Abstract
Purpose: The aim of the study was to evaluate the efficacy of high intensity focused ultrasound (HIFU) in the treatment of symptomatic breast fibroadenomas (FA) after 6 and 12 months.
Materials and methods: Between December 2013 and November 2014, 27 patients with histologically confirmed FA received one application of HIFU under local anesthesia (NCT02011919). Follow-up visits occurred after 6 and 12 months measuring the FA volume and clinical symptoms. A volume reduction of more than 65% was defined as success. Core needle biopsy (CNB) was offered after 12 months if indistinct residuals were visible on ultrasound (US).
Results: A successful reduction in FA volume after 12 months was achieved in 24/27 patients (89%). At baseline 16 patients (59%) had pain, which was resolved in 63% (10/16). All patients were satisfied with the cosmetic related outcome. Twenty-four patients (89%) would repeat the procedure. After 12 months 21 patients with sonographically indistinct residuals underwent a CNB. There were no vital cells in 86%. Three cases showed vital cells of FA. Retrospectively possible reasons in these three cases were an insufficient treatment due to bad visibility and insufficient fixation of the FA during HIFU and/or a too short follow-up time.
Conclusion: US-guided HIFU is an effective procedure and a minimally invasive alternative for the treatment of breast FA.
Introduction
Fibroadenomas (FA) are the most common benign breast lesions in women. While most commonly occurring in women under the age of 30 [Citation1], they are reported in approximately 10% of all women during their lifetime [Citation2]. FA account for between 30% and 75% of all breast biopsies [Citation3,Citation4]. Nationwide screening programs detect not just malignant but also benign breast lesions; however, one consequence is the increased rate of open excisional biopsies [Citation5]. 15.7% of all surgical breast procedures in Germany in 2015 involved benign lesions [Citation6]. Although surgical excision is still a widespread treatment option, it can lead to the development of scarring and sometimes poor cosmetic results. Minimal invasive techniques including thermoablative procedures such as High-Intensity Focused Ultrasound (HIFU) or cryoablation are becoming well-accepted alternatives [Citation4,Citation7].
Cryotherapy involves freezing the lesion by inserting a cryoneedle into its center under local anesthesia. Cryoablation for the treatment of fibroadenomas has been demonstrated to be safe and efficient with a high patient satisfaction rate [Citation8–11]. It has also been used to treat malignant lesions of the kidney, liver, prostate and adrenal gland [Citation12] and there are promising results in the field of breast cancer [Citation13,Citation14].
HIFU is a thermoablative technique that delivers energy to a confined space leading to protein denaturation and coagulative necrosis at a temperature of >65 °C [Citation15,Citation16]. It is a noninvasive technique, can be performed under local anesthesia and does not involve probe insertion, thus avoiding scars.
Recently, the feasibility and safety of HIFU were evaluated in a number of clinical trials on benign and malignant tumors of the prostate, thyroid, uterus, liver, kidney, pancreas, bone and brain [Citation17,Citation18]. Only a few studies exist on breast fibroadenomas. Studies proving the efficiency and feasibility of MR-guided HIFU ablation started in 2001 [Citation19], including only nine patients. Ultrasound-guided HIFU was established a decade later by Kovatcheva, who demonstrated a volume reduction of 72.5% after one year with good feasibility [Citation20] in 51 fibroadenomas. Recently, Kovatcheva et al found that a persistent volume reduction after two years of follow-up can be achieved and that a second HIFU treatment can significantly reduce the tumor volume even more. In 2016, Peek et al showed that with circumferential treatment, a good volume of 43.5% could be achieved after 6 months [Citation21]. Up to now, trials on breast cancer with MRI-guided HIFU have shown an insufficient ablation rate (17–79%) [Citation22–24], which is mandatory if HIFU is to replace surgery in certain cases.
The aim of this trial was to evaluate the efficacy and tolerability of HIFU in the treatment of breast fibroadenoma using the Echopulse® device (Theraclion, Malakoff, France). The efficacy was assessed by volume reduction of the FA, improvement of clinical symptoms, long-term cosmetic outcome and histological verification at 12 months’ follow-up. A special characteristic of this trial was that a core needle biopsy was offered to all patients with indistinct ultrasound presentation of the treated area after 12 months’ follow-up.
