Abstract
Introduction: The targeting of hepatocellular carcinomas (HCC) in the hepatic dome can be challenging during percutaneous thermal ablation (PTA). The aims of this study were (1) to evaluate the safety and efficacy of PTA of HCC in the hepatic dome that cannot be visualized under US, using artificial CO2 pneumothorax and CT-guidance and (2) to compare the results with US-visible HCC located in the liver dome treated under US-guidance.
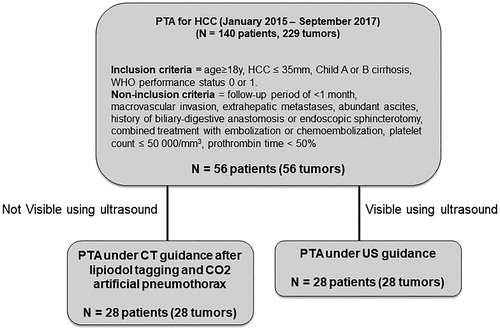
Materials: Over a 32-month period, 56 HCC located in the hepatic dome were extracted from a prospectively maintained database. Twenty-eight cases (US-guidance group) were treated under US-guidance, while the others (n = 28, CT-CO2 group) were treated under CT-guidance using artificial CO2 pneumothorax after lipiodol tagging of the tumor. The primary technical success and complications rates of this technique were retrospectively assessed. Local tumor progression (LTP), intrahepatic distant recurrence (IDR), local recurrence-free survival (LRFS) and overall survival (OS) were also compared between both groups.
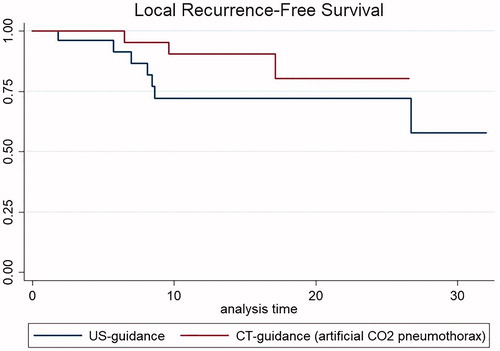
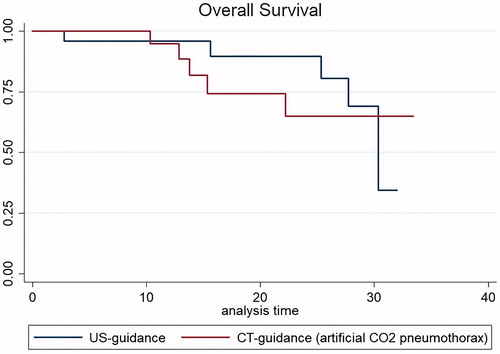
Results: Primary technical success was 100% in both groups. No major complications occurred. After a median follow-up of 13.8 months (range, 1–33.4 months), LTP occurred in 10.7% (3/28) in CT-CO2 vs. 25% (7/28) in the US-guidance group (p = NS). IDR occurred in 39.3% (11/28) in CT-CO2 vs. 28.6% (8/28) in the US-guidance group (p = NS). Death occurred in 17.9% (5/28) of patients in both groups. LRFS and OS did not significantly differ using Kaplan-Meier survival estimates.
Conclusion: CT-guided PTA after artificially induced CO2 pneumothorax is a safe and efficient technique to treat HCC located in the hepatic dome.
Introduction
Percutaneous thermal ablation (PTA) is a validated curative treatment option, that is, usually performed as first-line therapy for very-early and early stages of hepatocellular carcinoma (HCC) [Citation1]. However, this procedure cannot be performed in ∼30% of patients with HCC who are candidates for PTA because of a high-risk location of the tumor, a high-risk patient, or because the targeted nodule is not visible on ultrasound (US) [Citation2]. Thanks to the real-time visualization and guidance of the needle(s) and target, accurate and precise positioning of the needle(s) and because it is easily accessible, US remains the modality of choice for needle guidance during PTA [Citation3,Citation4].
