Abstract
Purpose: Brain metastasis is a common complication in cancer patients. Local recurrence after total resection of metastatic brain tumor has been frequently reported. In this study, we developed a new hyperthermia device and applied it to metastatic brain tumor patients intra-operatively to study if hyperthermia treatment could reduce local tumor recurrence.
Materials and methods: A total of 63 metastatic brain patients were enrolled in the study with an informed consent obtained from every patient. After total resection of the tumor, the hyperthermia device was applied intra-operatively to the resection cavity. The surrounding brain tissue at 5 mm in depth from the tumor resection margin was raised to 42.5 °C for a total of 60 minutes (Clinical Research Information Service Registration Number: KCT0001308).
Results: A total of 10 local recurrences were observed in 63 patients who received hyperthermia treatment showing a local recurrence rate of 15.8%. It was significantly lower than the local recurrence rate of those who received conventional treatment (34%) when analyzed with one tailed z-test (p value: .001). Kaplan–Meier analysis also showed a significantly lower recurrence rate in the hyperthermia treatment group (p value: .0003). Complications included two cases of seizures and two cases of wound infection.
Conclusions: Results of this study suggest that intra-operative hyperthermia treatment after total resection of metastatic brain tumor could reduce local recurrence of tumor. We believe that intra-operative hyperthermia treatment could be used as an adjuvant therapy to surgery and post-operative radiotherapy, or as a salvage treatment in patients who cannot receive further radiotherapy.
Introduction
Brain metastasis is a common complication in cancer patients affecting as many as one-third of cancer patients. The incidence of brain metastases seems to be rising with advancement in therapies that control systemic disease which ultimately prolongs patient’s survival period [Citation1]. Surgery is widely accepted as an appropriate treatment modality for patients with brain metastases, especially, for those with good performance level and a single, surgically accessible metastatic lesion. Surgical resection of metastatic lesion may help to stabilize neurological symptoms and provide survival benefit for patients [Citation2,Citation3]. A complete en bloc resection of tumor is preferred in most cases to reduce local recurrence rate. However, local recurrences have been reported in patients who receive total resection of the tumor. Local recurrence rate ranges from 10 to 34% in patients who have went through complete resection of the tumor followed by post-operative radiotherapy [Citation4–7]. Local recurrence is thought to be caused by clinically undetectable cancer cell islands infiltrated among the tumor bed. These tumor cells did not seem to infiltrate for more than 5 mm in depth from the tumor resection margin in previously reported studies [Citation8,Citation9].
The efficacy of hyperthermia treatment in tumor patients has been actively investigated over the past years. Hyperthermia treatment is defined as a procedure of artificially raising the target tissue temperature up to 40–44 °C. It has been used mostly as a supplementary therapy to other established cancer treatment such as radiotherapy and chemotherapy [Citation10–12]. Hyperthermia treatment can induce tumor cell deaths through heat-induced necrosis and protein inactivation [Citation13]. In addition, it is believed that hyperthermia treatment can sensitize cancerous cells to radiotherapy and chemotherapy [Citation12,Citation14]. Several studies have also demonstrated that hyperthermia treatment can increase immunological attacks against tumor [Citation15].
Different methods of delivering heat to tumor cells have been investigated [Citation16]. Interstitial hyperthermia treatment for brain tumors has been studied where heat is applied to the tumor by implanting interstitial catheters within or around the tumor. In general, these studies have concluded that hyperthermia treatment could provide local tumor control [Citation17]. However, acute complications, such as, focal seizure and cerebral edema have been reported [Citation18]. In addition, there were concerns involving increased likelihood of intracranial infection due to intracranial implantation of foreign bodies such as thermal probes [Citation19,Citation20]. Technical limitations in effectively achieving an even temperature (42–45 °C) distribution within the entire tumor were also reported [Citation21].
In this prospective study, we developed a new hyperthermia device to effectively raise the temperature of target areas to therapeutic level (42.5 °C) and monitor the effect of the treatment. The hyperthermia device was applied to metastatic brain tumor patients intra-operatively after total resection of the tumor. The primary goal of this clinical trial was to study if intra-operative hyperthermia treatment could provide better local tumor control compared to the conventional treatment, which consists of surgical resection, adjuvant radiotherapy and systemic chemotherapy.