Materials and methods
Study design
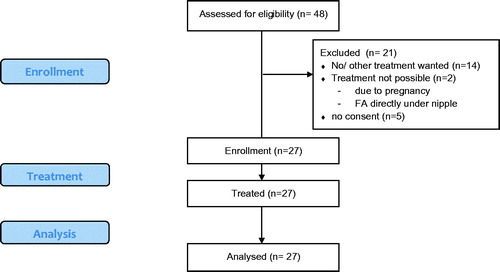
The Tuebingen HIFU trial was a prospective, mono-, non-randomized, open-label trial. From December 2013 until November 2014, 48 patients with histologically confirmed FA were screened. Twenty-seven of these women (56%) were enrolled in the trial and consecutively treated (). Follow-up visits were performed after 7 days (D7), 6 (M6) and 12 months (M12). A core needle biopsy (CNB) was provided if an indistinct residual volume was visible after 12 months on ultrasound (US).
Inclusion and exclusion criteria/patient selection
Inclusion criteria were women aged ≥18 years with a symptomatic FA histologically confirmed by CNB, with a maximum diameter of 25 mm and signed written informed consent. Exclusion criteria were women who were pregnant or breastfeeding, cryoablation or laser therapy to the ipsilateral side within 12 months, breast implants or a history of breast cancer and/or radiotherapy within the last 5 years. The study was approved by the local ethics committee (Trial number 441/2013MPG23) and registered under clinicaltrials.gov NCT02011919.
Hifu-treatment
All patients received a single HIFU application under local anesthesia (LA) using the ultrasound-guided Echopulse® device (Theraclion, Malakoff, France).
The device visualization and treatment unit (VTU) included diagnostic and HIFU transducers. The HIFU transducer was 56 mm in diameter with an 11 mm central aperture and produced therapeutic ultrasound energy at 3.0 MHz. A confocal 5–10 MHz diagnostic US transducer was set in the cavity for real-time US guidance. The HIFU transducer enabled delivery of 4-s pulses. Each pulse ablated an ellipsoid lesion of approximately 9 mm length and 2 mm width. A balloon filled with cooling fluid was installed as part of the standard configuration on the VTU. The fluid ensured both ultrasound coupling and skin cooling to prevent skin burns.
Local anesthesia (LA) was infiltrated around the FA. The first 14 patients received lidocaine 2%, while the remaining 13 patients received ropivacaine 0.2%. The switch was made due to ropivacaine’s more potent and longer lasting analgesic effect [Citation25].
Depending on the location of the FA, the patients were placed in a supine or lateral position with the arm placed over the patient’s head or close to the upper body. To avoid uncontrolled movements of the FA, a compression paddle or bonding technique was used. After locating the FA with a high-resolution handheld probe, the VTU was positioned ventral to the FA.
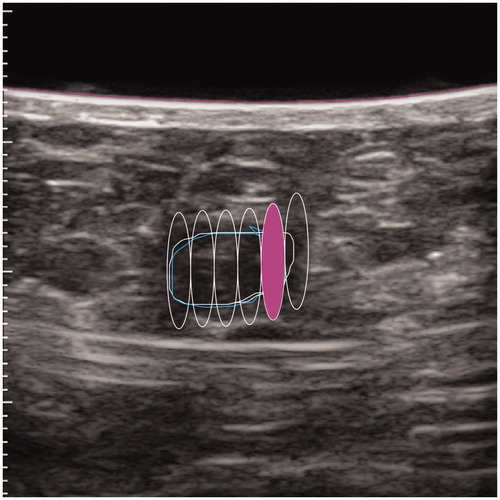
The FA was delineated on the US screen while the software calculated the overall energy per lesion and the number of pulses. The generated sonication has a fixed volume size. The software automatically proposes the target after the lesion is drawn on the screen to avoid leaving a portion of the FA uncovered, thus mimicking the way surgery is performed (). The treated margin around the FA should be as small as possible to avoid damages or adverse events to normal fat or breast tissue [Citation26].
