Abstract
Background: Surgery is the standard treatment for cervical metastases of medullary thyroid cancer (MTC) diagnosed after initial surgical treatment. Repeated neck dissections, however, carry an elevated risk of complications, have an adverse impact on the quality of life, and sometimes do not achieve cure of the disease
Clinical case: In a patient who had undergone two cervical neck dissections complicated by accessory nerve injury, an US-guided laser ablation (LA) of a lymph node metastasis of MTC was performed. LA was performed with two treatments during a five month period. The procedure was carried out with one optical fiber and an energy delivery of 3300 and 360 Joules. Treatments were well tolerated and resulted in complete structural and biochemical cure during a 12 month follow-up. No major complication was registered.
Conclusions: LA is a promising tool for the management of relapsing cervical metastases that are localized in non- critical areas and are characterized by low progression rate. Advantages of LA are the outpatient setting, the absence of general anesthesia, the tolerability and the safety of the procedure. Thus, LA may be considered as an alternative approach to surgery or active surveillance for the management of local recurrences of MTC in selected patients.
Introduction
Medullary thyroid cancer (MTC), a neoplasm derived from calcitonin producing parafollicular C cells, represents from 1 to 2% of thyroid carcinomas [Citation1]. MTC is classified as sporadic in 75–80% of the patients and as part of autosomal dominant hereditary syndromes in the remaining cases [Citation1]. The production of calcitonin is a characteristic feature of this tumor and the postoperative assessment of calcitonin provides a sensitive marker for disease persistence or progression [Citation1].
Sporadic MTC is a somewhat more aggressive tumor than differentiated thyroid cancers. The 5 year survival rate of MTC ranges from 70% to 90% and the 10 year survival rate ranges from 56% to 87% [Citation2]. In the majority of cases the disease has already metastasized at the time of diagnosis and up to 70% of patients have microscopic or clinically detectable cervical lymph node involvement [Citation3–4]. Consequently, after the initial surgical treatment, cervical disease persistence or recurrence may occur and additional surgery is required for lymph node metastases detected at follow-up [Citation5, Citation6]. Yet, repeated neck dissections carry an increased risk of complications (i.e. injury to the thoracic duct or to the phrenic, laryngeal and accessory nerves), have an unfavorable impact on the quality of life and may not attain the cure of the disease [Citation1, Citation3, Citation4, Citation6]. For these reasons, active surveillance is considered as a management option for small volume locoregional disease [Citation1].
Percutaneous laser ablation (LA) is a thoroughly assessed ultrasound (US)-guided thermal ablation technique, mainly employed for the treatment of benign thyroid nodules [Citation7, Citation8]. In recent years, LA and other thermal ablation procedures were reported as effective non-surgical techniques for local control of cervical nodal metastases of papillary thyroid carcinoma (PTC) that are radioiodine refractory [Citation7]. On the basis of the promising results in the management of PTC recurrences, we evaluated in this feasibility study the tolerability, safety, and clinical outcome of LA as a therapeutic tool for local control of a neck recurrence of MTC in a patient with accessory spinal nerve damage due to previous neck dissection [Citation9–11].
