOI:10.1080/02656736.2017.1362115
- Rempp H, Voigtländer M, Schenk M, et al. Internally gas-cooled radiofrequency applicators as an alternative to conventional radiofrequency and microwave ablation devices: an in vivo comparison. Eur J Radiol. 2013;82:e350–e355.