Materials and methods
Development of hyperthermia device
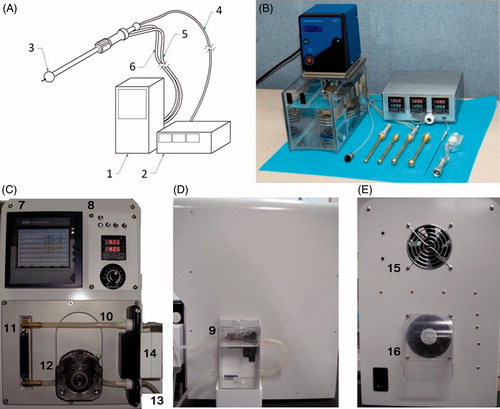
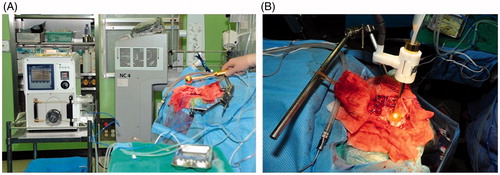
Hyperthermia device developed in this study consisted of a water tank, a water heater, a water pump, water tubes, a spherical implant, a temperature sensor and a temperature display monitor (). Water was heated by the heater then circulated into the spherical implant through the water tubes. The water tubes were made to be disposed after each treatment and be changed to new ones. After the pre-clinical animal experiment, modifications of the hyperthermia device were made. The water tank, water heater, water pump, and the temperature display monitor were all integrated into a single closed system to reduce the risk of infection (). The modified hyperthermia device was used in the clinical trial, and both Korean patent (no. 10–2006-0042) and US patent (no. 11–789059) were obtained for the device.
Figure 1. Schematic illustration and photographs of the newly developed hyperthermia device. (A) Schematic illustration of the prototype hyperthermia device used in the pre-clinical animal experiment. The device consists of a (1) water heater and pump, a (2) temperature display monitor, a (3) spherical hyperthermia implant, a (4) temperature sensor line, a (5) water inlet tube, and a (6) water outlet tube. (B) Photograph of the prototype hyperthermia device. Photograph of the (C) anterior (D) lateral and (E) posterior side of the modified hyperthermia device. The (7) temperature display monitor and the (8) control panels were on the anterior side. The (9) water tank was attached on the lateral side. The water would pass through the (10) upper tube from the water tank to the (11) water heater. The heated water would then pass through the lower tube and the (12) water pump. It would ultimately be pumped out through the (13) water outlet tube to heat up the spherical hyperthermia implant. The temperature sensor was connected to the (14) sensor connector. There were (15), (16) fans on the posterior side of the device for cooling.
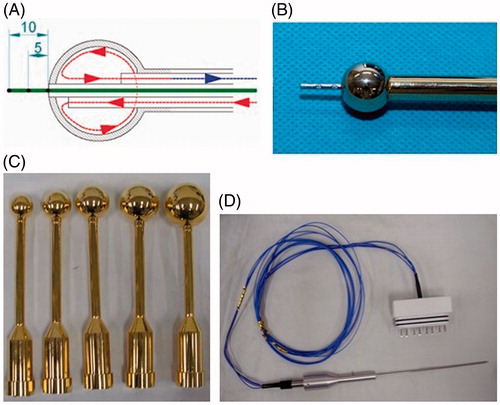
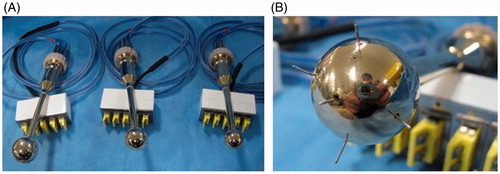
The hyperthermia implant was designed in a spherical shape in an effort to evenly raise the temperature of the surrounding brain tissues. It was made of brass to maximize the rate of heat conduction. It was then coated with gold to ensure biological safety when placed in the surgical resection cavity. The spherical implant was designed in 10 different sizes to accommodate various sizes of surgical resection cavity (15 mm, 22.5 mm, 25 mm, 27.5 mm, 30 mm, 32.5 mm, 35 mm, 40 mm, 45 mm in diameter). The shaft of the spherical implant was insulated from the inside to prevent it from heating up with the implant. The temperature sensor was assembled inside the shaft with the tip of the sensor extending out from the spherical implant. The temperature sensor had three different channels situated 5 mm apart from another (.
