Abstract
Purpose: Invasive pulmonary aspergillosis (IPA) is a life-threatening complication of microwave ablation (MWA) during the treatment of primary or metastatic lung tumors. The purpose of this study was to investigate the clinical, radiological and demographic characteristics and treatment responses of patients with IPA after MWA.
Materials and methods: From January 2011 to January 2016, all patients who were treated by MWA of their lung tumors from six health institutions were enrolled in this study. Patients with IPA secondary to MWA were identified and retrospectively evaluated for predisposing factors, clinical treatment, and outcome.
Results: The incidence of IPA secondary to lung MWA was 1.44% (23/1596). Of the 23 patients who developed IPA, six died as a consequence, resulting in a high mortality rate of 26.1%. Using computed tomography (CT), pulmonary cavitation was the most common finding and occurred in 87.0% (20/23) of the patients. Sudden massive hemoptysis was responsible for one-third of the deaths (2/6). Most patients (22/23) received voriconazole as an initial treatment, and six patients with huge cavities underwent intracavitary lavage. Finally, 17 patients (73.9%) achieved treatment success.
Conclusions: Lung MWA may be an additional host risk factor for IPA, particularly in elderly patients with underlying diseases and in patients who have recently undergone chemotherapy. Early and accurate diagnosis of IPA after MWA is critical for patient prognosis. Voriconazole should be given as the first-line treatment as early as possible. Bronchial artery embolization or intracavitary lavage may be required in some patients.
Introduction
Microwave ablation (MWA) has been developed over the past decade as a new image-guided percutaneous thermal ablation technique for the treatment of primary and metastatic lung tumors [Citation1]. MWA, with an electromagnetic field frequency ranging from 900–2450 MHz, utilizes dielectric hysteresis to generate energy that heats tumor tissues to lethal temperatures. Heating is based on agitation of polar molecules (primarily H2O) with an oscillating electric field to induce cell death by coagulation necrosis [Citation2]. MWA offers some advantages, such as a larger ablation zone, shorter ablation duration, lower heatsinking effect and synergistic action of multiple antennas [Citation3]. Furthermore, microwaves propagate effectively through air-filled lungs that are characterized by a high impedance, low electrical conductivity and low thermal transfer [Citation4]. Based on the above merits, MWA has been increasingly applied to various stages of pulmonary primary and metastatic tumors [Citation5–13].
Despite being a minimally invasive procedure, MWA can cause major complications [Citation5–7,Citation13]. Invasive pulmonary aspergillosis (IPA), a life-threatening and common complication secondary to MWA, was rarely reported previously [Citation14]. The goals of this retrospective multicenter study were to investigate the incidence and clinical and radiologic manifestations of IPA in patients who underwent MWA and to analyze the treatment outcomes, mortality rate and possible risk factors.
Materials and methods
This was a retrospective, multicenter review of patients included in a comprehensive lung MWA database was formed by six health institutions in China. From January 2011 to January 2016, all patients who were treated by MWA of their lung tumors were enrolled in this study. Patients with IPA secondary to MWA were identified and retrospectively evaluated for predisposing factors, clinical treatment and outcome. Approval from each institutional review board and written informed consent from the patients were obtained for the analysis of lung tumor MWA.
Percutaneous microwave ablation procedure
Immediate preoperative computed tomography (CT; Lightspeed 16; GE Healthcare, Waukesha, WI) was used to design individualized treatment plans considering the tumor location, size and adjacent structures. The MTC-3C microwave ablation system (Qi Ya Research Institute of Microwave Electronic, Nanjing, China. Registration standard: YZB/country 1408–2003. No: SFDA (III) 20073251059) or the ECO-2450B microwave ablation system (ECO Microwave Electronic Institute, Nanjing, China. Registration standard: YZB/country 1475–2013. No: SFDA (III) 20112251456) was used for MWA.
