Abstract
Purpose: To retrospectively review long-term oncologic outcomes after ultrasound (US)-guided percutaneous microwave ablation (MWA) of T1a renal cell carcinoma (RCC) and to identify the incidence and risk factors that predict local tumor progression (LTP) after MWA of RCC.
Materials and methods: The present study was approved by the institutional review board. A total of 162 patients with 171 RCC nodules (mean size, 2.6 ± 0.8 cm; range, 0.6–4.0 cm) were treated by MWA between April 2006 and January 2017. The influence of eight factors (age; sex; longest tumor diameter; tumor number, location and pathology type; ablation power and time) affecting the risk of LTP was assessed. Univariate Kaplan–Meier and Cox proportional hazard models were used for statistical analysis.
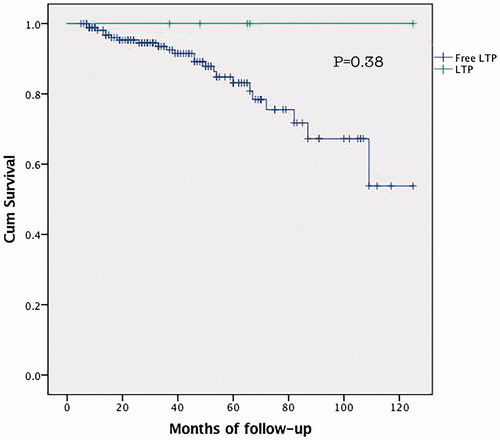
Results: LTP occurred in five patients (5 tumors) after US-guided percutaneous MWA of stage T1a RCC. The overall occurrence of LTP was 2.9% per tumor and 3.0% per patient with a median follow-up of 45.5 months. Among the 162 patients, there were no instances of LTP-related deaths; however, 20 patients died of other diseases. All patients with LTP survived through follow-up. The survival rate of LTP-free patients at 1, 3 and 5 years were 98.7%, 89.5% and 82.1%, respectively (p = .38). Univariate and multivariate analysis identified tumor location to be the only independent predictor of LTP.
Conclusions: US-guided percutaneous MWA for T1a RCC achieved a relatively low LTP incidence rate. Tumors adjacent to the renal pelvis or bowel increased the potential of LTP occurrence.
Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all human malignancies. It is the seventh most common cancer in men and the ninth in women [Citation1]. The incidence worldwide is about 209 000 new cases and 102 000 deaths per year [Citation2]. Traditionally, the reference standard for treatment of RCC has been radical nephrectomy [Citation3]. However, with the development of ablation technology, 2017 National Cancer Comprehensive Network Clinical Practice Guidelines in Oncology: Kidney Cancer point out that ablation strategies may be considered for small RCC [Citation4]. Currently, cryoablation (CA), radiofrequency ablation (RFA) and microwave ablation (MWA), especially with imaging guidance, are the main types of ablation treatments used for RCC.
Local tumor progression (LTP) is an important index for evaluating the efficacy of ablation therapy. LTP after percutaneous RFA with image guidance for T1a RCC is reported to range between 0.0% and 4.2% [Citation5–10] and tumor location and size were the most relevant risk factors for LTP after RFA of T1 RCC [Citation11–14]. LTP after percutaneous CA with image guidance for T1a RCC reportedly ranges between 1.6% and 4.9% [Citation9,Citation10,Citation15]. One large sample review demonstrated tumor size and growth pattern correlated with an increased risk of relapse after CA for T1 RCC [Citation16].
Though RFA and CA have been more widely used to treat T1a renal tumors, MWA is an encouraging ablation technology for RCC therapy with several advantages, including a higher temperature thermal field and larger ablation zone [Citation17]. Over the past decade, MWA for T1a RCC has made encouraging strides with an LTP <5.0% [Citation18–20]. However, published MWA studies are based on relatively small sample sizes, short follow-up times and lack analysis of risk factors for LTP. This is important to evaluate as the incidence of and risk factors for LTP after MWA of RCC may be different than RFA and CA. Therefore, the present study analyzed the incidence of LTP and related risk factors after ultrasound (US)-guided MWA of T1a RCC and verified whether LTP affects the survival of patients in a single high-volume center with a long-term follow-up.