Figure 2. Schematic illustration and photographs of spherical hyperthermia implants and temperature sensor. (A) Schematic illustration of the spherical hyperthermia implant. The implant was heated by circulating heated water into it. The temperature sensor tip extending from the implant had three different channels situated 5 mm apart from one another. (B) Photograph of the hyperthermia implant with temperature sensor. (C) Photograph of hyperthermia implants in different sizes. (D) Photograph of the temperature sensor.
Preclinical animal experiment and biological safety
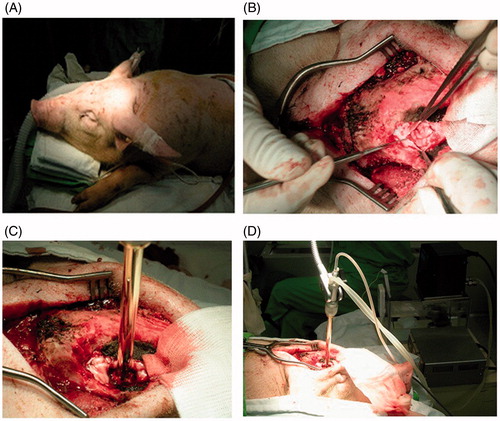
Preclinical animal experiments were conducted using the prototype hyperthermia device to determine the safety of the device. Right frontal craniectomy and partial resection of the frontal lobe were performed on a total of six pigs (). The resection cavity did not cross the midline of the brain. The spherical hyperthermia implant was then applied to the surgically made brain cavity (. The temperature of the surrounding brain tissues at 5 mm in depth from the cavity margin was elevated to 42.5 °C for a total of 60 min. All pigs received the hyperthermia treatment using the smallest spherical implant, which was 15 mm in diameter. The thermal distribution study showed that the water had to be heated between the ranges of 53–54 °C to maintain the temperature of the targeted area at a constant level of 42.5 °C. After the hyperthermia treatment, the dura was closed and the wound was closed without replacing the bone. All pre-clinical animal studies were approved and conducted under the supervision of the Institutional Animal Care and Use Committee of National Cancer Center.
Figure 3. Photographs of the hyperthermia device being applied to a pig in pre-clinical experiment. (A) A total of six pigs were used in the pre-clinical animal experiment. (B) Right frontal craniectomy and partial resection of the frontal lobe were performed on a pig. (C) The hyperthermia device was applied to the surgically made brain cavity. The temperature of the surrounding brain tissues at 5 mm in depth from the cavity margin was elevated to 42.5 °C for a total of 60 min. (D) Photograph illustrating how the hyperthermia device was set up and applied to a pig.
Two pigs were terminated immediately after the hyperthermia treatment, two pigs after 1 month, and two pigs after 6 months. All pathology studies of the pigs were done by experienced neuro-pathologists. The behavioral observations and neurological examinations of the pigs were done by a veterinarian. These included gait analyses, observation for abnormal head and forelegs positions, and cranial nerve function tests.
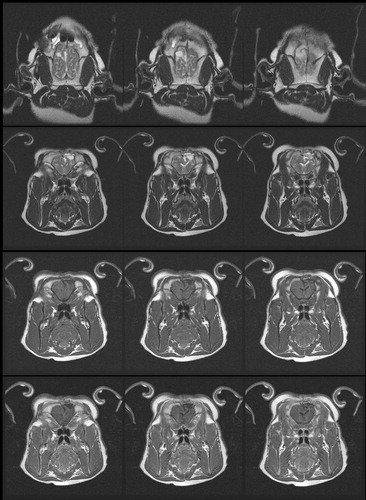
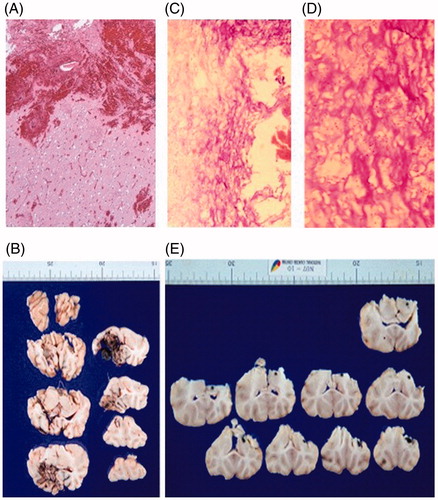
Pathology studies of the brain were done in pigs that were terminated immediately after the hyperthermia treatment () and in pigs that were followed up for a month after the hyperthermia treatment (. Heat-induced degenerative neuronal injury was not observed in either of the cases. Brain Magnetic Resonance Imaging (MRI) conducted on pigs after one month () and six months after the hyperthermia treatment did not show any significant findings. Behavioral and neurological abnormalities were not observed in any of the pigs during the experiment. After both the short-term and long-term safety of the hyperthermia device were proven by these pre-clinical animal experiments, the hyperthermia device was approved for clinical trial by the Korea Food and Drug Administration (KFDA) (KFDA board approval number: no. 199).