Appropriate body placement, puncture sites on the body surface, puncture trajectory, and antenna number were confirmed. The following drugs were administered prior to MWA as preemptive analgesia: 10 mg of morphine via subcutaneous injection, 10 mg of diazepam via intramuscular injection and 50 mg of flurbiprofen axetil via intravenous injection [Citation15]. In addition, 2% lidocaine was injected as a local anesthesia. After achieving satisfactory anesthesia, the MWA procedure was performed by inserting the antenna (14–15 G external diameter, 100–180 mm length with a 3-cm active tip and water-circulation cooling system) in the proper position along the planned trajectory. Then, a coaxial cable was used to connect the MW antenna to an MW generator with a water-circulation cooling system, and MWA was performed. The ablation power and duration were determined with reference to the manufacturers’ recommendations. Thereafter, the MW antenna was extracted, and the puncture wound was disinfected with iodine volts and then bandaged. Vital signs were monitored continuously for 6 h after the patients’ safe return to the ward. Prophylactic cefazolin sodium (4 g intravenously every 12 h) was administered starting 12 h before and discontinued 3 days after ablation. Unenhanced chest CT was performed 24–48 h post-MWA to assess the scope of ablation and to monitor for major complications. Patients were discharged 3–5 days after MWA if they recovered well. Changes in clinical condition were monitored via a follow-up CT examination, symptom enquiry, physical examination and laboratory testing.
Case definitions of IPA ()
IPA was defined according to the criteria of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group [Citation16] and was classified into one of three groups: proven, probable and possible. Proven IPA required proof demonstrating the presence of fungal elements in diseased tissue by microscopic analysis or by the culture of sterile material. Probable IPA required the presence of a host factor, a clinical criterion and a mycological criterion. Cases fulfilling the criteria for a host factor and a clinical criterion but that lacked a mycological criterion were considered possible IPA. In accordance with the guidelines, IPA assumes a diagnostic certainty of proven or probable cases [Citation16,Citation17]. In the present study, only patients with proven or probable IPA were included.
The response to antimycotic treatment was assessed using criteria established by the Mycoses Study Group and European Organization for Research and Treatment of Cancer [Citation18]. Treatment success was defined as a ≥25% reduction in the diameter of radiological lesions, plus patient survival, improvement of disease-related symptoms, and documented clearance of infected sites. Treatment failure was defined as patient survival with no/minor improvement in disease-related symptoms plus a 0–25% reduction in lesion diameter, deteriorating disease-related clinical symptoms plus new sites of disease or radiological exacerbation of preexisting lesions, persistent isolation of mold species from infected sites, or patient death during the prespecified period of evaluation.
Inclusion and exclusion criteria of IPA associated with MWA, and data collection
Inclusion criteria were as follows: (a) patients undergoing percutaneous lung MWA during the past two months; (b) symptoms of lower respiratory tract infection (fever, cough, expectoration, hemoptysis, chest pain, dyspnea, pleural effusion); (c) confirmed signs of infection at the ablation site by imaging; (d) a positive Aspergillus result by sputum smear and culture, smear and culture of aseptic specimens derived from the ablation zone, or galactomannan antigen detection; (e) the initial exclusion of a bacterial infection; (f) poor effect of broad-spectrum antibacterial treatment; (g) proven and probable cases of IPA; (h) good efficacy of the appropriate empirical therapy against molds. Exclusion criteria were: (a) infection that occurred more than 2 months after MWA; (b) infection of a non-ablation site; (c) the absence of aforementioned mycological evidence; (d) positive bacterial cultivation results from the original specimen; (e) excellent efficacy following antibacterial treatment.
From the hospitals’ data processing systems, we extracted and examined the hospital medical records, discharge reports and radiographic results of all participants. We recorded the demographic features, smoking history, comorbidities, pulmonary function test results, lung tumor characteristics, radiotherapy or chemotherapy histories, MWA details, clinical symptoms of IPA, laboratory test results, the evolution of imaging findings, hospitalization periods, responses to treatment and outcomes. Definitive diagnosis of proven and probable IPA was established by a multidisciplinary tumor board that included a medical oncologist, respiratory physician, radiologist, microbiologist, clinical laboratory technician and pathologists.
Statistical analysis
Continuous variables were summarized as the mean ± standard deviation (SD) and were assessed using an independent-samples t-test. Proportions were calculated for categorical variables. If a categorical variable was not fit for the χ2 test because 1 or more cells in a crosstab had an expected count of less than 5, then the 2-sided Fisher exact test was adopted. A p values of less than .05 was considered statistically significant. All statistical analyses were performed using SPSS version 17.0 packaged software (SPSS, Inc, Chicago, IL).