Certain areas of the liver, such as the dome, are known to be particularly difficult to target during PTA. Indeed, the interposition of the lung frequently affects tumor visibility and accessibility. In these cases, PTA may be contraindicated by some interventional radiologists (IR) due to the risk of complications from diaphragmatic and/or pleural injury [Citation5], while others will prefer CT-guidance. Although tumor visibility can be improved during CT by injecting ethiodized oil into liver arteries to tag the tumor [Citation6], access to tumors in the liver dome remains difficult with this technique because of lung interposition. Although several techniques, such as gantry angulation [Citation7], artificial ascites [Citation8–10], the epicardial fat pad approach [Citation11], or artificial pleural infusion [Citation12], have been described they have rarely been compared with standard US-guidance.
In 2005, De Baere et al. described an artificially induced pneumothorax procedure to clear the lung from the needle path [Citation3]. This technique involved introducing air into the pleural cavity, which was reached using an 18 G epidural needle. This technique has been shown to be safe in 6 patients. We used a slightly modified technique in patients with HCC located in the dome of the liver, and not visible under US, to create an artificial CO2 pneumothorax with a Veress needle.
The aims of this study were to (1) evaluate the safety and efficacy of PTA of HCC located in the hepatic dome and non visible at US using artificial CO2 pneumothorax and CT-guidance and (2) assess the performance of PTA with this technique compared with PTA of US-visible HCC located in the liver dome treated under US-guidance.
Materials and methods
Study design and patient selection
We retrospectively analyzed all patients from a prospectively maintained database with HCC located in the hepatic dome who underwent PTA in our department from January 2015 to September 2017. The ‘hepatic dome’ was defined as the interposition of lung parenchyma between the skin and the liver tumor through an anterior or lateral route on axial CT or MRI images [Citation4].
Inclusion criteria for the study were (i) age ≥18 years, (ii) HCC diagnosed either by pathology or by imaging according to European Association for the Study of the Liver (EASL) guidelines [Citation1], (iii) HCC ≤35 mm located in the hepatic dome, (iv) Child A or B cirrhosis, (v) WHO performance status 0 or 1. Noninclusion criteria were: follow-up period of <1 month, macrovascular invasion, extrahepatic metastases, abundant ascites, history of biliary-digestive anastomosis, or endoscopic sphincterotomy, combined treatment with embolization or chemoembolization, platelet count ≤50,000/mm3, prothrombin time <50%. A tumor board including IR, liver surgeons, oncologists, and hepatologists validated the therapeutic strategy for all patients included in the study, based on contrast-enhanced multiphasic CT and/or magnetic resonance imaging (MRI) performed within 1 month before the procedure. This retrospective study was approved by our institutional review board (Local Ethics and Research Committee: CLER, authorization number 2017_CLER-MTP_12–04). Informed consent for both the procedure and prospective anonymized data collection was obtained from all patients.
Percutaneous thermal ablation
All procedures were performed by the same IR (whatever the technique) with the patient under general anesthesia with endotracheal intubation. During the procedure, patients were mechanically ventilated in controlled volume mode. Tidal volume was set between 3 and 4 ml per kg of predicted body weight and respiratory rate was adjusted to maintain an end tidal CO2 between 35 and 45 mmHg. All procedures were performed in a multimodal interventional suite. From January 2015 to February 2017, we worked on a CT-scan (Optima 660, General Electric, Milwaukee, USA) with a combined mobile C-arm (General Electric, Milwaukee, USA). Since February 2017, we used an Angio-CT system (Infinix-I 4DCT, Canon Medical Systems, Tokyo, Japan). A US machine (Logiq E9, General Electric, Milwaukee, USA) was also available in both suites. PTA was performed using a radiofrequency or a microwave device depending on the operator’s choice and level of expertise:
Radiofrequency ablations were performed using a separable clustered electrode (Octopus®, STARmed, Goyang, Korea) and a three-channel dual-generator (VIVA Multi®, STARmed). The separable clustered electrode included three internally cooled 17 G electrodes. One or two electrodes were used at the discretion of the IR depending on tumor size and accessibility. When two electrodes were needed, the generator was used in dual-switch mode [Citation13].
Microwave ablations were performed using a single 15 G internally cooled electrode with the Acculis MTA system (Angiodynamics, Amsterdam, Netherlands). Output of the generator was up to 140 W at continuous wave of 2450 MHz.
The aim of treatment was to obtain complete ablation of the tumor with a margin of 5–10 mm. Track ablations were performed according to the manufacturer’s instructions.