Figure 4. Pathology studies performed on pigs during the pre-clinical animal experiment. (A) Hematoxylin & Eosin (H&E) stain and (B) formalin fixed brain slices of a pig that was terminated immediately after the hyperthermia treatment. Hemorrhage and mild edematous change around the surgical cavity was observed. However, heat-induced degenerative neuronal injury was not observed. (C) H&E stain 40 × (D) H&E stain 100× and (E) formalin fixed brain slices of a pig that was terminated one month after the hyperthermia treatment. Pyknosis and cytoplasmic degeneration surrounded by foam cells and inflammatory cells were observed. Reactive gliosis was observed in the adjacent brain tissues but heat-induced degenerative neuronal injury was not observed.
Study design
Metastatic brain tumor patients between the age of 18 to 80 who were either newly diagnosed with cancer or diagnosed with local recurrence after initial surgical resection and adjuvant treatments were selected as candidates for this study. Patients had to score 70 or more on the Karnofsky Performance Status Scale to be eligible for the study. Patients with more than three metastatic brain lesions, leptomeningeal carcinomatosis, or uncontrolled primary disease were excluded from the study.
Patients underwent total resection of the metastatic brain tumor, which was confirmed by intra-operative frozen section margin biopsy and post-operative MRI. The spherical hyperthermia implant compatible to the size of the resection cavity was then applied into the surgical cavity to raise the surrounding brain tissue to target temperature (. The implant was secured without the shaft touching the normal brain. The brain was left open during the intra-operative hyperthermia treatment. The attending surgeon was on standby without leaving the surgical field during the entire hyperthermia treatment. Intra-operative electroencephalography was done in all patients.
Figure 6. Photographs of the hyperthermia device being applied to a patient during the clinical trial. (A) Photograph illustrating how the modified hyperthermia device was applied to a patient in an operating room setting. (B) The hyperthermia device was applied intra-operatively after total resection of the metastatic brain tumor was performed.
Previous data have shown that the maximum temperature that could be applied to central nervous system without obvious complications is in the range of 42 to 42.5 °C for 40 to 60 min [Citation22]. Therefore, in this study the temperature of the surrounding brain tissues at 5 mm in depth from the tumor resection margin was raised to 42.5 °C for a total of 60 min.
The temperature sensor tip extending from the implant was carefully inserted into the surrounding brain tissue to monitor the temperature. The temperature of the surrounding brain tissue was kept constant at the target therapeutic by controlling the temperature of the heated water circulating the spherical implant.
After the surgical resection and intra-operative hyperthermia treatment, patients received adjuvant radiotherapy if possible, and appropriate systemic chemotherapy as clinically needed. Post-operative radiotherapy was started within a month from the operation after surgical wound has been fully healed.
This clinical trial was approved by the Institutional Review Board of National Cancer Center (IRB No. NCCCTS-MD-08–001). An informed consent was obtained from every patient in this study after the nature and possible consequences of the study were explained.
Patient population
It was difficult to choose a historical control cohort for this study as local recurrence rate for metastatic brain tumor patient varied greatly in previously reported studies. However, as this was a single center clinical trial, we chose the 94 patients who were treated for metastatic brain cancer at the National Cancer Center from 2001 to 2007 [Citation23] as our control cohort. Patients in the conventional treatment group underwent total resection of the tumor followed by adjuvant radiotherapy and systemic chemotherapy. The 65 patients listed as those who did not receive post-operative radiotherapy were those who underwent systemic chemotherapy first due to progression of the primary disease. These patients later received delayed radiotherapy when brain metastasis was detected.
The minimum number of patient subjects needed for this study was calculated by using Fleming’s single stage procedure formula for phase 2 clinical trials [Citation24]. After considering an estimated additional 10% of follow-up loss, a minimum of 62 patients were needed for the study. Ultimately, a total of 63 metastatic brain tumor patients were enrolled in the study from 2010 to 2016.