Clinical case
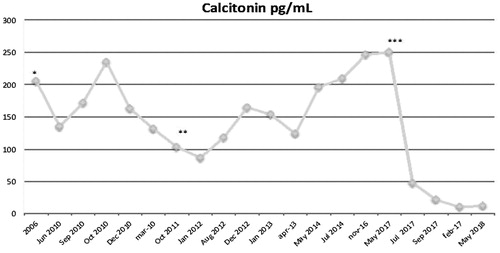
A 67-year-old woman underwent total thyroidectomy and central neck dissection in 2006 for a 8 × 7 mm thyroid nodule that at US-guided fine needle aspiration (FNA) was diagnosed as Bethesda Class VI [Citation12]. Preoperative serum calcitonin level was 250 pg/ml (normal values: 0–11.5 pg/ml; Immulite 2000, Siemens, Germany). Postoperative histological examination confirmed a medullary thyroid carcinoma of the right thyroid lobe (2018 AJCC stage: pT1N1aMx) [Citation13]. The germinal mutations of RET (REarranged during transfection) proto-oncogene analysis was negative. Serum calcium, parathyroid hormone, and 24-h metanephrine urinary excretion were within normal limits in accordance with the diagnosis of sporadic MTC. During postoperative follow-up calcitonin levels were persistently elevated (range 120–150 pg/ml, in serial determinations) but no structural evidence of disease was revealed by US, contrast-enhanced computerized tomography (CT) and radionuclide bone imaging. In June 2011, 18Fluoro-3,4-dihydroxyphenylalanine (18F-DOPA) PET/CT examination revealed two suspicious lymph nodes in the right latero-cervical area (neck level IV) and in the left superior mediastinum (neck level VI), respectively. No evidence of disease outside the neck was present. In August 2011 the patient was submitted to bilateral cervical dissection with histological confirmation of lymph node metastasis of MTC. Surgery was followed by bilateral accessory spinal nerve injury with impairment of flexion-extension and abduction movements of the arms. During the following years, a slow but progressive increase of serum calcitonin level was registered (from 140 to 247 pg/mL) and, in 2016, a second 18F-DOPA PET/CT revealed a new suspicious adenopathy in the right cervical area (. US examination confirmed the presence of a 12 × 7 × 7 mm suspicious lymph node (right level IV) and US-guided FNA provided a diagnosis of MTC lymph node metastasis. Cytological diagnosis was confirmed by the measurement of calcitonin (1278 pg/mL) on needle wash-out. Due to the complications occurred after the precedent neck dissection, the patient refused additional surgery and accepted the proposal of a palliative treatment with LA.
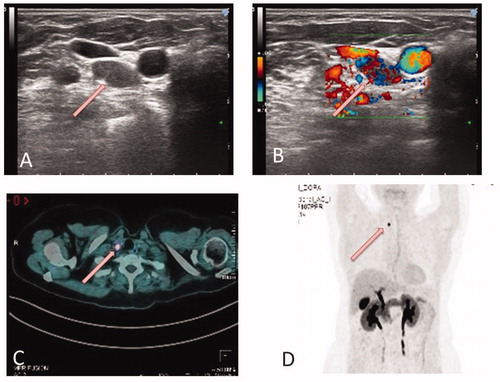
Figure 1 (A and B) US-guided FNA of a pathologic lymph-node, right level VI, 12 × 7×7 mm (white arrow). Cytologic diagnosis: lymph-node metastasis from medullary carcinoma. Calcitonin measurement on needle wash-out: 1278 pg/mL. (C and D) 18F-DOPA PET CT images (July 2016): pathologic adenopathy (7 × 6 mm) in the right upper thoracic passage (white arrows).
After local anesthesia with 2% xylocaine of superficial tissues and thyroid capsule, LA was performed with a 300 nm optical fiber inserted through a 21-gauge needle and with a Nd:YAG laser source (Modilight, Echolaser, Elesta, Florence, Italy), according to the previously described technique [Citation7, Citation14]. The treatment was carried out under US monitoring, with no preliminary hydro dissection, with 3 Watts output power. The total delivered energy was 3300 Joules in 18 min (. The procedure was well tolerated and was followed by mild cervical pain, well controlled by the administration of ketoprophen (Orudis, Sanophi, 50 mg ×2 by mouth) for 24 h. No local complication and no worsening of the pre-existent nerve injury were observed. After 24 h, color Doppler and contrast-enhanced US examination showed a marked decrease of vascular signals within the treated area (. Follow-up was performed with neck US and calcitonin measurement during the following 12 months. The mean volume of the lesion decreased from 12 × 7 × 7 mm (0.30 ml) at baseline to 9 × 7 × 4 mm (0.13 ml) at 1 month, and to 8 × 7 × 4 mm (0.11 ml) at 6 months (volume reduction vs baseline at 1 and 6 months: 56.7% and 63.4%, respectively). Due to the persistence of vascular signals in the lower part of the lesion, a second laser treatment was performed with the same procedure and an energy delivery of 360 Joules in 2 min. The volume of the lesion decreased to 7 × 4 × 3 mm (0.04 ml) nine months after the initial treatment and to 4 × 2 × 3 mm (0.01 ml) at 12 months (total decrease vs baseline: 86.7% and 96.7%, respectively). The treated lesion was visible at US examination as an ill-defined hyperechoic area devoid of vascular signals (. No re-growth was observed during follow-up and serum calcitonin level progressively decreased from 247 pg/mL at baseline to 48 pg/mL at 3 months, 21 pg/mL at 5 months, 10 pg/mL at 9 months and 12 pg/mL at 12 months (.