Results
A total of 1596 patients underwent CT-guided percutaneous MWA of lung tumors, as shown in . Of these, 23 (1.44%) developed proven (n = 6) or probable (n = 17) IPA, including nineteen men and four women, with a mean age of 64.5 ± 7.4 (range: 50.0–79.0) years. All 23 patients with IPA had histologically confirmed primary lung cancers (11 adenocarcinomas, 10 squamous carcinomas and two indeterminate tumors) spanning all TNM stages from I to IV (2 cases of Ia, 4 cases of Ib, 2 cases of IIa, 1 case of IIb, 4 cases of IIIa, 5 cases of IIIb and 5 cases of IV).
Table 2. Demographic and clinical characteristics of patients.
Tumor ablation and complications
The characteristics of the tumor, the ablation and the complications that arose due to the ablation in the 23 patients with IPA are shown in . A total of 25 lesions were ablated including ablation of single (n = 21) and double lesions (n = 2). Single-spot ablation (one antenna) was used for tumors with a maximum diameter of ≤3.0 cm while multiple-spot ablation (two antennae) was used for tumors >3.0 cm in diameter. Complications experienced after ablation, according to the classification of the Imaging-guided Tumor Ablation International Working Group of the Society of Interventional Radiology [Citation19], included the following: post ablation syndrome (n = 16, which was treated with nonsteroidal drugs, when necessary); pneumothorax (n = 9, of which six required catheterization for closed drainage); pleural effusion (n = 10, of which five underwent thoracentesis); hemoptysis (n = 3, which was managed with the appropriate administration of hemostatic drugs); and atelectasis (n = 3, which required no special treatment).
Table 3. Tumor, ablation, and complication characteristics of 25 lesions in 23 patients with IPA.
Clinical characteristics, diagnosis, treatment and prognosis of patients with IPA
The clinical characteristics of patients with IPA including symptoms and laboratory and radiological findings are shown in . It should be noted that the sputum of most patients was characterized as being smoke gray in color and containing a floc. Of the six cases of proven IPA, one was verified by visualization of the hyphae via direct microscopy and five were verified by positive culture of intracavitary drainage under sterile conditions.
Table 4. Symptoms and laboratory and radiological findings of 23 patients with IPA.
Regarding antifungal treatment, 22 cases initially received intravenous voriconazole while one case received itraconazole. Once clinical improvement was demonstrated with intravenous voriconazole, patients were discharged with oral itraconazole (n = 5) or oral voriconazole (n = 17). Six patients required intracavitary lavage due to necrotic liquefaction and five required thoracic drainage due to pleural effusion and bronchopleural fistula. Both procedures were performed via percutaneous catheterization. Bronchial artery embolization was performed in one patient to manage severe hemoptysis. Six patients (26.1%) died before completion of treatment and their treatment responses were categorized as failures. Sudden massive hemoptysis was responsible for a third of the deaths (2/6). Based on clinical and radiological improvement, 17 patients (73.9%) achieved treatment success, including five who died from tumor recurrence or metastasis, rather than IPA. In patients with treatment success, the mean duration of treatment was 44.6 days (vs. 18.5 days in those with treatment failure). Also of note, secondary bacterial infections developed in eight patients during antifungal treatment ().
Table 5. Diagnosis, antifungal therapy, and outcomes in 23 patients with IPA after lung MWA.
Discussion
Aspergillus, a saprophytic fungus, is a ubiquitous, hardy organism that grows best on organic debris and in humid environments while its natural ecological niche is the soil [Citation20]. Aspergillus releases thousands of airborne conidia into the atmosphere that can easily be inhaled into the distal airways and alveoli. In immunocompetent hosts without underlying lung disease, these conidia are normally eliminated by mucociliary clearance and innate immune mechanisms [Citation21]. MWA destroys tumors and a small amount of the surrounding normal lung tissue. Necrotic tissue debris in ablation sites offers excellent conditions for germination of dormant spores. Although hundreds of Aspergillus species have been identified, few have been implicated in human disease. A. fumigatus is the most common pathogenic species, accounting for 90% of the pulmonary aspergillosis cases. Although less common, A. terreus, A. flavus and A. niger also contribute to the pulmonary aspergillosis incidence [Citation22], as demonstrated in the present study.