Statistics
Mann–Whitney U test and chi-square tests were used to compare the demographic characteristics between those who received the hyperthermia treatment and those who received the conventional treatment. Local recurrence rate between these two groups were compared using the one-tailed z-test. One-tailed z-test was used instead of a two-tailed z-test as this was a comparison between the hyperthermia treatment group and the group of patients who were previously treated conventionally for metastatic cancer at the National Cancer Center. A Kaplan–Meier analysis was also performed to evaluate the risk of recurrence with appropriate censoring and identification of the numbers of patients at risk. A univariate Cox regression was performed to evaluate the hazard ratio. Results were considered statistically significant when the probability values (p values) were below .05. All statistical analyses were performed using SAS software, version 9.4 (SAS Inc., Cary, NC).
Results
Characteristics of patient population
The characteristics of patients are summarized in . A total of 40 male patients and 23 female patients were enrolled in the study. Their mean age was 58 years (33–77 years). Nonsmall cell lung cancer was the most common (66.7%) primary tumor, and breast cancer was the second most common (11.1%). Almost half (46%) of metastatic brain lesions were located in the frontal lobe. The diameter of the tumor ranged from 1.6 cm to 6.6 cm; 36.5% of the tumor had a diameter between 2.1 cm and 3 cm. Ten patients from the hyperthermia treatment group had more than one metastatic lesion; eight patients had two metastatic lesions and two patients had three metastatic lesions. Among 63 patients, 26 patients were able to undergo post-operative radiotherapy. The 37 patients who did not receive post-operative radiotherapy were either those who underwent chemotherapy first or those who were not suitable for adjuvant radiotherapy (ex. history of prior radiotherapy or worsened functional status after treatment). There were no significant difference between the hyperthermia group and the control group in regards to the demographic parameters of sex, age, tumor size and post-operative radiotherapy. The post-operative radiotherapy regimen and radiation dose received by both groups are described in .
Table 1. Baseline characteristics of the patients by types of treatment.
Table 2. RT regimen and radiation dose by types of treatment.
Post-operative follow up
The absence of residual tumor was confirmed by MRI within 48 h of the surgery. All patients underwent neuroimaging studies at 1 month after the surgery followed by a regular neuroimaging work up every 3 months or when it was clinically indicated. The mean overall follow-up duration for patients who received hyperthermia treatment was 13.7 months (0.8–64 months). The mean follow up duration for patients who received conventional treatment was 10.4 months (0.9–62.4 months). Local recurrence was defined as a tumor recurrence at the original site of resection within 3 years from the surgery, and was diagnosed using brain MRI by the neuro-radiologists at National Cancer Center. There were no demonstrable hyperthermia-associated MRI changes in patients who underwent intra-operative hyperthermia treatment.
Analysis of local recurrence
A total of 10 local recurrences were observed in 63 patients who received hyperthermia treatment (15.8%). Among these 10 patients, 7 patients underwent post-operative radiotherapy. The mean time to recurrence was 7.7 months (1.9–24.5 months). Patients who received conventional treatment showed local recurrence rate of 34% with 32 cases of local recurrence in 94 patients. One-tailed z-test showed that the local recurrence rate of those who received hyperthermia treatment was significantly lower than those who received conventional treatment (p values: .001) ().
Table 3. One tailed z-test of local recurrence.
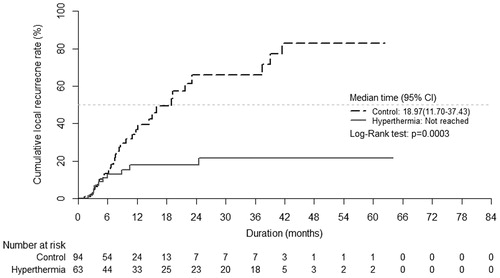
The local recurrence was also significantly lower in the hyperthermia treatment group compared to the conventional treatment group when Kaplan–Meier analysis was performed to account for those who may have died from systemic disease before local recurrence (p values: .0003) (. Univariate Cox regression analysis showed that the risk of local recurrence was 0.28 times lower in the hyperthermia treatment group compared to the conventional treatment group (95%CI: 0.13–0.59, p values: .0007).