The procedure started with a single pulse of 40 watts (peak intensity at focus 27 k watts/cm2) and could be reduced stepwise to a minimum of 30 watts (peak intensity at focus 20 k watts/cm2), if necessary for pain control. Such values have shown to be relevant according to Kovatcheva et al [Citation20]. The energy was applied stepwise using numerous pulses. Power values provided in the document are invariant by depth because they are derated values. During treatment planning, both the FA and the skin surface are outlined. The system calculates the depth for all individual lesions. The derated power for treatment at a certain depth is calculated by the system automatically and it applies the acoustic power to the HIFU transducer.
The mean energy for the complete HIFU treatment was 6.2 kilojoules (SD 2.7, median 6.5 min 1.2, maximum 11.3 kilojoules). The mean maximum power during treatment was 41 watts (SD 3, median 40, minimum 40, maximum 50 watts). The treatment duration was recorded.
The skin aspect was photographically documented after treatment. Patients were asked to indicate their pain on a standard visual analog scale (VAS) from 0 to 100 during the procedure and again 30 min afterward.
The patients were discharged 30 min after the treatment and 600 mg ibuprofen 3 times a day was recommended for one week for pain relief.
Patients were followed up after 7 days (D7), 6 months (M6) and 12 months (M12). Each visit included a physical examination, an ultrasound measurement of the lesion () and photographic documentation to evaluate cosmetic effects such as bruising, swelling, redness, hematoma, dimpling or skin burns. The volume reduction was calculated and a reduction of more than 65% was considered as effective treatment. The pain level was documented using the standard Visual Analog Scale (VAS) at each visit. After 12 months, a CNB was proposed if an indistinct lesion was visible on ultrasound.
Data collection and statistical analysis
All data was documented in case report forms and captured in a database; therefore double data entry was performed. All statistical analyses were performed using R, Version 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria). Data were summarized as means and standard deviations (SD), medians and ranges, numbers and percentages. The association between continuous variables like FA volume reduction and total energy used was estimated by Pearson’s correlation coefficient.
Results
Patient characteristics
Twenty-seven patients with symptomatic FA participated and successfully underwent HIFU treatment. Of these 27 patients, 23 (85%) had a palpable lump, 16 (59%) presented with pain and 19 (70%) reported anxiety related to the presence of the lump. The mean age was 28.9 years (SD 9.1 years, median 26, range 18–50 years). Thirteen patients had a previously treated FA, eight of these (62%) on the ipsilateral side. All treated FA in this trial were mobile and none of them altered the cosmetic appearance (). All 27 patients completed the intended treatment and follow-up.
Table 1. Patient’s characteristics.
Volume reduction
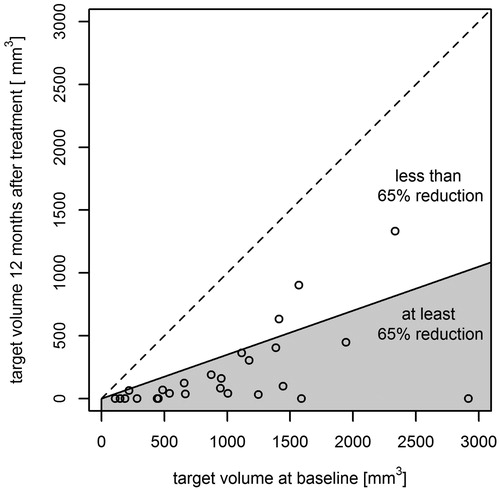
The mean volume at baseline was 1083.1 mm³ (SD 801.0, median 952.1 mm³, range 108.1–3138.1 mm³) and 203.2 mm³ after 12 months (SD 316.2, median 68.7 mm³, range 0–1 333.0 mm³). Effective treatment (65% volume reduction) after 6 months was reported in 19 patients (70%), and after 12 months in 24 patients (89%) (. The mean relative volume reduction after 6 months was 61.6% and after 12 months 84.8%. No significant change in blood supply was noticed in the treated fibroadenoma using Doppler-US before and after the treatment as well as during long-term follow-up (McNemar test p = .505).
Duration of treatment
The mean room occupation time for the treatment was 80 min (SD 19, median 80 min, range 46–120 min). The procedure itself had a mean duration of 38 min (SD 12, median 34 min, range 15–69 min).
