Abstract
Objective: To evaluate the feasibility and safety of vaginal delivery after ultrasound-guided high-intensity focused ultrasound (HIFU) ablation treatment for women with uterine fibroids of child-bearing ages.
Methods: A prospective study was conducted on women who underwent ultrasound-guided HIFU therapy for uterine fibroids at the Chinese PLA General Hospital from January 2008 to December 2014. Patients were interviewed yearly to assess their fertility outcomes, including conception method, delivery mode, neonatal outcomes and complications during pregnancy, labor, and delivery.
Results: A total of 174 patients with plans for future pregnancy were included, and 88 pregnancies in 81 women occurred. The pregnancy rate was 46.6% (81/174), and the median follow-up time was 76 months. The rate of pregnancies that ended in miscarriages was 10% (9/88), the rate of elective pregnancy termination was 6% (5/88), and 84% (74/88) of the pregnancies resulted in deliveries, with 3 of the 71 women having two deliveries. A cesarean section was requested by 50% (37/74) of the women, and 50% (37/74) opted for a vaginal delivery. Eleven of the 37 pregnancies were scheduled for elective cesarean sections, owing to pregnancy complications; the remaining 26 pregnancies were scheduled for vaginal delivery, and 21 (80.8%) were successful. None of the patients with a vaginal delivery experienced any complications during pregnancy and labor.
Conclusions: Ultrasound-guided HIFU ablation could be considered a promising clinical treatment for women with uterine fibroids and plans for future pregnancy, and vaginal delivery after ultrasound-guided HIFU ablation treatment appear to be feasible and safe.
Introduction
Uterine fibroids are among the most common uterine diseases, and they are found in approximately 12% to 25% of the women of reproductive age [Citation1,Citation2]. These benign neoplasms may produce symptoms such as menorrhagia, frequent urination, and dysmenorrhea and can even result in subfertility [Citation3]. The treatments for symptomatic uterine fibroids range from surgery to minimally invasive approaches. Myomectomy is the most common surgical treatment for those considering future pregnancies [Citation4], and improved pregnancy rates and outcomes have been reported following this procedure [Citation5]. However, the risk of uterine rupture during pregnancy and the formation of surgical adhesions may make women opt for less invasive alternatives [Citation6,Citation7].
High-intensity focused ultrasound (HIFU) ablation is a noninvasive technique for the treatment of solid tumors and has been shown in previous studies to be safe and effective for symptomatic uterine fibroids [Citation8–14]. Because of its unknown effect on future fertility, initial trials of HIFU ablation treatment for uterine fibroids were restricted to patients with no desire for future fertility. Interestingly, pregnancy has occasionally occurred, and dozens of successful delivery cases after HIFU ablation treatment have been published [Citation15–20]. Nevertheless, among the reported cases of successful delivery, little study has focused on the cases of vaginal delivery after ultrasound-guided HIFU ablation treatment for uterine fibroids. In addition, the feasibility and safety of vaginal delivery after ultrasound-guided HIFU ablation have been controversial and not been well studied prospectively.
The purpose of our study is to evaluate the feasibility and safety of vaginal delivery after ultrasound-guided HIFU ablation treatment for uterine fibroids.
Materials and methods
Patients
Women of reproductive age who underwent ultrasound-guided HIFU ablation treatment for uterine fibroids at the Chinese PLA General Hospital from January 2008 to December 2014 were included in the study if they met the following inclusion criteria: (1) had symptoms related to uterine fibroids such as menorrhagia, pelvic pressure, infertility and previous history of spontaneous abortion or were asymptomatic but were anxious about the negative effect of uterine fibroids (≥5 cm in diameter) in future pregnancy; (2) had received no other treatment for uterine fibroids before ultrasound-guided HIFU ablation treatment; and (3) had an intact myometrium surrounding the ablated fibroids when viewed on contrast-enhanced magnetic resonance imaging (MRI) 3 months after the treatment. The exclusion criteria were: (1) fibroids categorized as Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) type 0, type 7 or type 8; (2) nulliparous women with no birth plans or unmarried during follow-up (it is legal for women to have children after they get married in China); (3) acute pelvic inflammatory disease and infertility due to other reasons besides uterine fibroids, such as tubal obstruction and polycystic ovarian syndrome; (4) serious abdominal scars in the proposed acoustic pathway; and (5) contraindications to anesthesia, ultrasonic contrast agents and MRI contrast agents.