Materials and methods
Patients
The current prospective study included consecutive patients within a single institution. Written informed consent was obtained from all enrolled patients, and the study protocol was approved by the institutional Ethics Committee. From April 2006 to January 2017, a total of 162 patients (122 men; 40 women) with 171 RCCs treated by internally-cooled MWA were enrolled to analyse LTP data. Among them, there were 156 clear cell RCCs with 165 nodules, 5 papillary cell carcinomas with 5 nodules, and 1 cystic RCC with 1 nodule. The mean age of patients was 62.6 ± 14.5 years (range, 21–86 years). The maximum diameter of tumors ranged from 0.6 to 4.0 cm (mean, 2.6 ± 0.8 cm). Baseline characteristics of patients are described in . Patient information collected included tumor number, pathology type, location, and growth pattern, as well as longest tumor diameter, serum creatinine levels, complications, renal function before and after ablation, site and date of recurrence or metastasis, status and date at last follow-up, death date and reason, and ablation parameters (power, time, and session).
Table 1. Baseline characteristics of patients undergoing microwave ablation.
Preprocedural evaluation
Inclusion criteria for MWA were a single lesion of 4 cm or smaller, the absence of renal vein thrombus or extrarenal metastases by contrast-enhanced US (CEUS) and magnetic resonance imaging (CEMRI)/computed tomography (CECT), and a safe pathway for MWA treatment of the tumor, and according to the type of renal tumor pathology verified by US-guided percutaneous biopsy before ablation, only malignant kidney tumors were included and all metastatic and benign kidney tumors were excluded. MWA treatment criteria included a prothrombin time less than 25 s, prothrombin activity higher than 40% and platelet count higher than 40 × 109 cells/L.
US-guided percutaneous MWA
For a detailed procedure of US-guided percutaneous MWA for RCC, refer to our previous report [Citation20]. MWA was performed by six interventional radiologists (MWA experience: P.L. and X.L.Y., 18 years each; Z.G.C. and Z.Y.H., 7 years each; J.Y. and F.Y.L., 6 years each) with the patient under moderate sedation and local anesthesia. The microwave unit (KY-2000; Kangyou Medical, Nanjing, China) used can produce 100 W of power at 2450 MHz. The cool-tip needle antenna had a diameter of 1.9 mm (15-G) and length of 18 cm. For tumors less than 2.0 cm in diameter, a single antenna was advanced, whereas 2 or more antennas were required for tumors with diameters of 2.0 cm or greater [Citation21]. Intravenous anesthesia was administered by a combination of propofol (Diprivan; Zeneca Pharmaceuticals, Wilmington, DE, USA) and ketamine (Shuanghe Pharmaceuticals, Beijing, China) via the peripheral vein. The antenna was percutaneously inserted into the tumor and placed at the desired location under US guidance. Then, US-guided biopsy was performed by an automatic biopsy gun with an 18-G cutting needle, and 2–3 separate punctures were performed. A power output of 50 W for 10 min was routinely used during MWA. If the heat-generated hyperechoic water vapor did not completely encompass the entire tumor, prolonged microwave emission was applied until the desired temperature was reached.
Protective thermal monitoring
From April 2004 to April 2014, we used protective thermal monitoring technology to protect the adjacent bowel and renal pelvis. A thermal monitoring needle was inserted into the tumor margin and bowel or renal pelvis for real-time temperature monitoring during ablation under US guidance. The temperature cut-off of ablation therapy was set at 54 °C in patients without a history of prior laparotomy or 50 °C in patients with history of laparotomy. The emission of microwaves was reactivated after the temperature decreased to 45 °C [Citation20].
