Abstract
Purpose: The aim of this retrospective study was to assess the safety and effectiveness of laser ablation (LA) in patients with small renal cell carcinomas (RCC) and increased risk of bleeding.
Material and methods: From 2013 to 2017, nine patients (six males, three females, aged 68.5 ± 12.2 years) at high risk of bleeding underwent ultrasonography-guided LA for an RCC. Patients were considered at increased risk of bleeding because of impairment of coagulation parameters, concomitant antiplatelet therapy, or at-risk location of the tumor (one, five, and three patients, respectively). RCC diameter ranged from 11 to 23 mm. According to tumor size, two or three laser fibers were introduced through 21-gauge needles and 1800 J per fiber were delivered in 6 min with a fixed power of 5 W. Major and minor complications, technical success, and primary and secondary technical effectiveness and tumor recurrence were recorded.
Results: Just one Grade 1 complication was observed: a small asymptomatic hematoma that spontaneously resolved. Technical success was 100%, 1 month technical efficacy was 88.9% (8/9 patients). One patient with residual tumor was successfully retreated 1 month later, and secondary efficacy rate was 100%. No local tumor recurrence occurred during a median follow-up of 26 months (range 11–49 months).
Conclusions: LA is safe and effective in the treatment of small RCC and might represent a valid option in patients with increased risk of bleeding.
Introduction
The detection rate of renal cell carcinoma (RCC) has increased over the last few years, mainly due to the increased number of incidentally diagnosed cases during diagnostic cross-sectional studies. The majority of RCCs are serendipitously found when of small dimensions, in T1a stage [Citation1,Citation2]. Partial nephrectomy (open or laparoscopic) is the first therapeutic option whenever it is technically feasible, as total nephrectomy may often reduce renal function [Citation3], and is mainly reserved to centrally located tumors. Image-guided ablation therapies are increasingly used as a valid option for patients unsuitable for surgery in several different clinical scenarios. In particular, they have been proposed as a safe and effective alternative to surgery in small RCC, as these therapies can result in up to 100% of tumor ablation and are less invasive and more nephron-sparing [Citation4,Citation5]. Quite recently, the Cardiovascular and Interventional Radiology Society of Europe (CIRSE) published the guidelines for percutaneous ablation of small RCC, recommending radiofrequency ablation (RFA) and cryoablation (CRA) as the most studied and most suitable modalities to ablate tumors up to 5 cm in diameter, and mentioning also microwave ablation (MWA) as an interesting and increasingly used technique, although at present it is less investigated than RFA and CRA, and some concerns exist concerning its higher risk of pelvicalyceal injury [Citation2].
Laser ablation (LA) uses laser optical fibers to deliver energy to the tissue [Citation6,Citation7]. Although it is less investigated than RFA and MWA, LA is currently used in many centers to treat primary and metastatic liver cancers, with results that are comparable to MWA and RFA [Citation8–13]. The multifiber technique uses 300 μm bare optical fibers that are introduced into the tumor through 21-gauge needles [Citation14,Citation15]. The needles are considerably thinner than RFA, MWA, and CRA devices, which may be advantageous in particular clinical settings [Citation15–17], mainly due to the very low invasiveness of the device. In particular, LA might be the ideal ablative technique in patients at high risk of complications due to the presence of comorbidities or difficult technical access [Citation14,Citation18]. However, to date, LA has not been thoroughly investigated in the treatment of RCC, and the experiences in renal ablation reported in literature are sporadic and almost anecdotal [Citation1,Citation19–21].
In our Department, we usually use RFA or MWA to ablate RCC, but in patients with small renal tumors with increased procedural risk we prefer to use LA because of the smaller diameter of the device and its minimal invasiveness.
Thus, the aim of the present article was to retrospectively assess the safety and effectiveness of LA in patients with small RCCs at high risk of bleeding.