Tumor tagging technique
When the HCC nodule was not visible under US or unenhanced CT-scan, we performed tumor tagging as follows: an angiographic catheter (4 or 5 F) was inserted through a femoral artery to catheterize the coeliac trunk or the superior mesenteric artery. Then, a 2.7 F microcatheter (Progreat, Terumo, Tokyo, Japan) was used to reach the right or left hepatic branch, depending on anatomical variants and the location of HCC. A 1.5–2 ml volume of lipiodol (Lipiodol ultrafluide, Guerbet, Aulnay-sous-bois, France) was injected nonselectively in this lobar arterial branch to tag the tumor, as demonstrated on an immediate postinjection CT acquisition.
Technique of artificial CO2 pneumothorax
We used this technique each time the tumor was not visible under US (otherwise, we used US guidance). Under CT-fluoroscopy, a 14 G 150 mm-long Veress needle (pneumoperitoneum needle; Landanger, Chaumont, France), connected to a 5 l bottle filled with medical-quality CO2 (Air Liquide Santé, Paris, France) and a three way stop-cock (), was inserted using an anterior approach. The Veress needle () is a blunt-tipped needle with a spring-loaded inner stylet and a sharp outer needle to penetrate tissue. When the needle is advanced through the chest wall, the blunt-tip stylet is retracted to allow the needle to penetrate the tissue. However, when the outer needle reaches any space (i.e., between fascia and muscle, mediastinum space, pleural space,…), the blunt stylet can protrude beyond the sharp tip of the needle, thus preventing further needle damage. A “click” is heard when the blunt stylet protrudes. We then inject 20–30 cc CO2 to determine the location of the Veress needle on CT fluoroscopy. If this is not the pleural space, the needle is advanced further the needle until the next “click” and then 20–30 cc CO2 is again injected and the location of the needle is checked. When the needle is correctly in place, we inject between 200 and 2000 cc CO2 to create an artificial CO2 pneumothorax to clear the lung parenchyma from the path of the ablation needle. The antenna or electrode(s) are then inserted into the tumor under CT guidance on the axial plane using an extrapulmonary transthoracic transdiaphragmatic approach (). The nontumoral parenchyma is systematically interposed between the liver capsule and the tumor for subcapsular tumors. At the end of the procedure, intrapleural CO2 is withdrawn through the Veress needle using a 60 cc syringe and evacuated into the room using the 3-way tap. This manoeuvre is repeated until complete or quasi-complete resolution of CO2 pneumothorax.
Figure 1. Pictures of a bottle of medical quality CO2 (A) and of Veress needle connected with 60 ml syringe through a 3-way tap (B left) with zoomed view on the extremity of Veress needle (B right).
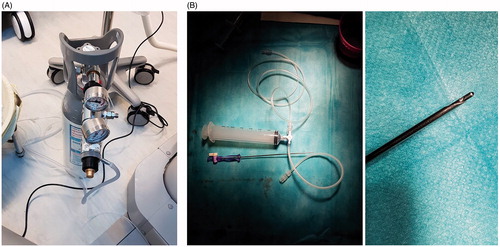
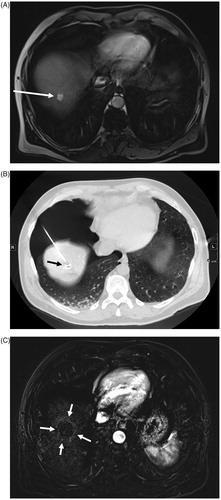
Figure 2. HCC (arrow) located in the dome (unvisible on US) on arterial T1-weighted MR image (A). PTA after HCC (arrow) tagging using lipiodol arterial injection and artificial CO2 pneumothorax (B). Follow-up 3 months after PTA showing the ablation zone (arrows) on subtraction T1-weighted MR image at the arterial phase (C).
Outcomes
The type and number of complications were recorded and classified according to Society of Interventional Radiology guidelines [Citation14]. Contrast-enhanced CT was performed immediately after the procedure to assess the ablation zone (i.e., area of low attenuation) and look for postprocedural complications. If PTA was incomplete, another session was immediately performed to achieve complete ablation during the same procedure. Primary technical success was defined as complete ablation of the target tumor, and secondary technical success was defined as complete ablation of the target tumor after repeated PTA [Citation15]. Patients were monitored overnight and were discharged on day one in the absence of complications. Patient follow-up was performed by radiologists specialized in both diagnostic and interventional liver oncology. All patients underwent a medical consultation and multiphasic liver MRI 6–8 weeks after the procedure and then every 3 months (for the first 2 years), and every 6 months (after 2 years) thereafter.