Symptom Evaluation
Palpable lump
In 23 (85%) patients a lump was palpable at baseline. Sixteen (70%) lesions were not palpable at 12-month follow up. Two non-palpable lesions at baseline were palpable after 12 months. One of these patients presented with a fatty cyst in the treated area on ultrasound which we interpreted as a thermoablative effect on the surrounding fatty tissue. The other patient showed sonographic signs for the development of scar tissue, which was histologically proven.
Pain
At baseline 16 (59%) patients reported pain with a mean value of 22.3 (SD 23.5, median 24, range 0–71) assessed by a 100 mm VAS. At the M12 visit, the pain had ceased in 10/16 (63%) of the cases. Two patients who were pain-free at baseline reported pain at M12. One of them experienced a few days of mild temporary pain. In total, the mean pain level decreased to 16.1 (SD 17.8, median 8, range 0–58) after 6 months and to 7.4 (SD 14.8, median 0, range 0–56) after 12 months. The mean maximum pain level during treatment was 60.3 (SD 23.9, median 64.5, range 0–91) and 29.6 (SD 20.0, median 31, range 0–69) 30 min after the treatment.
The local anesthetic used in the first 14 patients was lidocaine 2.0% with a mean volume of 16 ml (SD 5.9, median 14, range 8–30). To improve pain control, ropivacaine was used in the subsequent 13 patients because of its more potent and longer lasting analgesic effect. In the second group, patients reported less post-treatment pain (26 versus 23 on the VAS-Scale of 100) with comparable mean dosage of 15.3 ml, (SD 4.6, median 16, range 7–20 ml). However, the mean treated fibroadenoma volume and the delivered energy were smaller for patients treated with ropivacaine (1405.5mm3 versus 735.9mm3; 4.87 kJ versus 7.39 kJ).
Cosmetic results
After 1 week, 19 patients presented with mild bruising [Citation10], swelling [Citation10], redness [Citation2] or hematoma [Citation4]. These symptoms regressed completely over time. After 12 months, all 27 patients were satisfied with the cosmetic results.
Histological outcome after treatment
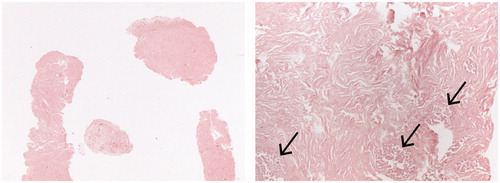
After 12 months, the lesion was no longer visible on ultrasound in four cases, one patient was pregnant and another patient refused CNB. The other 21 patients who presented with sonographically indistinct residues underwent CNB. In 18/21 cases (86%) no more vital fibroadenoma cells were reported (), while in 3/21 cases (14%) vital residues were found. Retrospectively we discovered the following explanations for the vital residues:
Figure 5. Low and high power view of a CNB specimen 12 months after HIFU with acellular stroma and ‘ghost glandular structures’ (arrows) as a sign of ischaemic necrosis. There was no evidence of viable lesional tissue.
Insufficient treatment due to poor visibility and insufficient fixation of the FA during treatment.
Insufficient follow-up time. We draw this conclusion because the patient received an open excisional biopsy due to pain 18 months after HIFU and no more vital cells were histologically detected.
Insufficient treatment because of deterioration of the image during treatment and insufficient follow-up time.
Adverse events
One patient (4%) presented with pain and sonographic signs of fat necrosis 9 months after treatment. Despite analgesia, the pain lasted until surgical excision 18 months after HIFU. Histologically, no vital FA cells were present.
Outcomes
All patients were satisfied with the cosmetic results. 24/27 patients (89%) were satisfied with the symptom-related outcome and would consider HIFU again. 26/27 patients (96%) would recommend this procedure. In 20 (74%) of the cases, the treatment was assessed as a total success – in terms of simultaneous total symptom reduction, side effects and histological vital findings. In 7 (26%) of the cases, only partial success was rated due to mild pain, indistinct imaging or the presence of vital FA cells at M12 ().
Table 2. Key results.
