Abstract
Background: Selecting colorectal patients for HIPEC-surgery needs improvement. The study aim was to improve the colorectal peritoneal score (COREP) and to compare it with three other scores: peritoneal-surface disease-severity score (PSDS), colorectal-peritoneal metastases prognostic-surgical-score (COMPASS), and the CEA/PCI ratio.
Method: Twelve preoperative factors were chosen to evaluate for COREP score modification. Criteria from logistical analyses were set to qualify for the modified COREP score (mCOREP). Odds ratios were used to assign score points for the eligible factors with open/close laparotomy prediction as endpoint. mCOREP was applied internally and compared with the original COREP, PSDS, COMPASS, and CEA/PCI ratio. Odds ratios, hazard ratios, and Kaplan–Meier curves were used for comparison.
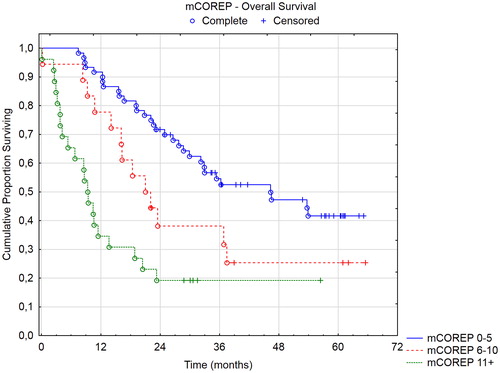
Results: Seven factors qualified for mCOREP: CEA, CA 19-9, CA-125, C-reactive protein, albumin, platelet count and signet-cell histology. mCOREP was superior to the original COREP. mCOREP and COMPASS scores were the only scores with independent prognostic value. The mCOREP had the best discriminatory ability between its prognostic groupings. mCOREP 11 + had 9 months survival with half of patients being open/close surgery.
Conclusion: The mCOREP has successfully been simplified while still improving its prognostic ability. The mCOREP and COMPASS scores have independent prognostic value. Patients with mCOREP 11 + may not benefit from treatment.
Introduction
Colorectal cancer with peritoneal metastases is a disease with varying prognosis. At an early stage, it is curable with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) [Citation1–6]. With later stages or more aggressive tumor biology, the prognosis is much poorer [Citation7]. In an attempt to improve the patient selection process, the COREP (colorectal peritoneal) score was developed [Citation8]. This score has been useful in identifying patients that will not benefit from the treatment [Citation8,Citation9]. The score requires a number of tumor markers and may be somewhat cumbersome to implement.
Besides the COREP score, there are a number of other prognostic scoring systems available. Even though the COREP score is designed as an objective solely preoperative score, it is relevant to compare its prognostic abilities with three other primarily postoperative scores: PSDS (peritoneal surface disease severity score) [Citation10], COMPASS (colorectal peritoneal metastases prognostic surgical score) [Citation11,Citation12] and the CEA/PCI ratio [Citation13].
This study has two aims. The first aim of this study is to investigate whether the COREP score can be simplified and yet remain robust in the patient selection process. The second aim is to compare COREP, PSDS, COMPASS and CEA/PCI ratio with each other. The main endpoints are the identification of open/close cases, the identification of patients with short survival (<12 months) and the ability to stratify the cohort into distinct prognostic groups.
Methods
All patients with colorectal cancer including appendiceal adenocarcinoma and isolated peritoneal metastases being accepted for cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) at the Uppsala Academic Hospital consecutively from 2009–2015 were included into the cohort. All low-grade adenomucinous neoplasms and pseudomyxoma peritonei syndrome patients were excluded. The method of CRS and HIPEC used has been previously described [Citation14,Citation15]. Variables were collected from the HIPEC database and verified from the original hospital charting. The following data were collected: age, gender, weight, height, serum tumor markers (STM), blood work, type of colorectal tumor, histopathology report, PCI and CC score. The following endpoints were registered: open and close laparotomies, overall survival (OS), recurrence-free survival (RFS), grade 3–4 postoperative in-hospital serious adverse events. The COREP score, PSDS score, COMPASS score and CEA/PCI ratio were calculated according to previous studies [Citation8–13]. The study was approved by the Uppsala Ethics Committee.