Treatment failure was defined as the presence of persistent enhancing foci at the tumor site on the first MR follow-up (i.e., at 6–8 weeks) [Citation15], while complete ablation during this first MR follow-up was considered to be a treatment success. Local tumor progression (LTP) was defined as any growing and enhancing tumor foci within or at the edge of (direct contact) the ablation zone, after at least one follow-up visit had confirmed complete ablation. Intrahepatic distant recurrence (IDR) was defined as any occurrence of a new nodule of HCC in the liver defined by EASL criteria [Citation15].
Statistical analysis
Categorical variables were described by percentages. Continuous variables were expressed as means ± SD or medians (range). Group comparisons were made using the Fischer’s exact test for categorical variables and a two-sided t-test or Kruskall-Wallis test as appropriate for continuous variables. Local recurrence-free survival (LRFS) was defined as the time from PTA to local tumor recurrence. Patients who had not experienced local recurrence at the last follow-up visit or who died were censored at the date of last follow-up or death. Overall survival (OS) was defined as the time from PTA to death (by any cause). Survival curves were estimated using the Kaplan-Meier method and compared using log-rank tests. All analyses were performed using Stata software version 14.0 (Stata corporation, College Station, TX, USA); p < .05 was considered to be significant.
Results
Patients
Fifty-six/229 HCC (24%) treated using PTA during the study period, were located in the hepatic dome and fulfilled inclusion/exclusion criteria for the study (). Half of them (n = 28) were visible on US and therefore treated by US-guidance, while the other half (n = 28) required CT-guided artificial CO2 pneumothorax PTA. Patients characteristics are shown in . Median age was 63 years old (range: 39–86) and 83.9% (47/56) were men, ASA 1–2 in 57.1% (32/56) of cases. PTA was the first HCC treatment in 39.3% (22/56) patients. All patients had cirrhosis, mainly of alcoholic origin (42.9%), Child-Pugh classification A in 98.2% (55/56). Median tumor size was 14 mm (range: 8–33). HCC was subcapsular in 32.1% (18/56). Microwave ablation was performed in 69.6% (35/56) of patients. None of the characteristics differed between both groups except for the type of imaging guidance and use of lipiodol tagging.
Table 1. Characteristics of the 56 included patients with HCC located at the hepatic dome.
Safety and tolerance of artificial CO2 pneumothorax
No grade 3 or higher complications were observed during or following the procedure. No modification in ventilation parameters was required during the procedure with artificial CO2 pneumothorax despite the use of low tidal volume ventilation. Oxygen saturation remained high and stable in all patients. No thoracic drain was left in place after the procedure. No case of pneumothorax, hemothorax, alveolar bleeding, or tumor seeding was reported. Moreover, no complications related to the femoral access were observed. All patients who underwent PTA following artificial CO2 pneumothorax were discharged the day after treatment (n = 100%, 26/26).
Outcomes
Data on primary technical success, follow-up, LTP, IDR, and patient death are shown in . Primary technical success was 100% in both groups. Median follow-up was 13.8 months (range: 1–33.4 months) and did not differ between the groups (13.1 months in CT-guidance group vs. 16.8 months in US-guidance group, p = NS). No differences in LTP, IDR, or patient death were observed between the groups.
Table 2. Outcomes of percutaneous thermal with CT-guidance after artificial CO2 pneumothorax compared to US-guidance.
Although there was trend towards longer LRFS in the CT-guidance (artificial CO2 pneumothorax) group using Kaplan-Meier survival estimates, LRFS, and OS did not significantly differ between the groups ( and ).