Discussion
Surgical excision is considered the gold standard for treating symptomatic fibroadenoma. However, open surgery is a costly, invasive procedure with surgical risks and reduced cosmetic outcome associated with scars [Citation4,Citation27]. HIFU – either guided with MRI or US – is a feasible and safe procedure for the treatment of benign and malignant tumors in numerous anatomic locations [Citation17,Citation28,Citation29], as well as for certain gynecological pathologies such as uterine fibroids and adenomyosis [Citation18,Citation30]. Some studies have demonstrated the treatment of benign and malignant breast lesions [Citation19,Citation20,Citation24,Citation31].
MRI-guided HIFU has the advantage of high sensitivity for the detection of lesions via excellent anatomical resolution. Therefore, it allows precise treatment planning and accurate evaluation of treatment efficacy in terms of volume reduction over time [Citation32]. In this trial, HIFU-treatment and all follow-up visits were done with ultrasound as it offers real-time visualization of the treated volume and gives direct feedback about the success of the applied energy through the appearance of a hyper-echoic mark (This mark shows the level of coagulative necrosis in real-time during treatment) [Citation15]. To avoid interobserver variability, US was mostly performed by the same person. Compared to MRI, US has the benefit of lower costs, is widely available and is independent of contrast media [Citation33].
Volume reduction
The results of this trial demonstrate a reduction or total regression in FA volume by more than 65% after 6 months in 70% and after 12 months in 89% of patients. The rationale in this trial was to retain a 65% volume threshold reduction as the success criterium, given that complete disappearance of the lesion could not be expected due to intramammary scars and necrosis.
The first multicenter study published in 2015 by Kovatcheva et al [Citation20] showed that a volume regression of more than 70% with a global pain level reduction after one year is achievable. Compared to our results, Kovatcheva et al [Citation20] achieved a smaller mean volume reduction after 6 months (59.2% versus 61.6%) and 12 months (72.5% versus 84.8%), although 8/51 (15.7%) of their patients underwent a second HIFU treatment. The reason might be the initially larger FA volume (mean volume 3890 mm³ versus 1083 mm³) or follow-up being too short.
In a recently published paper by Kovatcheva [Citation34], the mean volume reductions after one or two HIFU-treatments were compared. Twenty-four months after the first treatment, the mean volume reduction was 77.3% in the first group and 90.5% in the second. Consequently, the volume reduction can be increased with a second treatment and a longer follow-up.
In contrast to the goal of optimal volume reduction, Peek et al [Citation21] believe that complete thermal ablative treatment of FA is not mandatory. This group promotes circumferential HIFU treatment, leading to consistent pain and volume reduction (mean volume reduction of 43.5% (SD 38.8%) after 6 months).
In our trial, six cases of FA were only treated partially due to either proximity to the skin, thickness of the targeted FA greater than 9 mm or skipping sites due to pain. Nevertheless, the mean volume reduction in these cases, with coverage inferior to 100%, was 73.6% after 6 months and 86.4% after 12 months. A general problem of the US follow-up could be the inter-observer variability among different ultrasonographers.
Duration of treatment
The mean total room occupation time was 80 min (SD 19) (min 46, max 120) with a mean total HIFU procedure duration of 38 min. Peek et al [Citation21] reported similar treatment times (mean 34.6 min, SD 10.5), but they only performed circumferential ablation of larger fibroadenomas (mean FAD volume of 7.3 cm³, SD 10.1, 0.4–44.0). Kovatcheva et al [Citation20] reported average treatment duration of 118 min, ranging from 60 to 255 min, but they treated FA up to a volume of 196.6 cm³.
A systematic review by Peek et al [Citation35] showed that the major disadvantage of HIFU is the prolonged treatment time, which ranged between 79 and 171 min. Analysis of the treatment duration is quite difficult, as it depends on several factors such as patient movement during energy delivery, time spent for repositioning and, principally, the size of the FA. To convert HIFU into an attractive alternative in terms of cost-effectiveness, the room occupancy needs to be as short as possible. Therefore, optimal teamwork among all persons involved in the process and a standardized approach is mandatory. A handheld probe instead of the current computer-controlled VTU transducer could save some time, as fixation becomes unnecessary and makes the procedure more comfortable for the patient.