IPA primarily occurs in severely immunocompromised patients such as those with a hematologic malignancy, inherited immune deficiencies, connective tissue disease, or that are undergoing immunosuppressive therapy or have had a solid organ transplant [Citation16]. Recent studies have revealed additional risk factors including critical illness, malnutrition, end-stage liver disease, alcoholic hepatitis, COPD, chemotherapy and DM [Citation23–25]. In COPD patients that are heavy smokers, decreased mucociliary clearance of the airway delays the elimination of Aspergillus spores and long-term use of an inhaled corticosteroid weakens the local immunity thereby increasing the incidence of IPA. In this study, the IPA incidence after MWA was significantly increased in patients with COPD and in those that smoke (p <.05). In addition, chemotherapy is known to lead to neutropenia and to a decline in systemic immunity, thus facilitating the occurrence of IPA. These observations were also found to be consistent with our findings.
The clinical symptoms and routine blood test used to diagnose IPA are often nonspecific and indistinguishable from a bacterial infection. The most common sign of IPA is a persistent fever that responds poorly to broad-spectrum antibiotic treatment [Citation26]. Smoke-gray sputum that contains a floc is characteristic of IPA and may aid in IPA diagnosis. In this study, significant expectoration was attributed to the giant necrotic cavitation that was observed in 87.0% of the cases. Massive hemoptysis, attributable to the potent invasive ability of Aspergillus hyphae into the bronchial arterioles, was a life-threatening symptom. Chest CT provided evidence of IPA which included dense, well-circumscribed lesion(s) with/without a halo sign of ground glass attenuation, air-crescent signs and cavity formation [Citation16,Citation27]. A retrospective study demonstrated that macronodules (94%), a halo sign (61%), consolidation (30%), infarct-shaped nodules (27%), cavitary lesions (20%) and air crescent signs (10%) were present on chest CT imaging [Citation28]. In our study, the most common chest CT imaging feature, cavitation with uneven walls within a mass of irregular nodules or consolidation (87.0%), was observed approximately 3–4 weeks after MWA (). The cavity size was generally larger than that of GGO after MWA (7.36 ± 1.94 cm vs 6.44 ± 1.76 cm, p <.05), signifying the strong aggressive capability of Aspergillus. Other radiological findings included infiltration, nodules, consolidation and BRF, and these findings often coexisted ().
Figure 1. (a) A contrast-enhanced CT scan showing a single neoplasm, 3.5 × 2.4 cm, adjacent to mediastinal pleural in the right upper lobe before MWA. (b) MWA was performed at a power of 70 W for an accumulated total time of 11 min. (c) A follow-up CT scan at 24 hours after MWA showing a GGO-like reaction band around the lesion that almost surrounded the entire tumor. (d) At 28 days after MWA, the ablation zone was replaced by a large uneven thick-walled cavity containing a mass of irregular consolidation surrounded by patchy infiltration. (e) A chest CT scan at 6 weeks after MWA showing shrinkage of the cavity, incrassation of the wall, and aggravated infiltration. (f) A chest CT scan at 6 weeks after MWA also showing multiple patchy infiltrations and nodules scattered in both lung fields.
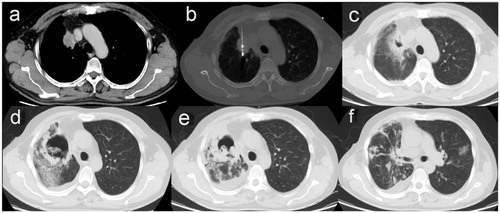
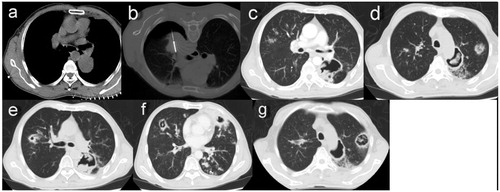
Figure 2. (a) A chest CT image showing a primary tumor 4.5 cm in diameter abutting the thoracic aorta and left lower pulmonary artery in the superior segment of the left lower lobe. (b) Despite a mild pneumothorax, MWA was successfully completed with a power of 70 W for a total of 21 min. (c) A CT scan 3 weeks after MWA showing a thin-walled cavity with an irregular luminal surface in the ablation zone. (d) The same CT scan 3 weeks after MWA showing coexistence of the cavity and nodules with a halo sign in the left upper lobe. (e) The same CT scan 3 weeks after MWA showing consolidation with cavitation in the right upper lobe and an uneven think-walled cavity containing pedunculated contents in the left lower lobe. (f) A CT scan 5 weeks after MWA showing diffuse consolidations with cavitation, infiltrations and nodules in bilateral lungs. (g) The CT scan 5 weeks after MWA showing the cavity emptying inside, as well as nodules enlarged and cavitated in the left upper lobe.