Materials and methods
The records of patients who underwent ultrasonography (US)-guided percutaneous ablation from August 2013 to January 2017 were retrospectively reviewed, and patients who were treated with LA due to increased risk of bleeding were analyzed. Institutional review board approval was obtained, and patients’ informed consent was waived. Patients gave their written informed consent to the ablation procedure after all were judged unsuitable for surgery by a panel of experts that included the urologist, anesthesiologist, oncologist, and interventional radiologist. Cause of exclusion from surgery were comorbidity (five patients) or advanced chronic renal failure (four patients). In four cases with inconclusive radiologic findings, RCC was diagnosed by US-guided biopsy; in the other cases the diagnosis was based on the imaging findings, and US-guided biopsy was performed immediately before the ablation procedure. According to prior recommendations [Citation22,Citation23], biopsy in these cases was aimed at obtaining information on the histology and grade of the tumor in order to plan surveillance after LA. Tumor size ranged from 11 to 23 mm in their largest diameter (median 18 mm). The patients underwent LA instead of other ablative techniques because they were considered at increased risk of bleeding: in three patients RCC was not exophytic, and a sizeable portion of renal parenchyma had to be passed through to reach the tumor (); one patient had low platelet count (50,000 platelets/mcL) and INR of 1.56 because of concomitant liver cirrhosis; five patients could not discontinue their antiplatelet therapy for ischemic heart disease with coronary stent implantation.
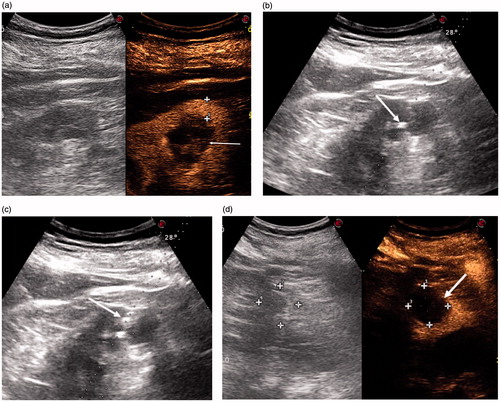
Figure 1. (a) CEUS scan showing a Bosniak III renal cyst cytologically proven to be RCC (arrow): 11.5 mm of renal parenchyma had to be passed through to reach the cyst (cross-shaped markers). (b) US scan showing the tip of a 21-G needle inside the BosnIak III cyst (arrow). (c) The steam produced by LA spreading inside the Bosniak III cyst (arrow). (d) Post-procedural CEUS scan showing a non-enhancing zone completely covering the Bosniak III cyst.
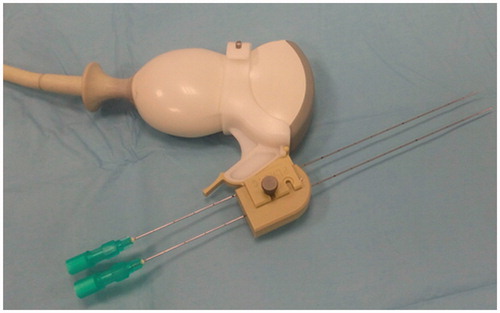
Ablation procedure
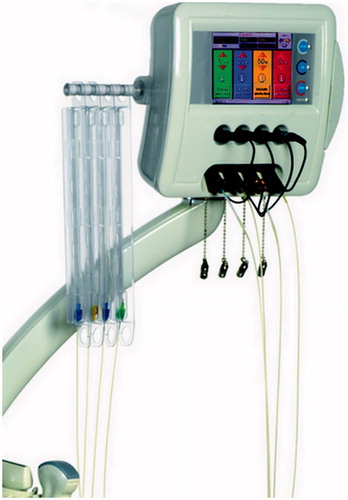
LA was performed on an inpatient basis by using a commercially available system composed of a US device and a laser unit (Echolaser; Elesta Srl, Florence, Italy). The laser source is a semi-conductor diode with a wavelength of 1064 nm, and a multi-source device () permits the use of up to four 300 μm fibers at once, making it possible to obtain ablation areas up to 4 cm in diameter [Citation9,Citation14,Citation15].
Figure 2. Laser ablation machine with multi-source device. Four 300 μm bare optical fibers are connected.