Discussion
This study shows that PTA can be safely performed to treat HCC in the hepatic dome using CT-guidance and artificial CO2 pneumothorax. The efficacy and patient outcome with this technique were similar to the standard technique, i.e., US-guided PTA for HCC in the same location. The PTA approach in our study improves the feasibility of treating nonvisible HCC in the hepatic dome and the clinical impact is important, because it improves access to liver lesions in areas that are usually difficult to treat. This technique could also allow treatment of earlier stage HCC because the visibility of liver lesions by US guidance is closely linked to the location and the size of the lesion. The high primary technical success rate without any complication confirm that this approach is safe. Moreover, the LTP rate (10.7%) after a median follow-up of 13.1 months was consistent with rate reported in large series of unselected HCC PTAs [Citation16,Citation17], and the IDR rate (39.3%) was close to that reported in previous studies [Citation18–20]. Interestingly, LTP and IDR rates, as well as LRFS and OS between patients treated under US-guidance and CT-guidance with artificial CO2 pneumothorax did not differ.
The technique of artificially induced pneumothorax was first described in 6 patients by De Baere et al. [Citation3]. In this article, the authors used a 18 G epidural needle to access the pleural space and injected air through a microporous system to create the pneumothorax. Although they reported a technical success rate of 100% without any complications, we chose (as suggested in their discussion section) a Veress needle to access the pleural cavity. Because of the needle design, <1 min was necessary to reach the pleural cavity compared to up to 8 min with the 18 G epidural needle [Citation3]. The Veress needle, which was developed in 1932 by Dr. Jano Veress to create pneumothorax to collapse lungs infected by tuberculosis, is now mainly used to induce pneumoperitoneum [Citation21]. A disposable Veress needle is an inexpensive and safe material to access the pleural cavity because of its blunt-stylet design that prevent tears in the visceral pleura or the lung parenchyma, which may occur with an 18 G epidural needle. Thus, there is no risk of alveolar bleeding or persistent pneumothorax compared to the transpulmonary approach [Citation22].
In a study by Fujiwara et al. [Citation23], artificial pneumothorax was performed in 16 patients for thermal ablation of HCCs located in the hepatic dome. They showed the good safety profile of this technique but here again, they used an epidural needle to access the pleural cavity, and air to create the pneumothorax. There is a potential risk of air embolism with the use of air for pleural insufflation [Citation24]. Moreover, air must be removed at the end of the procedure. Interestingly, CO2 is highly soluble (20 times greater than oxygen) in the blood. It can be safely injected in the pleural cavity [Citation25] and does not necessarily need to be aspirated at the end of the procedure. Artificial pneumothorax using CO2 insufflation has already been used for thermal protection of extrathoracic organs during radiofrequency ablation [Citation26,Citation27], for biopsy of the adrenal gland with lung interposition [Citation25], or for pain relief during RFA of peripheral lung tumors [Citation28]. This technique is safe, even in the context of low tidal volume ventilation used to limit liver movements. Indeed, oxygen saturation has never been affected in our 28 patients who had low tidal-volume ventilation during general anaesthesia. To note that expired CO2 monitoring can be influenced by the solubilization of intrapleural CO2 in the blood then evacuated by the respiratory system.
Several limitations to our study must be acknowledged. First, this is a retrospective study, although the data came from a prospectively maintained database. Second, the number of patients enrolled is relatively low but we aimed to select patients based on a clear definition of the hepatic dome. The term hepatic dome usually refers to the liver parenchyma close to the diaphragm [Citation4]. However, precise anatomical limits remain unclear. We chose to define a tumor located at the hepatic dome when the lung interposes between the skin and the tumor through an anterior or lateral route on the axial plane at baseline CT or MRI. Indeed, such definition basically reflects a technical difficulty to access the tumor using CT guidance. Third, post-PTA follow-up, although systematically performed using MRI, remains short. Forth, this study was not adequately powered to prove equivalency or superiority in outcome, regarding both techniques.
Conclusion
PTA under CT-guidance after artificially induced CO2 pneumothorax is a safe and efficient technique for treating HCC located in the hepatic dome.
| Abbreviations | ||
| HCC | = | hepatocellular carcinoma(s) |
| IR | = | interventional radiologists |
| MRI | = | magnetic resonance imaging |
| PTA | = | percutaneous thermal ablation |
| US | = | ultrasonography |
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- Kim J-E, Kim Y-S, Rhim H, et al. Outcomes of patients with hepatocellular carcinoma referred for percutaneous radiofrequency ablation at a tertiary center: analysis focused on the feasibility with the use of ultrasonography guidance. Eur J Radiol. 2011;79:e80–e84.