Statistics
The demographics and variables collected were represented with mean and median values with standard deviation and total range or with n-values and percentages. A univariate and multivariable logistical analysis was performed on all blood work results (CEA, CA 15-3, CA 19–9, CA 72-4, CA 125, hemoglobin, C-reactive protein, albumin level, platelet count, white blood cell count) and on two histopathology variables (signet-cell ring tumor and poor differentiation). A variable qualified to be included in the modified COREP score, if it was significantly associated with both open/close laparotomy prediction and short overall survival in the univariate analysis (p values <.05) or the variable was a statistically significant or near significant independent prognostic factor in the multivariable analyses for either of the two endpoints (p values < .1). The variables that fulfilled either of these two criteria were included in a new modified COREP scoring system. The continuous variables were then dichotomized according to the best specificity/sensitivity breaking point. p values are two-tailed.
Using the dichotomized variables for the new score, odds ratios were calculated for both open/close laparotomy and short overall survival. As the COREP score is intended to be a preoperative score with the hopes of improving patient selection, the odds ratio from the open/close univariate analyses were used to give scoring values to the variables. The closest whole number from the odds ratio was chosen. The modified COREP (mCOREP) was compared with the original COREP score in the same univariate and multivariable logistical analyses mentioned above. Furthermore, the mCOREP score at referral was compared to mCOREP at operation date in the same manner.
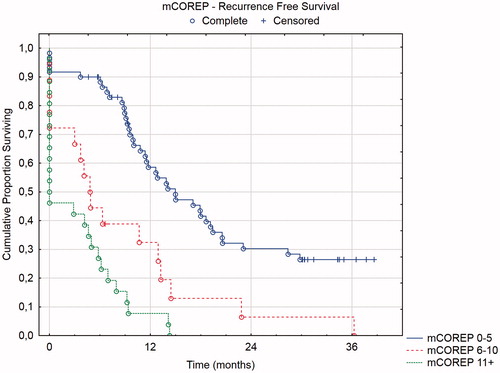
Three prognostic groups were identified in the mCOREP results: 0–5, 6–10, and 11+. These groups were compared in a Kaplan–Meier curve with log rank tests with overall survival and recurrence-free survival as endpoints.
Lastly, a comparison between mCOREP, PSDS, COMPASS and CEA/PCI ratio was performed with univariate and multivariable analyses between the four scores with open/close laparotomy and short overall survival as endpoints. The Harrell’s c-statistic was calculated from the area-under-the curve of the ROC curves from the logistical analyses. COMPASS and PSDS scores were divided into 4 prognostic groups as defined according to previous studies [Citation10–13] and represented with Kaplan–Meier curves to evaluate the scores discriminatory ability between the groups.
Results
A total of 128 patients were identified from the HIPEC registry. The demographics and variables collected are reported in . The univariate and multivariable logistical analyses with odds ratios for the blood work and histopathological prognostic indicators chosen are displayed in . Using the univariate odds ratio from the open/close analyses of the chosen dichotomized variables as described in the methods section, new scores for a modified COREP (mCOREP) was developed. The nearest whole number was chosen and the original and mCOREP scores are represented in . A comparison between the original and mCOREP is seen in as well as a comparison between mCOREP at referral and at the time of the operation. The mCOREP score is superior to the original COREP and the mCOREP score at the time of surgery is the one with most prognostic value.
Table 1. Demographics and variables collected.
Table 2. Univariate logistical analyses with odds ratios.
Table 3. COREP and modified COREP scoring systems.
In , four prognostic scores are compared with logistical and cox-proportional analyses: mCOREP, PSDS, COMPASS and CEA/PCI ratio. The only two scores that had independent prognostic value were mCOREP and COMPASS. The COMPASS had better open/close prediction than mCOREP despite mCOREP being based on this endpoint. However, the mCOREP had greater prognostic value in recurrence-free survival. Both scores had similar independent prognostic values for short overall survival.
Table 4. Comparison of four prognostic scoring systems with univariate and multivariable logistical analyses and cox-proportional hazards analyses (n = 104).