The study was approved by the Ethics Committee of the Chinese PLA General Hospital, Beijing, China. Before treatment, all patients were fully informed prior to the procedure of the potential risks and side effects of the treatment on fertility. In addition, pregnant women who attempted vaginal delivery after treatment were given a thorough explanation of the benefits and potential risks of this method. The explanation focused on the risk of uterine rupture during pregnancy and labor. However, vaginal delivery would not be attempted when pregnancy complications occurred such as fetal macrosomia, fetal malpresentation, and intrauterine growth retardation. In addition, an immediate cesarean section would be performed when vaginal trial failed for reasons such as arrest of labor, postdate infants and any emergency.
Equipment
Ultrasound-guided HIFU ablation treatment for uterine fibroids was performed using the JC HIFU system (model JC; Chongqing Haifu Technology, Chongqing, China) with real-time imaging monitoring through a diagnostic ultrasound probe (3.5 MHz to 5.0 MHz) integrated into the center of the therapeutic transducer. The focused ultrasound energy is produced by a therapeutic transducer with a focal length of 150 mm operating at a frequency of 0.9 MHz. The range of the focal region extends 9.8 mm along the beam axis and 1.3 mm in the transverse direction. The movement of the integrated transducer can be controlled in six directions, including three orthogonal directions (x, y and z), rotation along the ultrasound beam axis (θ), and rotation along the long (γ) or short (ψ) axis of the bed.
Ultrasound-guided HIFU ablation procedure
Before the treatment, contrast-enhanced MRI and ultrasound were essential for every patient to determine the location, dimension and blood supply of the targeted fibroids. In addition, careful bowel preparation was required of patients 2 days prior to the treatment, according to instructions, and an enema was given in the early morning of the treatment day following a 12-h fast. Ultrasound-guided HIFU ablation was performed under conscious intravenous sedation with the administration of fentanyl (0.8–1.0 μg/kg) and midazolam (0.02–0.03 mg/kg) every 30 min. Patients were placed in the prone position on the treatment table to ensure that their abdominal skin was in contact with the degassed water. To push away the bowel from the acoustic pathway, different sizes of degassed water balloons were used when necessary. The targeted fibroids were divided into sections with 5 mm of separation guided by real-time ultrasound. An acoustic power of 420–520 W was used. The treatment began from the center layer of the fibroids, and the focused spot was moved to the next layer when the gray-scale of the targeted layer was changed. The treatment was terminated when all the targeted lesions were ablated. During the treatment, the focused spot was kept at least 1 cm away from the margin of the fibroids to avoid thermal damage to adjacent structures. Post-treatment contrast-enhanced ultrasound (CEUS) was performed immediately after ultrasound-guided HIFU ablation for a preliminary evaluation of therapeutic efficacy. If blood perfusion was detected in the targeted fibroids, complementary ultrasound-guided HIFU ablation treatment was performed immediately.
Post-treatment follow-up and data collection
Every patient underwent a contrast-enhanced pelvic MRI scan within one week after treatment to estimate the none perfusion volume (NPV) and NPV ratio of the fibroids and 3 months later to assess the integrity of the myometrium surrounding the ablated fibroids. Conception planning was not recommended until the integrity of the myometrium surrounding the ablated fibroids was confirmed. Patients were then advised to return for an MRI or ultrasound examination at 6 months, 12 months and then once a year thereafter.
Basic information about the patients at the time of ultrasound-guided HIFU ablation treatment, including age, characteristics of the fibroids (number, location and type) and pregnancy history, was collected. According to the FIGO classification system, submucous fibroids are subclassified into type 0, 1, and 2 depending on the degree of intramural involvement. Type 0 lesions are entirely intracavitary with a stalk attached to the endometrium, with types 1 and 2 being ≤50% and >50% intramural, respectively. Intramural fibroids are subclassified into type 3 and 4. The type 3 lesions are extracavitary but abut the endometrium, while type 4 lesions are entirely within the myometrium. Subserosal fibroids are subclassified into type 5, 6 and 7, with type 5 being >50% intramural, type 6 ≤ 50% intramural, and type 7 attached to the serosa by a stalk. Type 8 lesions are not related to the myometrium at all [Citation21]. The locations of the fibroids were described as on the anterior or posterior wall or the fundus of the uterus. The measurement of the non perfusion area, fibroids and uterus was performed on MRI and the NPV, volume of fibroids and uterus was calculated using the equation: V = 0.5233 × D1 × D2 × D3 (D1 is the longitudinal dimension, D2 is the anterior–posterior dimension, and D3 is the transverse dimension) [Citation22]. The NPV ratio was calculated according to the following equation: NPV% = the volume of the non perfusion area/the volume of the targeted lesions ×100%. The symptom relief and changes of the treated fibroids were assessed. Obstetric outcomes were also recorded; data collected included the time interval between ultrasound-guided HIFU ablation treatment and pregnancy, conception method, delivery mode, obstetric complications and neonatal outcomes.