Hydrodissection
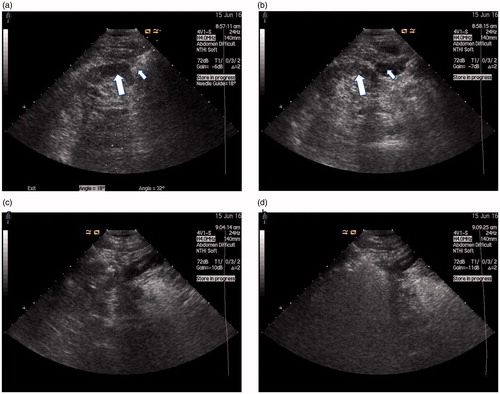
Since April 2014, hydrodissection technology has been used to treat renal tumors in the adjacent bowel or renal pelvis [Citation21–23]. For tumors adjacent to the bowl, after the administration of local anesthesia (i.e., 1% lidocaine) at the puncture site, a 15-cm long, 16-G intravenous catheter (BD Angiocath, Sandy, UT) was used to puncture the tissue between the index renal tumor and the bowel under US guidance. Saline solution (20–60 ml) was then rapidly injected; a small, local separation of the liquid between the tumor and the bowel was present. Then, the core needle was retracted, the catheter retained, and 1000–2000 ml of saline solution continuously injected [Citation21]. For tumors adjacent to the renal pelvis, a 20-cm long, 21-G percutaneous transhepatic cholangiography needle (Hakko Co., Ltd., Medical Device Division, Japan) was used to puncture the tissue between the index renal tumor and the renal pelvis under US guidance. To retain the guide needle after exiting the core, 100–500 ml of saline solution was rapidly injected in renal pelvis. The saline was continuously injected during ablation. ()
Figure 1. (a) US scan during ablation with tumor (thick arrows) adjacent to the bowel (thin arrow) that distance between tumor margin and renal pelvis <5 mm as measured by US. (b) US scan local separation of the liquid (thin arrow) between the tumor (thick arrows) and the bowel during hydrodissection. (c) US scan during ablation antenna was percutaneously inserted into the tumor and placed at the desired location. (d) US scan during ablation the heat-generated hyperechoic water vapor did completely encompass the entire tumor.
Definition of growth patterns for renal lesions and lesions in dangerous locations
The growth patterns of renal lesions were classified into three types: exophytic, parenchymal and endophytic. Exophytic nodules were considered nodules beyond the renal contour with no component extending into the renal sinus. Parenchymal nodules were considered nodules confined within the parenchyma without contour bulging or sinus extension. Endophytic nodules were considered nodules that extended into the renal sinus and were in close proximity to the collecting structures or ureters [Citation20]. When ablation was performed, US was used to determine the location of the lesion and the bowel. Lesions in dangerous locations were tumors adjacent to the renal pelvis and bowel (distance between tumor margin and bowel or renal pelvis <5 mm as measured by US) () .
Local tumor progression
After complete ablation, LTP was considered to have occurred if lesions were found inside or abutting the ablation zone with hyperenhancement at the arterial phase and hypoenhancement at the delayed phase by CEUS, CEMRI and/or CECT during the follow-up period.
Follow-up
According to the standardization of ablation terminology and reporting [Citation24], contrast-enhanced imaging (CT + CEUS or MRI + CEUS) was performed to evaluate the treatment efficacy. First, immediately after MWA, all patients underwent CEUS. These images were compared with those acquired before MWA to assess whether ablation was complete; nodular areas (cover tumor center and margin) of hypoechoic were not enhanced. Second, 1 month after MWA, at least 2 types of contrast-enhanced imaging (MRI + CEUS, CT + CEUS or CT + MRI + CEUS) were used to verify there was no enhancement of nodular areas. Based on complete ablation, routine contrast-enhanced imaging (CEUS and CEMRI) were used to evaluate LTP every 3 months within 6 months after complete MWA. If patients were LTP-free at this point, LTP was evaluated every 6 months after half a year by contrast-enhanced imaging.