Figure 2. Neck Ultrasound Examination during treatment. Ultrasound-guided percutaneous laser ablation of the pathologic lymph-node showed in Figure 1. (A) the US image demonstrates the insertion of the optical fibre in the pathologic lymph-node (white arrow). (B) colour Doppler monitoring of the target lesion during US-guided laser treatment (white arrow).
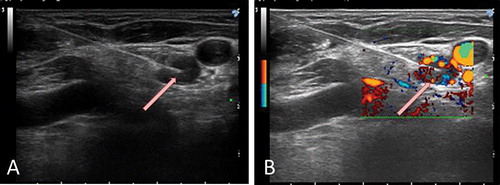
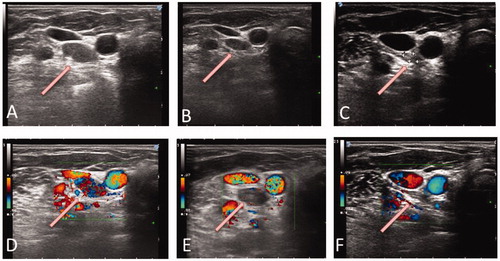
Figure 3. Neck ultrasound follow-up after LA. (A, B, C) Progressive volume decrease of the LA-treated lesion (A: at baseline; B. at 5 months; C: at 12 months). (D, E, F) Color-Doppler examination showed the marked decrease and, successively, the disappearance of the vascular signals within the treated area (D: at baseline; E: at 5 months; F: at 12 months). In all images, the arrows point toward the pathologic lymph-node.
Discussion
Sporadic MTC is frequently associated with cervical persistence or recurrence of disease after the initial surgical treatment [Citation6, Citation15, Citation16]. In absence of distant metastases, patients with recurrent cervical lymph node metastases after thyroidectomy may be successfully cured with re-operation. The systematic central and lateral lymph node dissection is reported to attain biochemical cure in 44% of patients who had no lymph node metastases at prior surgery and in 18% of patients who had up to five metastatic lymph nodes [Citation15]. So, even if surgical resection is the standard treatment of cervical metastases, reoperation carries an elevated risk of morbidity, has an unfavorable impact on the quality of life, is expensive, and is frequently not curative [Citation17]. Various non-surgical management options may be considered for the control of MTC metastasis: active surveillance, external beam radiation therapy (EBRT), directed therapies (such as radiofrequency, cryoablation, and embolization), or systemic therapies with tyrosine-kinase inhibitors (TKI) [Citation1, Citation18]. Due to the side-effects, cost and impact on the quality of life, the use of TKI and EBRT is not appropriate in patients with small size and slow growing recurrences. So, for most MTC patients with persistent, asymptomatic small volume locoregional disease, active surveillance is suggested as the best management option. Patients are generally followed with serial imaging examination at 6 month intervals, and surgical intervention is performed in case of structural disease progression [Citation1].