Discussion
Results of this study showed that intra-operative hyperthermia treatment after surgical resection of the metastatic brain tumor could reduce local recurrence. The local recurrence rate of patients who received hyperthermia treatment was significantly lower than those who received conventional treatment. Local control of metastatic brain tumor may not greatly affect the overall survival of the patients as the prognosis of these patients depends more heavily on the control of the primary tumor [Citation25]. However, better local tumor control may have several benefits primarily by preventing further neurologic deterioration. This may lead to better quality of life in patients, help to maintain adequate functional status for further adjuvant therapies, and also help to prevent secondary medical problems related to poor neurologic status such as pneumonia or pressure sores.
We assume that better local tumor control in the hyperthermia treatment group is most likely due to the heat-induced necrosis of surgically undetectable tumor cells. There was no evidence to suggest that intra-operative hyperthermia treatment improved distant brain failure. We believe that intra-operative hyperthermia treatment could have a few applications in treatment of metastatic brain patients. It could be used as an additional adjuvant therapy to surgical resection and post-operative radiotherapy. Also, it could be used as a salvage treatment when further post-operative radiotherapy is not possible, for example, when cumulative toxicities from previous radiotherapy limit additional radiotherapy for recurrent brain lesions. The question of whether intra-operative hyperthermia treatment could replace post-operative radiotherapy has not been tested. In order to test this hypothesis, we would have had to design a study comparing those who received post-operative radiotherapy and those who received intra-operative hyperthermia treatment without adjuvant radiotherapy after surgical resection of the tumor. However, as numerous studies [Citation26] show that local recurrence is significantly reduced by post-operative radiotherapy, it seemed unethical to deny patients from post-operative radiotherapy for the sake of the clinical trial.
Different methods of hyperthermia treatment for brain tumors are being actively investigated, including the use of magnetic nanoparticles [Citation27,Citation28] and laser for thermal ablation [Citation29]. Our hyperthermia device avoids direct injury to the surrounding normal brain tissues which may be a problem when directly ablating the tumor with a laser. In addition, as our method of hyperthermia treatment is applied intra-operatively, it may reduce the likelihood of infection compared to the previous method of inserting thermal probes into the brain. Although the total duration of the surgery was prolonged by at least 60 min, which was the amount of time hyperthermia device was applied to the tumor cavity, this did not seem to significantly increase the rate of post-operative infection. Among the 63 patients who received hyperthermia treatment, two cases of surgical site infection occurred. One of the patients later received a wound revision operation, whereas, the other patient recovered fully after being treated with empirical antibiotics. There were also two cases of post-operative seizure. Both seizures were controlled after intravenous injection of antiepileptic drugs.
This study has a few limitations. First, there are limitations to generalize the results of this study as this was a single-center, nonrandomized study. The group of patients who were treated with intra-operative hyperthermia was compared to a pool of patients who were treated at the National Cancer Center before the start of the hyperthermia clinical trial. Both groups were heterogeneous in nature consisting of patients with different sizes and numbers of metastatic lesions. Also, patients had different types of primary tumor which would have had impact on the efficacy of post-operative radiotherapy in some of the patients. The chemotherapy regimen varied among the patients according to the different types and genetic/molecular characteristics of the primary tumor. Secondly, although the temperature of the brain tissues around the temperature sensor tip was accurately monitored and kept constant, there were limitations in monitoring the temperature of the rest of the surrounding brain tissues. The hyperthermia implant was designed in a spherical shape to evenly raise the temperature. However, the heterogeneous environment of the brain tissues around the surgical cavity could have affected the thermal distribution. Toward the end of the clinical trial, we developed a modified thermal implant that could harbor five temperature sensor tips to better monitor the temperature of the surrounding tissues (. However, only one patient from this study was treated using the modified implant. Thirdly, although we developed different sizes of hyperthermia implant for various sizes of resection cavity, we came across a few cases where it was difficult to fit the implant into the resection cavity. Therefore, we found it necessary to develop a finer selection of implant in different sizes before utilizing the hyperthermia device in clinical practices.
Figure 8. Photographs of modified spherical hyperthermia implants. (A) Photograph of the modified hyperthermia implants in different sizes. (B) The modified implant had five temperature sensors tips extending around the implant to better monitor the temperature of the surrounding brain tissues.