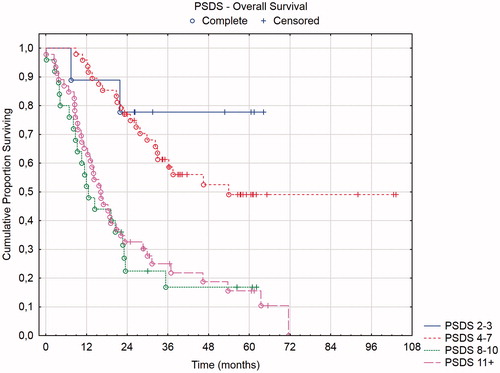
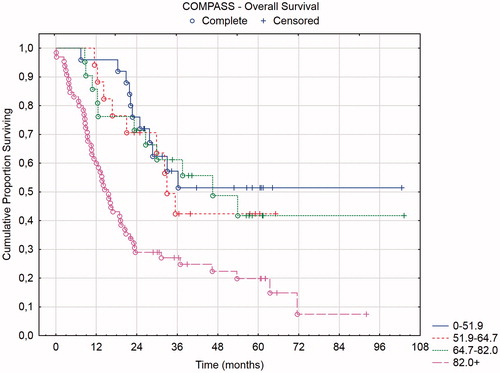
Three of the four prognostic scores have defined prognostic groups: mCOREP, COMPASS, and PSDS. In and , the overall and recurrence-free survival of the mCOREP score are displayed and in and the overall survival of the PSDS and COMPASS groups are displayed. The projected median OS of mCOREP 0–5 was 46 months, 22 months for 6–10 and 9 months for 11 + (statistically significant difference between the three groups with p values < .05). The median OS of PSDS group 2–3 was 32 months, 36 months for 4–7, 13 months for 8–10 and 16 months for 11+. The median OS of the COMPASS group 0–51.9 was 32 months, 32 months for 51.9–64.7, 38 months for 64.7-82, and 16 months for 82+. The PSDS could not discriminate between its four groups. Rather, two groupings were relevant in this cohort: PSDS 2–7 versus 8+. Likewise, the four COMPASS groupings were not discriminatory and also here two groupings were seen: COMPASS 0–82 versus 82+. The mCOREP did not have 4 groupings but rather 3. They were statistically separated in the overall survival and in the recurrence-free survival.
Discussion
A modification to the COREP score was successful in improving its prognostic ability. Besides being an improvement over the original score, it is somewhat simpler to implement as each STM has only one point level with one cut-off value. Furthermore, the mCOREP score at the time of surgery has more prognostic impact then at the time of referral. As such, there may be a place for downstaging neoadjuvant therapy to improve the survival of patients with higher mCOREP scores at referral. This aspect is currently being investigated in a new study on the effect of neoadjuvant chemotherapy for colorectal cancer with peritoneal metastases.
Besides removing the CA15–3 from the COREP score, the main modification to the score is in switching the white blood cell count to C-reactive protein and adding the platelet count and albumen level to the score. C-reactive protein albumin ratio (CAR) has been shown to be predictive of overall and disease-free survival in colorectal cancer [Citation16–17]. Likewise, thrombocytosis has been shown to have the same prognostic effect [Citation18]. The mCOREP score combines three STMs with three well-known inflammatory/hematological profile markers of poor prognosis. Together with signet cell histology, this scoring system is robust in differentiating separate prognostic groups. The use of three groups instead of four seemed more relevant clinically; where one group does well, one group may not benefit from the treatment, and a third intermediate group where the benefit is uncertain. As a preoperative scoring system, it could be used in the patient selection process. All patients in the poor prognostic group had recurrences within 15 months and more than half of them where open/close cases from the start (). While an external confirmation is needed from a separate cohort, the results seem to demonstrate that a patient with a preoperative mCOREP score of 11 or more is not going to benefit from the treatment.
The mCOREP was compared with COMPASS, PSDS and CEA/PCI in terms of prognosticating open/close laparotomies and short overall survival defined as survival <12 months. The mCOREP and COMPASS scores performed the best in this cohort and were the only two scores with independent prognostic value in . COMPASS had slightly better Harrell’s c statistic for open/close laparotomy while the mCOREP was slightly better in short survival.