LA procedures were performed by two physicians with more than 10 years of experience in US-guided ablations of liver and kidney tumors. If the tumor was adjacent to the colon, preliminary hydrodissection by instilling 5% dextrose solution was performed to obtain a 2 cm safety margin between the colon and the tumor. After local anesthesia with lignocaine 1% 10 ml and conscious sedation with intravenous midazolam and ramifentanil, laser fibers were introduced into the tumor under US guidance through 21-gauge Chiba needles (). Two laser fibers spaced 12 mm apart were used for lesions with a diameter up to 14 mm, and three fibers with isosceles triangle configuration spaced 6–12 mm apart were inserted if the diameter of the lesions exceeded 14 mm; the pullback technique [Citation14,Citation15] was used if the antero-posterior diameter of the nodules exceeded 12 mm. The needles were introduced by using a guidance device with separate channels at prefixed distance (from 2 to 18 mm) (Elesta Srl, Florence, Italy) (. The laser machine was set at a power of 5 W, and 1800 J per fiber were delivered in 6 min, with the spread of the steam inside the tumor monitored by real-time US (); a further 1800 J were delivered if the pullback technique was used. The completeness of the ablation was assessed by contrast-enhanced US (CEUS) performed about 10 min after the end of the procedure. An 8 μL/mL solution of sulfur hexafluoride microbubbles stabilized by a phospholipid shell (SonoVue®; Bracco, Milan, Italy) was used as contrast agent, and CEUS was performed by using a low mechanical index contrast-specific nonlinear technique (CnTI; Esaote, Genova, Italy). If no enhancing zone with diameters equal to or greater than those of the treated tumor was depicted, the treatment was considered complete (). If residual enhancing foci of tumoral tissue were identified, another one or two laser fibers were inserted into the viable foci under CEUS guidance, and a further 1800J per fiber were delivered to complete the treatment.
Post-procedural care and follow-up
After LA, the patients remained in the hospital overnight and their vital signs were monitored. The next morning, clinical evaluation and US examination of the abdomen were performed, and the patients were discharged in the afternoon if they were considered to be well.
All patients were followed up until death, dropout, or the time the data were censored (22 December 2017). The patients underwent contrast-enhanced computed tomography (CECT), or CEUS if they had impaired renal function, 1 month after LA, and were subsequently monitored with alternating CECT and CEUS (or only CEUS in presence of reduced renal function) every 3 months for the first 2 years, then every 6 months.
Variables
The number of fibers used in each case, with pullback when needed, the presence or absence of residual disease at immediate CEUS and number of patients immediately retreated, and the number of cases requiring hydrodissection were recorded. The duration of energy application and the duration of the entire procedure (time from the patient’s admission into the interventional suite to patient’s discharge to the recovery unit) were also recorded. Technical success, technical efficacy, primary and secondary efficacy rates, and local tumor progression were recorded and defined according to the recommendations of the International Working Group on the Image-guided Tumor Ablation [Citation24]. In particular, technical success addressed whether the tumor was treated according to protocol and whether it was completely covered by the ablation zone. Technical efficacy was defined as a non-enhancing area with diameters equal to or greater than those of the treated tumors, assessed by a contrast-enhanced imaging technique (CECT or CEUS) performed 1 month after LA (). If residual viable foci were detected, the patient underwent further CEUS-guided LA and the result was assessed by CEUS or CECT performed 1 month later. Secondary efficacy rate was defined as the percentage of tumors successfully eradicated following the first ablation session or the ablation session performed to treat residual tumor foci detected 1 month after the initial LA. Local tumor progression was defined as the detection, 1 month after the initial or repeated LA, of viable tumor foci within or close to the ablated zone after complete ablation had been documented.
Complications were recorded and classified according to the CIRSE classification system for complications reporting [Citation25].
Clinical data were retrospectively retrieved from the hospital digital archive and images reviewed from the hospital picture archiving and communication system.
Results
Patients and tumor characteristics are reported in detail in .
Table 1. Patients and tumor characteristics.
Two laser fibers, three laser fibers, and three laser fibers with the pullback technique were used in four, two, and three patients, respectively. Hydrodissection was performed in two cases. Post-procedural CEUS documented residual viable foci in two patients, both of whom underwent further CEUS-guided LA during the same treatment session, and technical success was 100% (); CECT performed after 1 month confirmed the completeness of the ablation ().