In this study, clear diagnosis of IPA depended on microbiological evidence that was mainly obtained by sputum smear and culture or by intracavitary lavage smear and culture. However, negative smear cultures do not exclude IPA when it is highly suspected clinically [Citation29], thus a serum GM test may aid IPA diagnosis. GM is released by Aspergillus spp. during hyphal growth as opposed to the conidia, hence GM testing potentially allows for differentiation between an active infection and colonization [Citation30–32]. A positive serum GM test combined with a pathogenic culture of specimens was conducted to distinguish between the Aspergillus species and exclude colonization.
At present, there are mainly three classes of antifungal agents active against Aspergillus spp.: triazoles, polyenes and caspofungin [Citation23,Citation33]. Voriconazole and itraconazole are triazoles, while amphotericin B is a polyene macrolide antibiotic. Voriconazole and amphotericin B are the initial treatments of choice for invasive aspergillosis, whereas caspofungin is only approved for salvage treatment [Citation17,Citation23,Citation33]. In an international, multicenter randomized open-label trial, voriconazole was compared with amphotericin B deoxycholate as an initial treatment. Voriconazole proved beneficial in terms of the response rate (53% vs. 32%), mortality rate (29% vs. 42%) and with respect to the rates of severe adverse reactions [Citation34,Citation35]. Hence, voriconazole is recommended for first-line treatment of IPA [Citation17]. Voriconazole is initially dosed intravenously (6 mg/kg every 12 h for one day and then 4 mg/kg every 12 h) and is then dosed orally when clinical improvement is observed. Oral itraconazole (400–600 mg/d) may serve as an alternate therapy once clinical improvement has been demonstrated with intravenous voriconazole [Citation28].
In cases of IPA that were complicated by massive hemoptysis that threatened clinical stability, bronchial artery embolization [Citation23] was an appropriate treatment option. In addition, intracavitary lavage by catheterization may be appropriate for some patients with huge cavities accompanied by inadequate drainage. Voriconazole was used to lavage cavities in this study, as opposed to amphotericin [Citation36]. In the present study, the mortality rate of IPA was attributed to massive hemoptysis and multiple organ failures that were induced by Aspergillus infection with/without secondary bacterial infection.
Conclusions
In conclusion, lung MWA may be an additional host risk factor for IPA, particularly in male patients with underlying diseases (especially COPD), heavy smokers or in patients who are undergoing perioperative chemotherapy. Early and accurate diagnosis of IPA after MWA was critical for the prognosis of patients. IPA should be considered in patients post-MWA that present with fever, cough that produces smoke-gray sputum, cavitation with/without infiltration on chest CT, positive Aspergillus culture, positive GM test results and poor response to broad-spectrum antibiotics. Furthermore, voriconazole, rather than itraconazole, should be used as the first line treatment and should be initiated as early as possible. Bronchial artery embolization or intracavitary lavage may be required. In this study, the relatively low incidence of IPA limited the analysis and identification of additional susceptibility and influencing factors. The participation of additional research institutions is required to expand the sample size. In addition, as a multicenter retrospective study, a minor bias might exist in selection of the IPA cases, which may have a slight effect on the results.
Disclosure statement
No potential conflict of interest was reported by the authors.
Table 1. Criteria for proven and probable invasive pulmonary aspergillosis.
References
- Schneider T, Heussel CP, Herth FJ, et al. Thermal ablation of malignant lung tumors. Dtsch Arztebl Int. 2013;110:394–400.
- Song Z, Qi H, Zhang H, et al. Microwave ablation: results with three different diameters of antennas in ex vivo bovine and in vivo porcine liver. J Cancer Res Ther. 2017;13(5):737–741.
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192–S203.