- de Baère T, Dromain C, Lapeyre M, et al. Artificially induced pneumothorax for percutaneous transthoracic radiofrequency ablation of tumors in the hepatic dome: initial experience. Radiology. 2005;236:666–670.
- Kambadakone A, Baliyan V, Kordbacheh H, et al. Imaging guided percutaneous interventions in hepatic dome lesions: tips and tricks. World J Hepatol. 2017;9:840–889.
- Curley SA, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–458.
- Zheng X-H, Guan Y-S, Zhou X-P, et al. Detection of hypervascular hepatocellular carcinoma: comparison of multi-detector CT with digital subtraction angiography and Lipiodol CT. World J Gastroenterol. 2005;11:200–203.
- Maher MM, Gervais DA, Kalra MK, et al. The inaccessible or undrainable abscess: how to drain it. Radiographics. 2004;24:717–735.
- Kang TW, Rhim H, Lee MW, et al. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy. Am J Roentgenol. 2011; 196:907–913.
- Lee EJ, Rhim H, Lim HK, et al. Effect of artificial ascites on thermal injury to the diaphragm and stomach in radiofrequency ablation of the liver: experimental study with a porcine model. Am J Roentgenol. 2008;190:1659–1664.
- Song I, Rhim H, Lim HK, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630–2640.
- Brennan DD, Ganguli S, Brecher CW, et al. Thinking outside the abdominal box: safe use of the epipericardial fat pad window for percutaneous radiofrequency ablation of hepatic dome tumors. J Vasc Interv Radiol. 2008;19:133–136.
- Kondo Y, Yoshida H, Tateishi R, et al. Percutaneous radiofrequency ablation of liver cancer in the hepatic dome using the intrapleural fluid infusion technique. Br J Surg. 2008;95:996–1004.
- Choi TW, Lee JM, Lee DH, et al. Percutaneous dual-switching monopolar radiofrequency ablation using a separable clustered electrode: a preliminary study. Korean J Radiol. 2017;18:799–808.
- Cardella JF, Kundu S, Miller DL, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189–S91.
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7 Suppl):S377–S390.
- Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684–692.
- Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900–909.
- Kim Y, Rhim H, Cho OK, et al. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59:432–441.
- Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–577.
- Kim Y, Lim HK, Rhim H, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89–97.
- Veress J. Neues instument zur ausführung von brust-oder bauch-punktionen und pneumothoraxbehandlung. Dtsch Med Wochenschr. 1938;64:1480–1481.
- Akahane M, Koga H, Kato N, et al. Complications of percutaneous radiofrequency ablation for hepato-cellular carcinoma: imaging spectrum and management. Radiogr Rev Publ Radiol Soc N Am Inc. 2005;25:S57–S68.
- Fujiwara H, Arai Y, Ishii H, et al. Computed tomography-guided radiofrequency ablation for sub-diaphragm hepatocellular carcinoma: safety and efficacy of inducing an artificial pneumothorax. Acta Med Okayama. 2016;70:189–195.
- Okuma T, Matsuoka T, Tutumi S, et al. Air embolism during needle placement for CT-guided radiofrequency ablation of an unresectable metastatic lung lesion. J Vasc Interv Radiol. 2007;18:1592–1594.
- Favelier S, Guiu S, Cherblanc V, et al. Transthoracic adrenal biopsy procedure using artificial carbon dioxide pneumothorax as outpatient procedure. Cardiovasc Intervent Radiol. 2013;36:1184–1187.
- Buy X, Tok C-H, Szwarc D, et al. Thermal protection during percutaneous thermal ablation procedures: interest of carbon dioxide dissection and temperature monitoring. Cardiovasc Intervent Radiol. 2009;32:529–534.
- Kariya S, Tanigawa N, Kojima H, et al. Radiofrequency ablation combined with CO2 injection for treatment of retroperitoneal tumor: protecting surrounding organs against thermal injury. Am J Roentgenol. 2005;185:890–893.
- Hiraki T, Gobara H, Shibamoto K, et al. Technique for creation of artificial pneumothorax for pain relief during radiofrequency ablation of peripheral lung tumors: report of seven cases. J Vasc Interv Radiol. 2011;22:503–506.