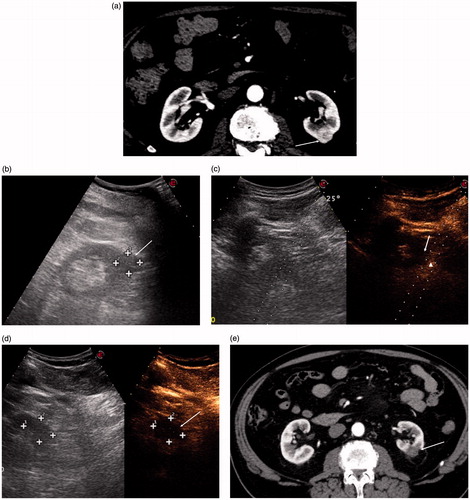
Figure 4. (a) CT scan showing an exophytic hyperenhancing nodule in the middle third of the left kidney (arrow). (b) US scan showing the same nodule (arrow). (c) Post-procedural CEUS scan showing residual enhancing tissue (arrowhead) in the inferolateral margin of the ablation zone (arrow). The residual viable tissue is targeted for immediate retreatment under CEUS guidance (dotted lines). (d) CEUS scan performed after the retreatment showing disappearance of the residual enhancing tissue (arrow). (e) CECT scan performed 1 month after LA showing complete disappearance of enhancement of the nodule with fibrosis of the surrounding fat (arrow).
Just one Grade 1 complication was observed: a small, asymptomatic hematoma (maximum thickness 4 mm) that spontaneously resolved and did not require any intervention. All the patients were discharged from the hospital the day after the procedure.
Five patients were followed-up by alternating CECT and CEUS, while four patients underwent only CEUS because of reduced renal function. At 1 month, technical efficacy was 88.9% (eight out of nine patients). One patient showed a residual viable tumor focus at CEUS performed 1 month after LA procedure and was successfully retreated. Therefore, secondary efficacy rate was 100%. No local tumor progression was demonstrated during a median follow-up of 26 months (range 11–49 months). One patient died 17 months after LA owing to acute cholecystitis and pneumonia; CECT performed 2 months before the death had documented complete tumor ablation and partial involution of the ablation zone. Three patients dropped out at 13, 33, and 49 months after LA, respectively; none of them had local tumor progression at the time they dropped out. Five patients were alive and disease-free at the time the data were censored (22 December 2017).
On the whole, the duration of energy application ranged from 6 to 18 min, and the duration of the entire procedure ranged from 60 to 95 min (mean 72 min). All these data are detailed in .
Table 2. LA procedure characteristics.
Discussion
The CIRSE guidelines recommend percutaneous ablation as an effective and safe alternative to partial nephrectomy in patients with T1a RCC who are not fit or are not willing to undergo surgical treatment [Citation2]. To date, RFA and CRA are the most extensively used and studied ablation techniques, with complete ablation rates up to 100% in tumors <3 cm and 90% for sizes between 3 and 5 cm, with over 90% 5 year disease-free survival for T1a lesions [Citation4,Citation5,Citation26–29]. MWA is also increasingly used for the treatment of RCC. In comparison with RFA, it can obtain larger ablation areas and is not limited by the heat sink effect, desiccation, or charring [Citation30,Citation31]. However, MWA produces ablation zones that are often ovoid [Citation32], and even with the use of the novel antennas that offer more spherical ablation zones than did the earlier ones, MWA seems to have higher risk of pelvicalyceal injury than the other ablation techniques [Citation33].
Even though LA has been less investigated than RFA and MWA, it is currently used in many centers to treat hepatocellular carcinoma (HCC) and liver metastases (LM), with very good results that are comparable to those of RFA and MWA for either HCC [Citation8,Citation34,Citation35] or LM from colorectal cancer [Citation10,Citation11,Citation36]. Moreover, no significant differences between LA and RFA in terms of local tumor control, overall survival, and safety were found in two randomized trials that compared the two techniques [Citation13,Citation35]. According to the multifiber technique [Citation14,Citation15], 300 μm bare optical fibers are introduced into the tumor through 21-gauge needles, and the possibility of using very thin needles makes LA particularly suitable for ablating lesions in an at-risk location or in locations that are difficult to reach [Citation9,Citation16–18].