Statistical analysis
LTP-free intervals and survival were estimated using the Kaplan–Meier method. Continuous variables were compared between those with and without LTP by Student’s t-test. A χ2 or Fisher’s exact test was applied to compare categorical variables between groups. LTP risk factors were analyzed using a Cox proportional hazards regression model. All statistical analyses were performed using SPSS 16.0 for Windows statistical package. Differences with a p values of <0.05 were considered statistically significant.
Results
Over an 11-year period, 162 patients with 171 RCCs underwent US-guided MWA of renal tumors with a 2450-MHz microwave generator. The median follow-up period was 44 months (range, 5–132 months). The mean ablated renal tumor size was 2.9 cm (range, 1.9–3.7 cm), and all tumors were clinical stage T1a. Among the 162 patients who underwent MWA, tumor enhancement disappeared after a single MWA session in 159 patients (98.1%, 159/162). In the other three patients (1.9%, 3/162), contrast-enhanced imaging three day after MWA revealed that the ablation zone edge occurred as an irregular peripheral enhancement in a scattered, nodular or eccentric pattern. Tumor enhancement disappeared after the second MWA session. Therefore, the primary and secondary technique effectiveness rates for percutaneous MWA of stage T1a RCC were 98.2% and 100%.
Overall LTP occurrence rate
LTP was diagnosed in 5 patients with 5 lesions (3.1% per tumor, 2.9% per patient). The pathological type of all patients with LTP was clear cell RCC. The median LTP occurrence time was 29.4 months (range, 19–43 months) after MWA (). The overall LTP occurrence rates at 1, 3 and 5 years were 0%, 2.3% and 2.9%, respectively. Three cases of LTP were treated by MWA, and another two cases received nephrectomy because they refused MWA. The overall survival rates of the 162 patients at 1, 3 and 5 years were 98.1%, 92.8% and 85.9%, respectively. The overall survival rates of LTP patients at 1, 3 and 5 years were 98.8%, 92.5% and 85.2%, respectively. The survival rate of LTP patients at 1, 3 and 5 years was 100% (. The survival rates of LTP-free patients at 1, 3 and 5 years were 98.7%, 89.5% and 82.1%, respectively, with no significant difference between the 2 groups (p = .38). To date, there have been no instances of LTP-related deaths.
Relationship between LTP and tumor size
There were 3 cases of LTP for tumors less than 3.0 cm, and 2 for 3.0- to 4.0-cm tumors. For tumors less than 3.0 cm, LTP occurred 26, 30 and 43 months after MWA, respectively. For 3 to 4 cm tumors, LTP occurred 19 and 29 months after MWA, respectively. There was no significant difference between the incidence of LTP between tumors of these sizes for T1a RCC (p = .82).
Relationship between LTP and tumor location
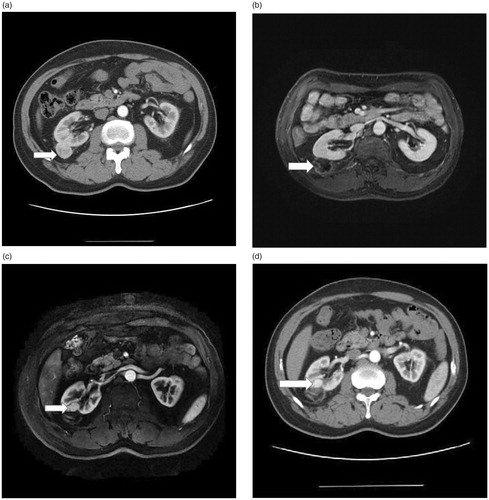
Among 171 RCC nodules treated by US-guided MWA, 50 were situated in dangerous locations, and 121 were situated in safe locations. The incidence of LTP for tumors in either safe or dangerous locations was as follows: 4 LTP tumors (8%, 4/50) were situated in dangerous locations (3 occurred in tumors adjacent to the gastrointestinal tract, and 1 occurred in a tumor protruding to the renal pelvis), and 1 LTP tumor (0.8%, 1/121) was situated in a safe location. According to χ2 test results, there was a significant difference in LTP incidence rate between tumors located in dangerous and safe locations (p = .03; . Tumors in dangerous locations were significantly (p = .03) associated with a higher LTP incidence rate compared with tumors in safe locations.