Study endpoints
The primary endpoint of the study was the successful delivery rate within women who opted for vaginal birth.
Secondary endpoints were fibroid related symptoms remission, ablated fibroid volume changes and maternal or neonatal complications after HIFU ablation treatment.
Statistical analysis
Statistical analyses were performed using SPSS version 17.0 (IBM, NY, USA). Continuous variables were described as the mean ± SD or medians with ranges. Differences at p < .05 were statistically significant.
Results
Patients characteristics
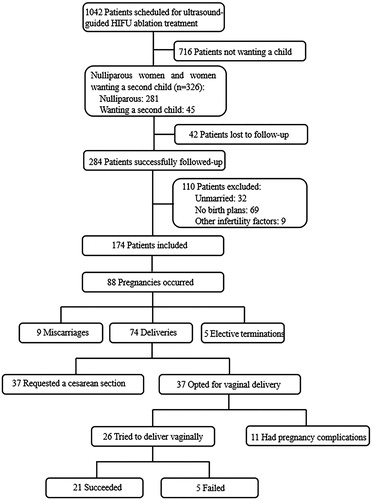
A total of 1042 women were scheduled for ultrasound-guided HIFU ablation treatment for uterine fibroids in the Chinese PLA General Hospital between January 2008 and December 2014. Of these patients, 281 women were nulliparous, and 45 women wanted a second child. A total of 284 women were successfully followed-up, and 174 women without contraception (having a normal sex life) were ultimately included in this study. Details about the excluded patients are shown in the flow chart (). Among the 174 women, 155 (89.1%) women were nulliparous and 123 (70.7%) women had never been pregnant. A total of 154 (88.5%) women had symptoms related to fibroids and 29 women had spontaneous abortion or infertility history, and 137 patients (89.0%) achieved symptom remission after the treatment. The median follow-up time was 76 months (range, 29–117 months).
Pregnancy outcomes after ultrasound-guided HIFU
At the end of the follow-up 88 pregnancies in 81 women had occurred, resulting in a pregnancy rate of 46.6% (81/174). Of the 88 pregnancies, 83 cases were spontaneous pregnancies and the remaining 5 cases involved in vitro fertilization. The mean age of the 81 pregnant women at the time of treatment was 31.1 ± 3.8 years (range, 24–41 years); the rate of never having been pregnant was 70.4% (57/81), and 90.1% (73/81) of the women had never had a delivery. The median interval between HIFU treatment and pregnancy was 16 months (range, 1–66 months). Of the 81 patients, 67 patients (82.7%) had symptoms related to fibroids, and 60 patients (89.6%) achieved symptom remission after the treatment up to the end of follow-up. A single fibroid was treated in 77.8% (63/81) of cases, and multiple fibroids were treated in 22.2% (18/81) of cases. The mean largest diameter and volume of the ablated fibroids was 5.9 ± 1.6 cm (range, 2.6–9.6 cm) and 90.0 ± 76.9 cm3 (range, 6.1–344.2 cm3), respectively. Ten patients had subserosal fibroids, 56 had intramural fibroids, and 15 had submucosal fibroids. The mean NPV and NPV ratio of the ablated fibroids within one week after the treatment was 81.3 ± 68.0 cm3 (range, 4.8–285.7 cm3) and 89.9 ± 0.1% (range, 39.0%–100%), leading to an average fibroid volume shrinkage of 72.5 ± 20.7% (30.5–100%) up to the end of follow-up. () Pregnancies ended in miscarriages at a rate of 10% (9/88), elective pregnancy termination was 6% (5/88), and 84% (74/88) of pregnancies resulted in deliveries, with 3 women having two deliveries among the 71 women. Regarding delivery outcomes, 7% (5/74) of the deliveries ended in preterm deliveries, 1% (1/74) resulted in post-term deliveries and 92% (67/74) ended in full-term deliveries. A total of 21 (28.4%) vaginal deliveries occurred in 19 women, and 53 (71.6%) cesarean sections occurred in 52 women (). Among the 52 women who underwent cesarean sections, 69.2% (36/52) requested the procedure for the following social reasons: 15 patients were worried about uterine rupture during vaginal delivery after HIFU ablation treatment, 10 were concerned about fear of pain during natural labor, and 11 had received a recommendation from obstetricians who took a conservative approach to managing labor in pregnant women with a history of HIFU ablation treatment.