In this cohort, the mCOREP and COMPASS scores were superior to PSDS and CEA/PCI ratio. The mCOREP score is built on preoperative factors that are available from blood work and a tumor biopsy. The mCOREP can provide a very good prognostic grouping already preoperatively. This information can be used in the multi-disciplinary setting as part of the patient selection process and can also be used during patient consultation for the patients to make informed decisions concerning their own treatment alternatives. The COMPASS score relies on some factors that may be difficult to correctly assess preoperatively, namely the PCI score and N2 nodal status. The PCI score as determined by preoperative radiology may incorrectly assess the PCI limit of 20 in 12% of cases [Citation19]. It can sometimes be assessed properly through a preoperative laparoscopy if there are not too many adhesions from previous surgery [Citation20]. However, a laparoscopic assessment is also resource-demanding. The N2 status is difficult to assess properly both radiologically or laparoscopically. Patients with their primary tumor still intact at referral will not have an assessable nodal status. Thus, the COMPASS score is more relevant as a postoperative prognostic assessment. While this assessment may be relevant for postoperative prognostic information, it may not be as useful in the preoperative patient selection setting. In the clinical setting, the mCOREP score could be used for preoperative patient selection assessment, while the COMPASS score is used for postoperative prognostication.
In conclusion, the mCOREP scores has successfully been simplified while still improving its prognostic ability. The mCOREP score is superior to PSDS and CEA/PCI ratio at predicting open/close laparotomy or poor survival of <12 months. It is similar to the COMPASS score with both scores having independent prognostic value. However, the mCOREP was superior in differentiating three separate prognostic groups from this cohort, while the PSDS and COMPASS could not discriminate between their 4 prognostic groups. An external validation study from a separate institution is ongoing now to validate if the mCOREP scoring system is the best for preoperative assessment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Goéré D, Malka D, Tzanis D, et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257:1065–1071.
- Cashin PH, Dranichnikov F, Mahteme H. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy treatment of colorectal peritoneal metastases: Cohort analysis of high volume disease and cure rate. J Surg Oncol. 2014;110:203–206.
- Cashin PH, Mahteme H, Spång N, et al. Cytoreductive surgery and intraperitoneal chemotherapy vs. systemic chemotherapy for colorectal peritoneal metastases: a randomised trial. Eur J Cancer. 2016;53:155–162.
- Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681–685.
- Franko J, Ibrahim Z, Gusani NJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756–3762.
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743.
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68.
- Cashin PH, Graf W, Nygren P, et al. Patient selection for cytoreductive surgery in colorectal peritoneal carcinomatosis using serum tumor markers: an observational cohort study. Ann Surg. 2012;256:1078–1083.
- Cashin PH, Graf W, Nygren P, et al. Comparison of prognostic scores for patients with colorectal cancer peritoneal metastases treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2013;20:4183–4189.
- Chua TC, Morris DL, Esquivel J. Impact of the peritoneal surface disease severity score on survival in patients with colorectal cancer peritoneal carcinomatosis undergoing complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:1330–1336.
- Simkens GA, van Oudheusden TR, Nieboer D, et al. Development of a prognostic nomogram for patients with peritoneally metastasized colorectal cancer treated with cytoreductive surgery and HIPEC. Ann Surg Oncol. 2016;23:4214–4221.
- Demey K, Wolthuis A, de Buck van Overstraeten A, et al. External validation of the prognostic nomogram (COMPASS) for patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2017;24:3604–3608.
- Kozman MA, Fisher OM, Rebolledo BJ, et al. CEA to peritoneal carcinomatosis index (PCI) ratio is prognostic in patients with colorectal cancer peritoneal carcinomatosis undergoing cytoreduction surgery and intraperitoneal chemotherapy: A retrospective cohort study. J Surg Oncol. 2018;117:725–736.
- Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42.
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996; 82:359–374.
- Egenvall M, Mörner M, Martling A, et al. Prediction of outcome after curative surgery for colorectal cancer: preoperative haemoglobin, C-reactive protein and albumin. Colorectal Dis. 2018;20:26–34.
- Haruki K, Shiba H, Horiuchi T, et al. Impact of the C-reactive protein to albumin ratio on long-term outcomes after hepatic resection for colorectal liver metastases. Am J Surg. 2017;214:752–756.
- Long Y, Wang T, Gao Q, et al. Prognostic significance of pretreatment elevated platelet count in patients with colorectal cancer: a meta-analysis. Oncotarget. 2016;7:81849–81861.
- Esquivel J, Chua TC, Stojadinovic A, et al. Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol. 2010;102:565–570.
- Marmor RA, Kelly KJ, Lowy AM, et al. Laparoscopy is safe and accurate to evaluate peritoneal surface metastasis prior to cytoreductive surgery. Ann Surg Oncol. 2016;23:1461–1467.