Figure 3. (a) Axial contrast-enhanced T1-weighted fat saturation MR image shows exophytic 2.7 cm clear cell RCC (arrow) located in safe location. (b) Axial contrast-enhanced T1-weighted fat saturation MR image shows no enhancement for nodular areas after US-guided percutaneous MWA. (c) (d) Axial contrast-enhanced T1-weighted fat saturation MR image and Axial contrast-enhanced CT obtained 30 months after MWA. A LTP nodular occurred in the anterior deep part of the ablation area.
Relationship between LTP and assistive technology in dangerous locations
Assistive technology was used for the 50 tumors situated in dangerous locations. Of them, protective thermal monitoring was used for 29, of which, 4 developed LTP (13.8%, 4/29). Hydrodissection was used for the remaining 21 tumors, and none of these tumors showed evidence of LTP (0%, 0/21). According to χ2 test results, there was no significant difference between use of either assistive technology and occurrence of LTP for tumors situated in dangerous locations (p = .13).
Risk factors for LTP
Nine possible tumor-related and treatment-related risk factors (age; sex; longest tumor diameter; tumor location, number and pathology type; ablation power and time) for LTP were evaluated by univariate analysis. Tumor location was a statistically significant risk factor (). Multivariate analysis () showed that tumor location (p = .043; 95% confidence interval) was the only independent risk factor associated with LTP occurrence.
Table 2. Univariate analysis of risk factors associated with local tumor progression treated by MWA.
Table 3. Independent risk factors associated with local tumor progression with Cox Proportional Hazards Model.
Table 4. Local tumor progression cease characteristic after MWA/RFA/LCA of renal malignancies.
Discussion
There have been remarkable achievements in local ablative therapies for T1a-stage RCC in recent decades. For all ablation techniques, successful treatment requires an adequate tumor-free margin and complete ablation of the viable tumor tissue. Thus, LTP is the best measure of the technical success of ablation modalities. MWA is an exciting advancement in the field of thermoablative techniques. However, little attention has been given to the incidence of LTP and related risk factors after complete MWA treatment. Because MWA can achieve higher thermal efficiency and a larger ablation zone compared with RFA [Citation25], it is possible that the incidence of and risk factors for LTP after MWA differ from that of other ablative therapies used for renal malignancies.
According to the current results (), US-guided percutaneous MWA of T1a RCC is a safe and effective technique. The incidence of LTP after MWA was 2.9% per tumor and 3.0% per patient during the median follow-up period (44 months). Compared with other MWA reports, the LTP incidence rate after MWA with image guidance for stage T1a RCC was 0.0–4.2%. Moreover, our research is consistent with other MWA studies on inactivation. Other studies with large sample sizes and long-term follow-up periods have reported the LTP incidence rate after percutaneous RFA with image guidance for T1a RCC was 2.7% and 4.2%, respectively [Citation5,Citation9]. The LTP incidence rate after percutaneous CA with image guidance for stage of T1a RCC has been reported as 1.6% and 3%, respectively [Citation9,Citation15]. Most studies of RFA, MWA, and CA have been guided by CT, and the LTP incidence rate after US-guided MWA in the present study was consistent with previous results of CT guidance for T1a RCC. Although CT density and spatial resolution is better than that of US during surgery, we used more than two types of contrast-enhanced imaging (MRI + CEUS, CT + CEUS, or CT + MRI + CEUS) to better design and evaluate our ablation option. This approach helped ensure the LTP incidence rate in the current study was the same as other CT-guided studies. Moreover, US guidance has the advantage of real-time dynamic monitoring without radiation. In previous reports [Citation26–32], the efficacies of percutaneous RFA, CA, and MWA of T1a RCC were 91–100%, 96.9–100%, and 98–100%, respectively. Likewise, the efficacy of percutaneous MWA treatment of T1a RCC in the current study was 98.2%.