Table 1. Baseline characteristics of 81 pregnant women at the time of ultrasound-guided HIFU ablation treatment.
Results of vaginal delivery after ultrasound-guided HIFU
Of the 74 delivered pregnancies in 71 women, 37 (50%) cases opted for a vaginal delivery (). Eleven of the 37 pregnancies underwent elective cesarean sections for the following indications: 5 with fetal macrosomia, 4 with fetal malpresentation, 1 with placenta previa, and 1 with intrauterine growth retardation. The remaining 26 pregnancies without complications were scheduled for vaginal delivery, and 21 (80.8%) were successful, with two women having two deliveries (). The characteristics and obstetric outcomes of the 19 women achieving successful vaginal delivery are shown in . None of the patients who delivered vaginally experienced any maternal or neonatal complications. Adhesion and placental abnormalities, such as placenta accrete, placental abruption and placenta remnant were not observed in any of the patients. No uterine rupture or postpartum hemorrhage occurred in present observation. The 5 women who experienced a failed vaginal delivery underwent cesarean sections: 1 had not developed spontaneous labor at 42 weeks of gestation; 3 had developed spontaneous labor but failed to progress; and 1 had intrauterine fetal distress during labor. Patient no. 20 developed spontaneous labor but failed to progress due to an intramural fibroid that obstructed the pelvic outlet. The fibroid was inadequately ablated and recurred 12 months after the treatment. During the cesarean section, she underwent a myomectomy of the fibroid of 178.4 cm3 in volume. represents the basic information of the 5 women who experienced a failed vaginal delivery; however, there were no maternal or neonatal complications.
Figure 2. Images for a 28-year-old woman with two vaginal deliveries after ultrasound-guided HIFU ablation treatment for a fibroid. (a) Contrast-enhanced ultrasound (CEUS) obtained before treatment showed enhancement of the fibroid (white arrow) on the posterior wall of the uterus. (b) Immediate CEUS obtained after treatment showed a non-enhanced region (53.7 cm3). The margins of the non-enhanced region (white arrow) were shown at the ablated region. (c) and (d) Sagittal view of MRI obtained before treatment showed enhancement of the fibroid (red arrow) on the posterior wall of the uterus. (e) and (f) Sagittal view of an MRI obtained 3 months after ultrasound-guided HIFU ablation showed a decreased size of the fibroid (red arrow). The volume of the fibroid was 35.5 cm3. The woman was pregnant after 6 months and 24 months following treatment and gave birth to two healthy babies weighing 3.3 kg and 3.0 kg, respectively.
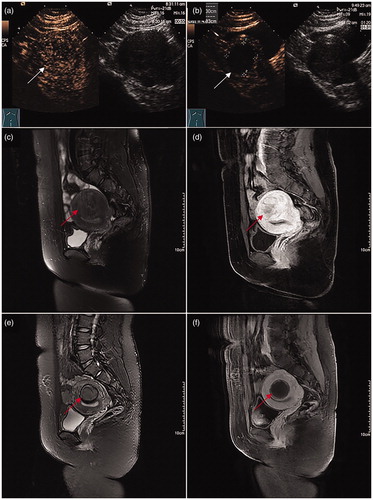
Table 2. Clinical characteristics and obstetric outcomes of 19 women achieving successful vaginal delivery after ultrasound-guided HIFU ablation treatment with summary statistics.
Table 3. Clinical characteristics and obstetric outcomes of 5 women who experienced failed vaginal delivery after ultrasound-guided HIFU ablation treatment with summary statistics.