US-guided thermal ablation procedures are thoroughly assessed loco-regional treatments for thyroid lesions and are reported to effectively control local recurrences of PTC with low risk of complications [Citation19]. Among them, LA seems to be ideal due to the smallest needle caliber (21 G) and the high precision of the technique, usually not requiring preliminary hydrodissection [Citation9, Citation20]. So, for patients with slow growing lymph node metastases, LA may represent a safe and effective alternative to simple surveillance [Citation1]. On the basis of these considerations, we preferred US-guided laser treatment in a patient who had undergone repeated neck dissection followed by injury of accessory nerves. The treatment was well-tolerated, with no complication, and achieved structural and biochemical cure during a 12 month follow-up.
This is the first case report on the use of LA for non-surgical management of MTC cervical metastases. The long-term efficacy of the treatment was demonstrated by the structural changes of the lesion at US examination and by the nearly complete and persistent normalization of serum calcitonin levels. A partial limitation of the study is the absence of contrast-enhanced US images after LA due, in this case, to technical reasons.
In conclusion, surgery remains the standard treatment for local recurrences of MTC but LA appears as a promising tool for the management of asymptomatic cervical metastasis with low progression rate. Relevant advantages of LA are the outpatient setting, the absence of general anesthesia, the safety and the tolerability of the technique. LA should be considered as an appropriate therapeutic approach especially in patients who are poor surgical candidates and in patients with surgical complications after a previous lymphadenectomy.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wells SA, Jr, Asa SL, Dralle H, et al. American thyroid association guidelines task force on medullary thyroid carcinoma. revised american thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610.
- Abraham DT, Low TH, Messina M, et al. Medullary thyroid carcinoma: long-term outcomes of surgical treatment. Ann Surg Oncol. 2011;18:219–225.
- Kebebew E, Greenspan FS, Clark OH, et al. Extent of disease and practice patterns for medullary thyroid cancer. J Am Coll Surg. 2005;200:890–896.
- Scollo C, Baudin E, Travagli JP, et al. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2003;88:2070–2075.
- NCCN Clinical Practice Guidelines in Oncology (2016) Medullary thyroid carcinoma. https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf
- Konstantinidis A, Stang M, Roman SA, et al. Surgical management of medullary thyroid carcinoma. Updates Surg. 2017;69:151–160.
- Pacella CM, Bizzarri G, Guglielmi R, et al. Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology. 2000;217:673–677.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100:3903–3910.
- Papini E, Bizzarri G, Bianchini A, et al. Percutaneous ultrasound-guided laser ablation is effective for treating selected nodal metastases in papillary thyroid cancer. J Clin Endocrinol Metab. 2013;98:E92–E97.
- Mauri G, Cova L, Tondolo T, et al. Percutaneous laser ablation of metastatic lymph nodes in the neck from papillary thyroid carcinoma: preliminary results. J Clin Endocrinol Metab. 2013;98: E1203–E1207.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39: 1023–1030.
- Cibas ES, Ali SZ. NCI Thyroid FNA state of the science conference. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658–665.
- American Joint Committee on Cancer. Thyroid–Medullary. In: Rosen JE, Lloyd RV, Brierly JD, et al., editors. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017: 891.
- Pacella CM, Bizzarri G, Spiezia S, et al. Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology. 2004;232: 272–280.
- Machens A, Dralle H. Benefit-risk balance of reoperation for persistent medullary thyroid cancer. Ann Surg. 2013; 257:751–757.
- Machens A, Gimm O, Ukkat J, et al. Improved prediction of calcitonin normalization in medullary thyroid carcinoma patients by quantitative lymph node analysis. Cancer. 2000; 88:1909–1915.
- Van Heerden JA, Grant CS, Gharib H, et al. Long-term course of patients with persistent hypercalcitoninemia after apparent curative primary surgery for medullary thyroid carcinoma. Ann Surg. 1990;212:395–400.
- Owen RP, Silver CE, Ravikumar TS, et al. Techniques for radiofrequency ablation of head and neck tumors. Arch Otolaryngol Head Neck Surg. 2004;130:52–56.
- Jeong SY, Baek JH, Choi YJ, et al. Ethanol and thermal ablation for malignant thyroid tumours. Int J Hyperthermia. 2017;33: 938–945.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33:911–919.