Table 5. Local tumor progression studies after MWA/RFA/LCA of renal malignancies for stage of T1a reported since 2007.
To our knowledge, this is the first study to analyze risk factors for LTP after MWA of stage T1a RCC. Wah et al. reported that the most relevant risk factors for LTP after RFA or CA of T1-stage tumors were tumor location and size. Because all the patients in the present study were stage T1a RCC, there was no correlation between tumor size and LTP occurrence. However, tumor location was the most relevant risk factor for LTP after MWA, as with other studies. On the other hand, Klapperich et al. [Citation19] showed that MWA is an effective treatment method regardless of T1a RCC tumor complexity [Citation11,Citation14,Citation16]. Our LTP-related results were comparable based on a larger sample and longer follow-up compared with Klapperich et al. According to the biological behavior of RCC, development of LTP after US-guided MWA of renal tumors is rare, progresses slowly and is slow-growing (mean volume doubling time, 25.7 months; growth rate, 4.4 mm/year) [Citation33]. Hence, long-term follow-up research regarding LTP is important.
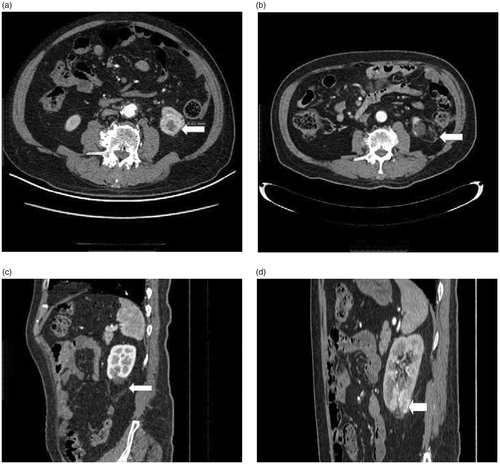
Next, we analyzed the causes of the five LTP cases. Only 1 case of LTP occurred in a safe location (. He was a 59-year-old male with clear cell RCC (longest tumor diameter of 2.7 cm), wherein LTP occurred 30 months after ablation. Due to a lack of early experience with MWA for RCC, only one needle was selected to ablate this nodule. Therefore, development of LTP in this case was attributed to our lack of understanding regarding the heat field. The remaining 4 LTP cases occurred in tumors that were situated in dangerous locations (. For these cases, we selected two needles for those whose longest tumor diameter was more than 2 cm. In three of these cases, protective thermal monitoring was used as an assistive therapy in the early stage. In 1 case, the tumor was too close to the bowel (59-year-old male with clear cell RCC; longest tumor diameter, 2.6 cm), and LTP occurred 26 months after ablation. After initial hydrodissection to separate the tumor and bowel under US guidance failed in this case, we used protective thermal monitoring. The reason for 4 LTP cases was relatively conservative in treatment in dangerous location.
Figure 4. (a) Axial contrast-enhanced CT image shows exophytic 2.6 cm clear cell RCC (arrow) located in dangerous location (The edge of the tumor is 0.5 cm from bowel). (b) (c) Axial and sagittal contrast-enhanced CT image shows no enhancement for nodular areas after US-guided percutaneous MWA. (d) sagittal contrast-enhanced CT image obtained 29 months after MWA. A LTP nodular occurred in the above of the ablation area.
Although thermal monitoring can reduce the incidence of complications after MWA, the temperature at the edge of the tumor does not reach temperatures greater than 50 °C after 4–6 min of treatment. Due to the small number of cases with tumors situated in dangerous areas in the current study, there was no significant difference between use of protective thermal monitoring and hydrodissection for tumors situated in dangerous locations that developed LTP. However, LTP did not occur in cases where hydrodissection was successfully applied, which has certain significance for US-guided percutaneous MWA of stage T1a RCC.