Palpation
Our results demonstrate a conversion of the initially palpable FA in 70% (16/23) to non-palpable at M12. However, two patients with initially non-palpable FA presented with a palpable lump at M12. There are only few available data about post-procedural change in palpability in the literature. Peek et al [Citation21] achieved in 20% a reduction in palpability in the treated area (4/20) with circumferential ablation. Because of these findings, patients should be informed that impalpability of the treated area is not always achievable and that non-palpable lesions at baseline or intramammary scars could become palpable over time.
Pain
Most patients requested rapid and simple treatment without general anesthesia or additional conscious sedation. From our experience, most young women appreciate receiving quick, uncomplicated treatment with a swift return to their normal daily activity. Therefore, the procedure was performed exclusively under local anesthesia (LA). The mean pain score measured on a VAS during the treatment was 60.3 (on a scale of 0–100).
Peek et al [Citation21] treated all patients with subcutaneous local anesthesia (1.0% lidocaine with adrenaline and 0.25–0.5% bupivacaine, ratio 1:1, mean 23.1 ml, SD 8.1 ml) with a similar volume. This group reported a mean pain score during treatment of 6.4 (SD 3.2) on a 0–10 VAS. A mean energy of 134.6 joules (SD 19.3 joules) and a mean power per treatment of 33.3 watts (SD 4.8 watts) was applied. Although higher energy (mean energy of 164 joules, SD 12 J) was applied and mean power was 41 watts (SD 3 watts) in this study, comparable pain level results were obtained. Consequently, the applied level of energy has limited influence on pain. Other authors such as Kovatcheva et al [Citation20] used conscious sedation in 42 patients and reported a lower mean pain score during treatment of 29.7 (SD 27.5) on a 0–100 mm VAS.
Future studies are necessary to investigate the optimal form of anesthesia for pain control. From our series of 16 patients presenting with pain before treatment, pain was completely suppressed in 10 cases (63%) after 12 months. However, two patients who were initially pain-free reported pain after 12 months. Peek et al [Citation21] also reported two patients who developed post-treatment pain which resolved within 3 months. One of the limitations of our study is that pain levels are generally difficult to distinguish due to individual variability in pain perception which is often mingled with discomfort. Pain levels can depend on several factors such as menses, physical activity etc. and the question concerning the maximum pain level since the last visit remains difficult to answer.
Cosmetic results
One advantage of HIFU compared with all other procedures is the avoidance of a skin incision. All short-term changes such as swelling, redness or hematomas resolved spontaneously. After 12 months all patients were satisfied with the cosmetic results. In the literature, there are reports about superficial skin burns, subcutaneous induration and hyperpigmentation [Citation20,Citation21]. From our experience these complications can be avoided by attentive recruitment, targeting patients with FA less than 25 mm in diameter and at least 5 mm from the skin.
Histological results
To our knowledge, this is the first trial with a CNB-proven post-treatment histology result. The histological outcome mostly confirmed the sonographic diagnosis as scar tissue or necrosis. In three cases, residuals of the original fibroadenoma were found at 12-month follow-up.
One case probably occurred because of incomplete treatment of the FA due to limited visibility and insufficient breast fixation during the treatment. However, the relative volume reduction after 12 months was 42%. The second case, with vital cells present at M12, showed no more vital cells histologically after 18 months when the patient underwent excisional surgery due to persistent mild pain. Our conclusion is that in some cases, a follow-up duration of 12 months might be too short to achieve a complete thermoablative effect. The third case had a relative volume reduction of 94% at M12 with almost no sonographically detectable residuals. Retrospectively, the positive histology might be associated with follow-up after HIFU being too short or insufficient ablation due to deterioration of the image during the treatment.
A limitation of this study was that the location of the treated FA was not clip marked, which could result in a false negative histology due to inaccurate localization. To avoid a false biopsy, ultrasound documentation of all visits specifying the precise lump location was compared. Another limitation is that a core needle biopsy might provide a histologically false negative result as it does not excise the complete lesion, but just takes a representative biopsy of three specimens. Another drawback could be that a duration of 12 months is possibly too short for analyzing the complete thermoablative effect in some patients.