Neonate outcomes and pregnancy complications
The mean birth weight of the neonates was 3.3 ± 0.4 kg (range, 1.4–4.4 kg), with two low-birth-weight infants. The median Apgar score at 1-min was 10 (8–10). One woman experienced a massive hemorrhage caused by placenta previa at 30 weeks of pregnancy; a premature baby weighing 1.4 kg was born after emergency cesarean section. Another woman with pregnancy-induced hypertension underwent cesarean section at 34 week of pregnancy due to fetal intrauterine growth retardation, and the weight of the baby was 2.3 kg. All the babies, including the two low birth weight babies, were healthy during follow-up. Malpresentation of the fetus occurred in 5.4% (4/74) of births. Two women experienced postpartum hemorrhage after the cesarean section, and one of them required blood transfusions. The remaining patients had unremarkable postpartum complications.
Discussion
Symptomatic uterine fibroids are increasingly common during the later reproductive years, and the impact of their treatment on fertility remains a topic for debate. Myomectomy is the most common surgical treatment for women with uterine fibroids of child-bearing ages, and vaginal delivery after myomectomy is controversial because the incidence of uterine rupture in women who experienced a trial of labor after myomectomy was 0.03–0.47% [Citation23–25]. During myomectomy, the continuity of the normal myometrium was interrupted, and the formation of myometrial scars, which were strained and thinner than the normal myometrium, may exert a negative effect on uterine contractions and increase the risk of uterine rupture during pregnancy and labor [Citation26,Citation27]. HIFU ablation treatment for uterine fibroids is a noninvasive tissue ablation technique with similar efficacy, faster recovery, and fewer complications compared to myomectomy [Citation14,Citation28]. During treatment, an ultrasound probe is used to provide real-time imaging of the patient’s anatomy surrounding the uterus and to monitor the thermal effect of the ablation on the targeted fibroids. By moving the focused spot, the fibroids are ablated conformally, preventing an unnecessary thermal damage to the adjacent structures, such as the normal myometrium and the endometrium; this is accomplished by keeping the focused spot at least 1 cm away from the margin of the fibroids. Without incisions and sutures, scar formation and collagen fiber hyperplasia in the myometrium occur less frequently after HIFU ablation treatment for fibroids. In addition, women were only allowed to conceive once the integrity of the myometrium surrounding the ablated fibroids was confirmed on contrast-enhanced MRI three months after the treatment in the present study. Previous case reports have shown that the pregnancies delivered vaginally at term after MRgFUS were without complications [Citation15,Citation16,Citation29], and another study reported a 64% vaginal delivery rate without low birth infants and stillbirths after MRgFUS among 54 pregnancies in 51 women [Citation19]. In the present study, vaginal delivery was successfully completed in 21 (80.8%) pregnancies in 19 women after HIFU ablation treatment without complications. Nevertheless, in this study, a total of 52 women underwent cesarean sections; 69.2% (36/52) requested a cesarean section for the following factors: 69.4% (25/36) were afraid to try vaginal delivery for fear of pain and complications, such as uterine rupture during vaginal delivery, and the remaining 30.6% (11/36) had received a recommendation from an obstetrician regarding the uncertain safety of vaginal delivery owing to the limited reports on pregnancy outcomes and the safety of vaginal delivery after HIFU treatment, leading to a significant reduction in vaginal delivery. Similar results were reported in two studies showing that social factors led to cesarean delivery, accounting the selection of this procedure in 72.1% (49/68) and 79.1% (53/67) of cases, respectively [Citation30,Citation31]. Along with the maturation of HIFU ablation treatment and publication of more clinical data on the pregnancy outcomes and safety of vaginal delivery, it may be highly probable that the cesarean delivery rate after HIFU ablation treatment will decrease.
The success rate of vaginal delivery after myomectomy reportedly has ranged from 25.5% to 80.6% [Citation25,Citation26,Citation32–35]. Studies have found that failed vaginal delivery may be attributed to the opened endometrium during the myomectomy [Citation35], and cesarean section is advantageous under these circumstances [Citation26]. As for HIFU ablation treatment, besides effective symptom relief, thermal damage to the endometrium is also a potential risk of the treatment for submucosal fibroids [Citation12]. Among the 15 women with submucosal fibroids in the present study, two women had to undergo a cesarean section for obstetric complications, and six women achieved successful vaginal delivery, indicating that the thermal damage to the endometrium may not be irreversible after HIFU ablation treatment and may not exert a negative effect on the development of spontaneous labor. Nevertheless, this issue needs to be further examined in a larger number of patients owing to the small number of the vaginal deliveries in the present study.
Among the 21 successful vaginal deliveries in our study, the mean birth weight of the infants was 3.3 kg, and there were no low birth weight infants and any complications occurred during pregnancy, and labor. No postpartum hemorrhage or placenta remnant occurred, indicating that vaginal delivery after ultrasound-guided HIFU ablation treatment may not increase the risk of obstetric and postpartum complications.