However, at present, LA is not currently used to treat RCC, and the studies reported in literature have been sporadic and have involved very small series of patients [Citation1,Citation19–21]. Moreover, in all these studies, LA was performed under magnetic resonance (MR) guidance by using large diameter devices requiring the introduction of a 9 French sheath into the tumor. Instead, in our series, LA was performed under US-guidance by using very thin needles. The kidney is a hypervascular organ and is considered more at risk of bleeding than the liver when interventional procedures are performed [Citation37,Citation38], and needle size has been reported to be a risk factor of hemorrhagic complications [Citation39,Citation40]. Therefore, the use of thinner needles could provide some advantage when thermal ablation is performed to treat RCC, in particular if the risk of bleeding is increased. Indeed, LA was preferred in our patients as they were considered at increased risk of bleeding because of impairment of coagulation parameters, concomitant antiplatelet therapy, or at-risk location of the tumor. No major complication was observed, and all tumors were completely ablated in one session (eight cases) or two sessions (one case) of treatment. Moreover, no local tumor progression occurred during the follow-up period. Unlike in our series, in the few previous reports on LA of RCCs the procedure was performed under MR guidance [Citation1,Citation19,Citation20]. The main advantage of MR over other imaging modalities is its sensitivity to thermal changes [Citation10,Citation41], which make it possible to monitor the heating process in near real time to ensure that the entire lesion has been treated and to reposition the applicators in case of residual tumor. However, post-procedural CEUS in early evaluation of the ablation procedures has been proven to be as accurate as both CEUS and CT performed at 24 h [Citation42,Citation43], and at present CEUS is recommended as a valid tool in the post-procedural assessment of the completeness of ablation [Citation44]. Indeed, post-procedural CEUS documented an unsatisfactory ablation area in two out of our nine patients (22.2%), both of whom were successfully retreated under CEUS guidance in the same treatment session, with a 100% technical success. Therefore, we think that post-procedural CEUS can counterbalance the advantage of MR in terms of correct evaluation of the completeness of the treatment, allowing for US-guided ablation whenever the tumor can be well-visualized and confidently targeted by US, with obvious cost savings and shorter duration of the procedure. Indeed, in our series the mean duration of the entire procedure was 72 min versus a reported duration of 3.5 h using MR-guided LA [Citation1].
Our study has some limits. In particular, it is retrospective, and the number of patients treated with LA is quite low. It follows that our results can only be regarded as preliminary. Moreover, the follow-up was relatively short in comparison with the trials that investigated RFA and CRA of RCC. However, a study on a 14 year experience of RFA in the treatment of renal cancer has recently demonstrated that the majority of recurrences are detected within the first 24 months after the procedure, with a significant number being diagnosed within the first 3 months after the ablation sessions [Citation45]. Therefore, it is likely that our median 26 month follow-up is long enough to infer good long-term efficacy of LA.
In conclusion, our retrospective study suggests that US-guided percutaneous LA of small RCC is safe and effective in the treatment of small RCC, and that it might also be successfully used in cases at high risk of bleeding. Our preliminary results justify the planning of larger prospective trials aimed at investigating whether LA could represent a valid alternative to RFA, CRA, or MWA in the treatment of Stage T1a RCC, in particular in patients at increased risk of bleeding.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kariniemi J, Ojala R, Hellstrom P, et al. MRI-guided percutaneous laser ablation of small renal cell carcinoma: initial clinical experience. Acta Radiol. 2010;51:467–472.
- Krokidis ME, Orsi F, Katsanos K, et al. CIRSE guidelines on percutaneous ablation of small renal cell carcinoma. Cardiovasc Intervent Radiol. 2017;40:177–191.
- McKiernan J, Simmons R, Katz J, et al. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59:816–820.