Outcome
The physicians involved defined HIFU treatment as successful in 74% of cases and as a noninvasive method allowing intra-operative visibility of the lesion with a low side-effect profile. Nevertheless, the immobilization system needs to be further developed for achieving effective fixation of the breast.
Conclusion
Our results demonstrated that HIFU treatment is effective in reducing clinical symptoms and tumor volume after one year. Therefore, HIFU is a safe alternative to surgery and a noninvasive procedure for selected patients with symptomatic FA.
Future studies are needed to assess long-term effectiveness, to compare different thermoablative and other noninvasive techniques, and to develop standardized pain control.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Larsen TK, Faurschou JP, Bak M, et al. Fibroadenoma of the breast–modern strategy of treatment. Ugeskr Laeger. 2003;165:1979–1983.
- Fine RE, Staren ED. Percutaneous radiofrequency-assisted excision of fibroadenomas. Am J Surg. 2006;192:545–547. doi: 10.1016/j.amjsurg.2006.06.011.
- Dent DM, Cant PJ. Fibroadenoma. World J Surg. 1989;13:706–710.
- Greenberg R, Skornick Y, Kaplan O. Management of breast fibroadenomas. J Gen Intern Med. 1998;13:640–645.
- Croce S, Bretz-Grenier MF, Mathelin C. Most common benign epithelial breast diseases: diagnosis, treatment and cancer risk. Gynecol Obstet Fertil. 2008;36:788–799. doi: 10.1016/j.gyobfe.2008.02.029.
- (IQTIG) IfQuTiG. Qualitätsreport 2015. In: (G-BA) GB, editor. Berlin: Gemeinsamer Bundesausschuss, 2015. p. 102–108. Retrieved from https://iqtig.org/downloads/berichte/2015/IQTIG-Qualitaetsreport-2015.pdf
- Hahn M, Krainick U, Peisker U, et al. Eignet sich das Hand Held Mammotome zur kompletten Entfernung benigner Läsionen der Brust? Geburtshilfe Frauenheilkd. 2004;64:719–722. doi: 10.1055/s-2004-821006.
- Whitworth PW, Rewcastle JC. Cryoablation and cryolocalization in the management of breast disease. J Surg Oncol. 2005;90:1–9. doi: 10.1002/jso.20201.
- Kaufman CS, Bachman B, Littrup PJ, et al. Cryoablation treatment of benign breast lesions with 12-month follow-up. Am J Surg. 2004;188:340–348. doi: 10.1016/j.amjsurg.2004.06.025.
- Golatta M, Harcos A, Pavlista D, et al. Ultrasound-guided cryoablation of breast fibroadenoma: a pilot trial. Arch Gynecol Obstet. 2015;291:1355–1360. doi: 10.1007/s00404-014-3553-5.
- Hahn M, Pavlista D, Danes J, et al. Ultrasound guided cryoablation of fibroadenomas. Ultraschall in Med. 2012;34:64–68. doi: 10.1055/s-0032-1325460.
- Gage AA, Baust JG. Cryosurgery for tumors - a clinical overview. Technol Cancer Res Treat. 2004;3:187–199. doi: 10.1177/153303460400300212.
- Simmons RM, Ballman KV, Cox C, et al. A phase ii trial exploring the success of cryoablation therapy in the treatment of invasive breast carcinoma: results from ACOSOG (Alliance) Z1072. Ann Surg Oncol. 2016;23:2438–2445. doi: 10.1245/s10434-016-5275-3.
- Sabel MS, Kaufman CS, Whitworth P, et al. Cryoablation of early-stage breast cancer: work-in-progress report of a multi-institutional trial. Ann Surg Oncol. 2004;11:542–549. doi: 10.1245/ASO.2004.08.003.
- Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23:89–104.
- Kim SH, Jung SE, Kim HL, et al. The potential role of dynamic MRI in assessing the effectiveness of high-intensity focused ultrasound ablation of breast cancer. Int J Hyperthermia. 2010;26:594–603. doi: 10.3109/02656736.2010.481275.