Three patients with LTP were treated using MWA, and another two patients underwent nephrectomy because they refused MWA. Recurrence did not occur in the three patients with LTP after MWA, and there were no instances of LTP-related deaths. LTP patients that corresponding treatment had no evident effect on patient survival compared with LTP-free patients (p = .95). These results are consistent with a previous long-term follow-up study by Georgiades et al. who documented the longest follow-up efficacy and safety of percutaneous CA for stage 1A/B RCC. Although they reported that two patients had residual disease, LTP did not affect patient survival [Citation15]. Moreover, Caputo et al. reported that local tumor recurrence after CA of RCC did not affect cancer-specific survival [Citation34]. The occurrence of LTP after US-guided MWA renal tumors is rare, progresses slowly and is slow-growing (mean volume doubling time, 25.7 months; growth rate, 4.4 mm/year) [Citation33]. Thus, in this study, we concluded that LTP after timely treatment did not affect patient survival.
Our study has some limitations. First, development of LTP after percutaneous MWA of T1a RCC is rare compared with RFA and CA, making it difficult to draw conclusions about an exact risk factor. Second, because renal tumor growth is slow, results may differ in LTP-free intervals. Thus, a longer follow-up time is necessary to more completely observe the characteristics of LTP. Lastly, our study is limited by its single-center design, especially with respect to learning how MWA may impact LTP incidence rates.
Conclusion
US-guided percutaneous MWA for T1a RCC yields a relatively low LTP occurrence rate. Although the risk of LTP significantly increases for tumors adjacent to the renal pelvis and bowel, assistive techniques, such as hydrodissection, can be used to reduce occurrence. Development of LTP after US-guided percutaneous MWA of T1a RCC tumors did not affect to patients’ survival prognoses. Considering the relatively low LTP incidence rate for T1a RCC and patient quality of life, we recommend that US-guided percutaneous MWA is considered an effective and relatively safe treatment option.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rini BI, Campbell SC. Renal cell carcinoma. Shelton, Conn.: People's Medical Pub. House; 2009.
- Gupta K, Miller JD, Li JZ, et al. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205. DOI:10.1016/j.ctrv.2007.12.001.
- Permpongkosol S, Chan DY, Link RE, et al. Laparoscopic radical nephrectomy: long-term outcomes. J Endourol. 2005;19:628–633. DOI:10.1089/end.2005.19.628.
- Crawford J, Becker PS, Armitage JO, et al. Myeloid Growth Factors, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1520–1541.
- Psutka SP, Feldman AS, McDougal WS, et al. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol. 2013;63:486–492.
- Takaki H, Yamakado K, Soga N, et al. Midterm results of radiofrequency ablation versus nephrectomy for T1a renal cell carcinoma. Jpn J Radiol. 2010;28:460–468.
- Lucas SM, Stern JM, Adibi M, et al. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol. 2008;179:75–79. discussion 79-80.
- Kim HJ, Park BK, Park JJ, et al. CT-guided radiofrequency ablation of T1a renal cell carcinoma in Korea: mid-term outcomes. Korean J Radiol. 2016;17:763–770. PubMed PMID: 27587966; PubMed Central PMCID: PMCPMC5007404.
- Thompson RH, Atwell T, Schmit G, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67:252–259.
- Atwell TD, Schmit GD, Boorjian SA, et al. Percutaneous ablation of renal masses measuring 3.0 cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. AJR Am J Roentgenol. 2013;200:461–466.
- Wah TM, Irving HC, Gregory W, et al. Radiofrequency ablation (RFA) of renal cell carcinoma (RCC): experience in 200 tumours. BJU Int. 2014;113:416–428. PubMed PMID: 24053769; PubMed Central PMCID: PMCPMC4233988.
- Balageas P, Cornelis F, Le Bras Y, et al. Ten-year experience of percutaneous image-guided radiofrequency ablation of malignant renal tumours in high-risk patients. Eur Radiol. 2013;23:1925–1932.