- Gervais DA, McGovern FJ, Arellano RS, et al. Radiofrequency ablation of renal cell carcinoma: part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:64–71.
- Ma Y, Bedir S, Cadeddu JA, et al. Long-term outcomes in healthy adults after radiofrequency ablation of T1a renal tumours. BJU Int. 2014;113:51–55.
- Loffroy R, Estivalet L, Favelier S, et al. Interventional radiology therapies for liver cancer. Hepatoma Res. 2016;2:1–9.
- Vogl TJ, Farshid P, Naguib NN, et al. Thermal ablation of liver metastases from colorectal cancer: radiofrequency, microwave and laser ablation therapies. Radiol Med. 2014;119:451–461.
- Pacella CM, Francica G, Di Lascio FM, et al. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. J Clin Oncol. 2009;27:2615–2621.
- Di Costanzo GG, Francica G, Pacella CM. Laser ablation for small hepatocellular carcinoma: state of the art and future perspectives. World J Hepatol. 2014;6:704–715.
- Vogl TJ, Straub R, Zangos S, et al. MR-guided laser-induced thermotherapy (LITT) of liver tumours: experimental and clinical data. Int J Hyperthermia. 2004;20:713–724.
- Vogl TJ, Dommermuth A, Heinle B, et al. Colorectal cancer liver metastases: long-term survival and progression-free survival after thermal ablation using magnetic resonance-guided laser-induced interstitial thermotherapy in 594 patients: analysis of prognostic factors. Invest Radiol. 2014;49:48–56.
- Eichler K, Zangos S, Gruber-Rouh T, et al. Magnetic resonance-guided laser-induced thermotherapy in patients with oligonodular hepatocellular carcinoma: long-term results over a 15-year period. J Clin Gastroenterol. 2012;46:796–801.
- Ferrari FS, Megliola A, Scorzelli A, et al. Treatment of small HCC through radiofrequency ablation and laser ablation. Comparison of techniques and long-term results. Radiol Med. 2007;112:377–393.
- Pacella CM, Bizzarri G, Francica G, et al. Percutaneous laser ablation in the treatment of hepatocellular carcinoma with small tumors: analysis of factors affecting the achievement of tumor necrosis. J Vasc Interv Radiol. 2005;16:1447–1457.
- Di Costanzo GG, D'Adamo G, Tortora R, et al. A novel needle guide system to perform percutaneous laser ablation of liver tumors using the multifiber technique. Acta Radiol. 2013;54:876–881.
- Tombesi P, Di Vece F, Sartori S. Radiofrequency, microwave, and laser ablation of liver tumors: time to move toward a tailored ablation technique? Hepatoma Res. 2015;1:52–57.
- Sartori S, Di Vece F, Ermili F, et al. Laser ablation of liver tumors: an ancillary technique, or an alternative to radiofrequency and microwave? World J Radiol. 2017;9:91–96.
- Francica G, Petrolati A, Di Stasio E, et al. Effectiveness, safety, and local progression after percutaneous laser ablation for hepatocellular carcinoma nodules up to 4 cm are not affected by tumor location. AJR Am J Roentgenol. 2012;199:1393–1401.
- de Jode MG, Vale JA, Gedroyc WM. MR-guided laser thermoablation of inoperable renal tumors in an open-configuration interventional MR scanner: preliminary clinical experience in three cases. J Magn Reson Imaging. 1999;10:545–549.
- Dick EA, Joarder R, De Jode MG, et al. Magnetic resonance imaging-guided laser thermal ablation of renal tumours. BJU Int. 2002;90:814–822.
- Giesbrandt K, Walser E. MR imaging-guided laser ablation of hepatic and renal tumors. AJR Am J Roentgenol. 2012;198:E642.
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924.
- Tsivian M, Rampersaud EN Jr, del Pilar Laguna Pes M, et al. Small renal mass biopsy–how, what and when: report from an international consensus panel. BJU Int. 2014;113:854–863.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. J Vasc Interv Radiol. 2014;25:1691–1705.e4.