- Esnault O, Franc B, Menegaux F, et al. High-intensity focused ultrasound ablation of thyroid nodules: first human feasibility study. Thyroid. 2011;21:965–973. doi: 10.1089/thy.2011.0141.
- Marinova M, Rauch M, Schild HH, et al. Novel non-invasive treatment with high-intensity focused ultrasound (HIFU). Ultraschall in Med. 2015;37:46–55. doi: 10.1055/s-0035-1553318.
- Hynynen K, Pomeroy O, Smith DN, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219:176–185. doi: 10.1148/radiology.219.1.r01ap02176.
- Kovatcheva R, Guglielmina JN, Abehsera M, et al. Ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma-a multicenter experience. J Ther Ultrasound. 2015;3:1. doi: 10.1186/s40349-014-0022-3.
- Peek MC, Ahmed M, Scudder J, et al. High intensity focused ultrasound in the treatment of breast fibroadenomata: results of the HIFU-F trial. Int J Hyperthermia. 2016;32:881–888. doi: 10.1080/02656736.2016.1212278.
- Gianfelice D, Khiat A, Amara M, et al. MR imaging-guided focused ultrasound surgery of breast cancer: correlation of dynamic contrast-enhanced MRI with histopathologic findings. Breast Cancer Res Treat. 2003;82:93–101. doi: 10.1023/B:BREA.0000003956.11376.5b.
- Gianfelice D, Khiat A, Amara M, et al. MR imaging-guided focused US ablation of breast cancer: histopathologic assessment of effectiveness– initial experience. Radiology. 2003;227:849–855. doi: 10.1148/radiol.2281012163.
- Gianfelice D, Khiat A, Boulanger Y, et al. Feasibility of magnetic resonance imaging-guided focused ultrasound surgery as an adjunct to tamoxifen therapy in high-risk surgical patients with breast carcinoma. J Vasc Interv Radiol. 2003;14:1275–1282.
- Boeer B, Brucker SY, Dezulian J, et al. Ist die Behandlung von symptomatischen Fibroadenomen der Brust mittels sonographisch gesteuertem hochintensivem Ultraschall (HIFU) in Lokalanästhesie möglich?: 61. Kongress der DGGG - Postersession; 2016.
- Alizadeh Z, Halabchi F, Mazaheri R, et al. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int J Endocrinol Metab. 2016;14:e36727. doi: 10.5812/ijem.36727.
- Cerrato F, Labow BI. Diagnosis and management of fibroadenomas in the adolescent breast. Semin Plast Surg. 2013;27:023– 025. doi: 10.1055/s-0033-1343992.
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–327. doi: 10.1038/nrc1591.
- Zhang L, Wang ZB. High-intensity focused ultrasound tumor ablation: review of ten years of clinical experience. Front Med China. 2010;4:294–302. doi: 10.1007/s11684-010-0092-8.
- Griffiths A, terHaar G, Rivens I, et al. High-intensity focused ultrasound in obstetrics and gynecology: the birth of a new era of noninvasive surgery? Ultraschall in Med. 2012;33:E8–15. doi: 10.1055/s-0031-1299407.
- Deckers R, Merckel LG, Denis de Senneville B, et al. Performance analysis of a dedicated breast MR-HIFU system for tumor ablation in breast cancer patients. Phys Med Biol. 2015;60:5527–5542. doi: 10.1088/0031-9155/60/14/5527.
- Peek MCL, Wu F. High-intensity focused ultrasound in the treatment of breast tumours. ecancer. 2018;12:794. doi: 10.3332/ecancer.2018.794.
- Cavallo Marincola B, Pediconi F, Anzidei M, et al. High-intensity focused ultrasound in breast pathology: non-invasive treatment of benign and malignant lesions. Expert Rev Med Devices. 2015;12:191–199. doi: 10.1586/17434440.2015.986096.
- Kovatcheva R, Zaletel K, Vlahov J, et al. Long-term efficacy of ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma. J Ther Ultrasound. 2017;5:1. doi: 10.1186/s40349-017-0083-1.
- Peek MC, Ahmed M, Napoli A, et al. Systematic review of high-intensity focused ultrasound ablation in the treatment of breast cancer. Br J Surg. 2015;102:873–882 doi: 10.1002/bjs.9793.