- Iannuccilli JD, Dupuy DE, Beland MD, et al. Effectiveness and safety of computed tomography-guided radiofrequency ablation of renal cancer: a 14-year single institution experience in 203 patients. Eur Radiol. 2016;26:1656–1664.
- Alguersuari A, Mateos A, Falco J, et al. Percutaneous radiofrequency ablation of renal tumors in high-risk patients: 10 years' experience. Radiologia. 2016;58:373–379.
- Georgiades CS, Rodriguez R. Efficacy and safety of percutaneous cryoablation for stage 1A/B renal cell carcinoma: results of a prospective, single-arm, 5-year study. Cardiovasc Intervent Radiol. 2014;37:1494–1499.
- Tsivian M, Lyne JC, Mayes JM, et al. Tumor size and endophytic growth pattern affect recurrence rates after laparoscopic renal cryoablation. Urology. 2010;75:307–310.
- Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480–488.
- Guan W, Bai J, Liu J, et al. Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol. 2012;106:316–321.
- Klapperich ME, Abel EJ, Ziemlewicz TJ, et al. Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology. 2017;284:272. PubMed PMID: 28076721; PubMed Central PMCID: PMCPMC5495130.
- Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology. 2012;263:900–908.
- Cheng Z, Yu X, Han Z, et al. Ultrasound-guided hydrodissection for assisting percutaneous microwave ablation of renal cell carcinomas adjacent to intestinal tracts: a preliminary clinical study. Int J Hyperthermia. 2018;34(3):315–320. DOI:10.1080/02656736.2017.1338362
- Breen DJ, Rutherford EE, Stedman B, et al. Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Intervent Radiol. 2007;30:936–942. PubMed PMID: 17573550; PubMed Central PMCID: PMCPMC2700242.
- Farrell MA, Charboneau JW, Callstrom MR, et al. Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. AJR Am J Roentgenol. 2003;181:1315–1317.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273:241–260. PubMed PMID: 24927329; PubMed Central PMCID: PMCPMC4263618.
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79:124–130.
- Tracy CR, Raman JD, Donnally C, et al. Durable oncologic outcomes after radiofrequency ablation: experience from treating 243 small renal masses over 7.5 years. Cancer. 2010;116:3135–3142.
- Lyrdal D, Andersson M, Hellstrom M, et al. Ultrasound-guided percutaneous radiofrequency ablation of small renal tumors: clinical results and radiological evolution during follow-up. Acta Radiol. 2010;51:808–818.
- Zagoria RJ, Pettus JA, Rogers M, et al. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011;77:1393–1397.
- Mues AC, Okhunov Z, Haramis G, et al. Comparison of percutaneous and laparoscopic renal cryoablation for small (<3.0 cm) renal masses. J Endourol. 2010;24:1097–1100.
- Rodriguez R, Cizman Z, Hong K, et al. Prospective analysis of the safety and efficacy of percutaneous cryoablation for pT1NxMx biopsy-proven renal cell carcinoma. Cardiovasc Intervent Radiol. 2011;34:573–578.
- Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer. 2008;113:2671–2680. PubMed PMID: 18816624; PubMed Central PMCID: PMCPMC2704569.
- Chalasani V, Martinez CH, Lim D, et al. Surgical cryoablation as an option for small renal masses in patients who are not ideal partial nephrectomy candidates: intermediate-term outcomes. CUAJ. 2010;4:399–402. PubMed PMID: 21191499; PubMed Central PMCID: PMCPMC2997831.
- Jilg CA, Neumann HP, Glasker S, et al. Growth kinetics in von Hippel-Lindau-associated renal cell carcinoma. Urol Int. 2012;88:71–78.
- Caputo PA, Ramirez D, Zargar H, et al. Laparoscopic cryoablation for renal cell carcinoma: 100-month oncologic outcomes. J Urol. 2015;194:892–896.