- Wasser EJ, Dupuy DE. Microwave ablation in the treatment of primary lung tumors. Semin Respir Crit Care Med. 2008;29:384–394.
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247:871–879.
- Carrafiello G, Mangini M, Fontana F, et al. Microwave ablation of lung tumours: single-centre preliminary experience. Radiol Med. 2014;119:75–82.
- Belfiore G, Ronza F, Belfiore MP, et al. Patients’ survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol. 2013;82:177–181.
- Wei Z, Ye X, Yang X, et al. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38:135–142.
- Acksteiner C, Steinke K. Percutaneous microwave ablation for early-stage non-small cell lung cancer (NSCLC) in the elderly: a promising outlook. J Med Imaging Radiat Oncol. 2015;59:82–90.
- Yang X, Ye X, Huang G, et al. Repeated percutaneous microwave ablation for local recurrence of inoperable Stage I nonsmall cell lung cancer. J Cancer Res Ther. 2017;13(4):683–688.
- Li B, Wang Z, Zhou K, et al. Safety and feasibility within 24 h of discharge in patents with inoperable malignant lung nodules after percutaneous microwave ablation. J Cancer Res Ther. 2016 Dec;12(Suppl):C171–C175.
- Ni Y, Bi J, Ye X, et al. Local microwave ablation with continued EGFR tyrosine kinase inhibitor as a treatment strategy in advanced non-small cell lung cancers that developed extra-central nervous system oligoprogressive disease during EGFR tyrosine kinase inhibitor treatment: a pilot study. Medicine (Baltimore). 2016;95:e3998.
- Zheng A, Wang X, Yang X, et al. Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg. 2014;98:243–248.
- Huang G, Liu Q, Ye X, et al. Invasive pulmonary aspergillosis: a rare complication after microwave ablation. Int J Hyperthermia. 2014;30:412–417.
- Ong CK, Lirk P, Seymour RA, et al. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. 2005;100:757–773.
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821.
- Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360.
- Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47:674–683.
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–739.
- Perfect JR, Cox GM, Lee JY, et al. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis. 2001;33:1824–1833.
- Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350.
- Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus–what makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013;9:e1003743.
- Patterson KC, Strek ME. Diagnosis and treatment of pulmonary aspergillosis syndromes. Chest. 2014;146:1358–1368.
- Liss B, Vehreschild JJ, Bangard C, et al. Our 2015 approach to invasive pulmonary aspergillosis. Mycoses. 2015;58:375–382.
- Chabi ML, Goracci A, Roche N, et al. Pulmonary aspergillosis. Diagn Interv Imaging. 2015;96:435–442.
- Schwartz S, Thiel E. Clinical presentation of invasive aspergillosis. Mycoses. 1997;40 (Suppl 2):21–24.
- Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–379.
- Samarakoon P, Soubani A. Invasive pulmonary aspergillosis in patients with COPD: a report of five cases and systematic review of the literature. Chron Respir Dis. 2008;5:19–27.
- Denning DW. Chronic forms of pulmonary aspergillosis. Clin Microbiol Infect. 2001;7:25–31.
- Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42:1417–1427.
- Hage CA, Knox KS, Davis TE, et al. Antigen detection in bronchoalveolar lavage fluid for diagnosis of fungal pneumonia. Curr Opin Pulm Med. 2011;17:167–171.
- Metan G, Koç AN, Atalay A, et al. What should be the optimal cut-off of serum 1,3-β-D-glucan for the detection of invasive pulmonary aspergillosis in patients with haematological malignancies? Scand J Infect Dis. 2012;44:330–336.
- Bassetti M, Pecori D, Della Siega P, et al. Current and future therapies for invasive aspergillosis. Pulm Pharmacol Ther. 2015;32:155–165.
- Blot F, Edé C, Nitenberg GM. Voriconazole versus amphotericin B for invasive aspergillosis. N Engl J Med. 2002;347:2080–2081.
- Karthaus M. Voriconazole versus amphotericin B for invasive aspergillosis. N Engl J Med. 2002;347:2080–2081.
- Kravitz JN, Berry MW, Schabel SI, et al. A modern series of percutaneous intracavitary instillation of amphotericin B for the treatment of severe hemoptysis from pulmonary aspergilloma. Chest. 2013;143:1414–1421.