- Filippiadis DK, Binkert C, Pellerin O, et al. CIRSE quality assurance document and standards for classification of complications: the CIRSE classification system. Cardiovasc Intervent Radiol. 2017;40:1141–1146.
- Zagoria RJ, Hawkins AD, Clark PE, et al. Percutaneous CT-guided radiofrequency ablation of renal neoplasms: factors influencing success. AJR Am J Roentgenol. 2004;183:201–207.
- Georgiades CS, Rodriguez R. Efficacy and safety of percutaneous cryoablation for stage 1A/B renal cell carcinoma: results of a prospective, single-arm, 5-year study. Cardiovasc Intervent Radiol. 2014;37:1494–1499.
- Breen DJ, Bryant TJ, Abbas A, et al. Percutaneous cryoablation of renal tumours: outcomes from 171 tumours in 147 patients. BJU Int. 2013;112:758–765.
- Schmit GD, Schenck LA, Thompson RH, et al. Predicting renal cryoablation complications: new risk score based on tumor size and location and patient history. Radiology. 2014;272:903–910.
- Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139.
- Di Vece F, Tombesi P, Ermili F, et al. Coagulation areas produced by cool-tip radiofrequency ablation and microwave ablation using a device to decrease back-heating effects: a prospective pilot study. Cardiovasc Intervent Radiol. 2014;37:723–729.
- Knavel EM, Hinshaw JL, Lubner MG, et al. High-powered gas-cooled microwave ablation: shaft cooling creates an effective stick function without altering the ablation zone. AJR Am J Roentgenol. 2012;198:W260–W265.
- Horn JC, Patel RS, Kim E, et al. Percutaneous microwave ablation of renal tumors using a gas-cooled 2.4-GHz probe: technique and initial results. J Vasc Interv Radiol. 2014;25:448–453.
- Pacella CM, Bizzarri G, Magnolfi F, et al. Laser thermal ablation in the treatment of small hepatocellular carcinoma: results in 74 patients. Radiology. 2001;221:712–720.
- Di Costanzo GG, Tortora R, D’Adamo G, et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma in cirrhosis: a randomized trial. J Gastroenterol Hepatol. 2015;30:559–565.
- Puls R, Langner S, Rosenberg C, et al. Laser ablation of liver metastases from colorectal cancer with MR thermometry: 5-year survival. J Vasc Interv Radiol. 2009;20:225–234.
- Taslakian B, Georges Sebaaly M, Al-Kutoubi A. Patient evaluation and preparation in vascular and interventional radiology: what every interventional radiologist should know (part 1: patient assessment and laboratory tests). Cardiovasc Intervent Radiol. 2016;39:325–333.
- Babaei Jandaghi A, Lebady M, Zamani AA, et al. A randomised clinical trial to compare coaxial and noncoaxial techniques in percutaneous core needle biopsy of renal parenchyma. Cardiovasc Intervent Radiol. 2017;40:106–111.
- Chunduri S, Whittier WL, Korbet SM. Adequacy and complication rates with 14- vs. 16-gauge automated needles in percutaneous renal biopsy of native kidneys. Semin Dial. 2015;28:E11–E14.
- Corapi KM, Chen JL, Balk EM, et al. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis. 2012;60:62–73.
- Germain D, Chevallier P, Laurent A, et al. MR monitoring of laser-induced lesions of the liver in vivo in a low-field open magnet: temperature mapping and lesion size prediction. J Magn Reson Imaging. 2001;13:42–49.
- Goldberg SN. Science to practice: Can we expand focal interventional oncologic ablation treatments into an effective systemic therapy? Radiology. 2013;267:321–323.
- Meloni MF, Andreano A, Zimbaro F, et al. Contrast enhanced ultrasound: roles in immediate post-procedural and 24-h evaluation of the effectiveness of thermal ablation of liver tumors. J Ultrasound. 2012;15:207–214.
- Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver – update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound. Med Biol. 2013;39:187–210.
- Iannucilli J, Dupuy D, Beland M, et al. Effectiveness and safety of computed tomography-guided radiofrequency ablation of renal cancer. Eur Radiol. 2015;26:1656.16664.