Our current clinical trial involves a limited number of heterogeneous patients. We acknowledge that a larger prospective controlled study is required. However, given the adequate local tumor control achieved in the hyperthermia treatment group, we believe that intra-operative hyperthermia treatment could be considered as one of the treatment options for patients with metastatic brain tumors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arvold ND, Lee EQ, Mehta MP. Updates in the management of brain metastases. Neuro Oncol. 2016;18:1043–1065.
- Stark AM, Tscheslog H, Buhl R, et al. Surgical treatment for brain metastases: prognostic factors and survival in 177 patients. Neurosurg Rev. 2005;28:115–119.
- Paek SH, Audu PB, Sperling MR, et al. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005;56:1021–1034.
- Hoskin PJ, Crow J, Ford HT. The influence of extent and local management on the outcome of radiotherapy for brain metastases. Int J Radiation Oncol Biol Phys. 1990;19:111–115.
- Mintz AP, Cairncross JG. Treatment of a single brain metastasis: the role of radiation following surgical resection. JAMA. 1998; 280:1527–1529.
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489.
- Sundaresan N, Sachdev VP, DiGiacinto GV, et al. Reoperation for brain metastases. J Clin Oncol. 1988;6:1625–1629.
- Baumert BG, Rutten I, Dehing-Oberije C, et al. A pathology-based substrate for target definition in radiosurgery of brain metastases. Int J Radiation Oncol Biol Phys. 2006;66:187–194.
- Neves S, Mazal PR, Wanschitz J, et al. Pseudogliomatous growth pattern of anaplastic small cell carcinomas metastatic to the brain. Clin Neuropathol. 2001;20:38–42.
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hypertherm. 2001;17:1–18.
- Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol). 2007;19:418–426.
- Morita K, Tanaka R, Kakinuma K, et al. Combination therapy of rat brain tumours using localized interstitial hyperthermia and intra-arterial chemotherapy. Int J Hypertherm. 2003;19:204–212.
- Jorritsma JB, Burgman P, Kampinga HH, et al. DNA polymerase activity in heat killing and hyperthermic radiosensitization of mammalian cells as observed after fractionated heat treatments. Radiat Res. 1986;105:307–319.
- Kong G, Dewhirst MW. Hyperthermia and liposomes. Int j hypertherm. 1999;15:345–370.
- Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hypertherm. 2012;28:528–542.
- Chicheł A, Skowronek J, Kubaszewska M, et al. Hyperthermia – description of a method and a review of clinical applications. Rep Pract Oncol Radiother. 2007;12:267–275.
- Sneed PK, Stauffer PR, McDermott MW, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/- hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:287–295.
- Stea B, Kittelson J, Cassady JR, et al. Treatment of malignant gliomas with interstitial irradiation and hyperthermia. Int J Radiat Oncol Biol Phys. 1992;24:657–667.
- Moran CJ, Marchosky JA, Wippold FJ, 2nd, et al. Conductive interstitial hyperthermia in the treatment of intracranial metastatic disease. J Neuro-Oncol. 1995; 26:53–63.
- Seegenschmiedt MH, Karlsson UL, Black P, et al. Thermoradiotherapy for brain tumors. Three cases of recurrent malignant astrocytoma and review of clinical experience. Am J Clin Oncol. 1995;18:510–518.
- Hulshof MC, Raaymakers BW, Lagendijk JJ, et al. A feasibility study of interstitial hyperthermia plus external beam radiotherapy in glioblastoma multiforme using the Multi ELectrode Current Source (MECS) system. Int J Hyperthe. 2004;20:451–463.
- Missios S, Bekelis K, Barnett GH. Renaissance of laser interstitial thermal ablation. Neurosurg Focus. 2015;38:E13.
- Yoo H, Kim YZ, Nam BH, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg. 2009;110:730–736.
- Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151.
- Soffietti R, Rudā R, Mutani R. Management of brain metastases. J Neurol. 2002;249:1357–1369.
- Mahmood U, Kwok Y, Regine WF, et al. Whole-brain irradiation for patients with brain metastases: still the standard of care. Lancet Oncol. 2010;11:221–222.
- Jordan A, Scholz R, Maier-Hauff K, et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neurooncol. 2006;78:7–14.
- Maier-Hauff K, Rothe R, Scholz R, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol. 2007;81:53–60.
- Ivan ME, Mohammadi AM, De Deugd N, et al. Laser ablation of newly diagnosed malignant gliomas: a meta-analysis. Neurosurgery. 2016;79:S17–S23.