There are several limitations in the present study. First, the number of patients with a vaginal delivery was too small to allow reliable statistical analysis for factors attributed to failure between the successful and failed groups; this deserves further examination in larger samples and multi-center studies. In addition, a randomized controlled trial of myomectomy versus ultrasound-guided HIFU ablation treatment is required to optimally evaluate the safety of vaginal delivery.
Conclusions
In conclusion, ultrasound-guided HIFU ablation could be considered a promising clinical treatment for women with uterine fibroids and plans for future pregnancy. Moreover, vaginal birth after ultrasound-guided HIFU ablation treatment appears to be feasible and safe, an observation that deserves further validation in a larger number of patients.
Acknowledgments
The authors wish to thank doctors from all the clinical centers and the radiology department for their skilled technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Downes E, Sikirica V, Gilabert-Estelles J, et al. The burden of uterine fibroids in five European countries. Eur J Obstet Gynecol Reprod Biol. 2010;152:96–102.
- Koo YJ, Lee JK, Lee YK, et al. Pregnancy outcomes and risk factors for uterine rupture after laparoscopic myomectomy: a single-center experience and literature review. J Minim Invasive Gynecol. 2015;22:1022–1028.
- Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91:1215–1223.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99–108.
- Clark NA, Mumford SL, Segars JH. Reproductive impact of MRI-guided focused ultrasound surgery for fibroids: a systematic review of the evidence. Curr Opin Obstet Gynecol. 2014;26:151–161.
- Somigliana E, Vercellini P, Daguati R, et al. Fibroids and female reproduction: a critical analysis of the evidence. Hum Reprod Update. 2007;13:465–476.
- Yoon SW, Kim KA, Kim SH, et al. Pregnancy and natural delivery following magnetic resonance imaging-guided focused ultrasound surgery of uterine myomas. Yonsei Med J. 2010;51:451.3.
- Stewart EA, Rabinovici J, Tempany CM, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85:22–29.
- Taran FA, Tempany CMC, Regan L, et al. Magnetic resonance-guided focused ultrasound (MRgFUS) compared with abdominal hysterectomy for treatment of uterine leiomyomas. Ultrasound Obstet Gynecol. 2009;34:572–578.
- Kim HS, Baik JH, Pham LD, et al. MR-guided high-intensity focused ultrasound treatment for symptomatic uterine leiomyomata: long-term outcomes. Acad Radiol. 2011;18:970–976.
- Gorny KR, Woodrum DA, Brown DL, et al. Magnetic Resonance–guided Focused Ultrasound of Uterine Leiomyomas: Review of a 12-month Outcome of 130 Clinical Patients. J Vasc Interv Radiol. 2011;22:857–864.
- Wang W, Wang Y, Wang T, et al. Safety and efficacy of US-guided high-intensity focused ultrasound for treatment of submucosal fibroids. Eur Radiol. 2012;22:2553–2558.
- Dobrotwir A, Pun E. Clinical 24 month experience of the first MRgFUS unit for treatment of uterine fibroids in Australia. J Med Imaging Radiat Oncol. 2012;56:409–416.
- Chen J, Li Y, Wang Z, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG: Int J Obstet Gy. 2018;125:354–364.
- Gavrilova-Jordan LP, Rose CH, Traynor KD, et al. Successful term pregnancy following MR-guided focused ultrasound treatment of uterine leiomyoma. J Perinatol. 2007;27:59–61.
- Hanstede MM, Tempany CM, Stewart EA. Focused ultrasound surgery of intramural leiomyomas may facilitate fertility: a case report. Fertil Steril. 2007;88:497:e5–497.
- Zaher S, Lyons D, Regan L. Uncomplicated term vaginal delivery following magnetic resonance-guided focused ultrasound surgery for uterine fibroids. Biomed Imaging Interv J. 2010;6:e28.
- Bouwsma EV, Gorny KR, Hesley GK, et al. Magnetic resonance-guided focused ultrasound surgery for leiomyoma-associated infertility. Fertil Steril. 2011;96:e9–e12.
- Rabinovici J, David M, Fukunishi H, et al. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;Jan93:199–209.
- Qin J, Chen JY, Zhao WP, et al. Outcome of unintended pregnancy after ultrasound-guided high-intensity focused ultrasound ablation of uterine fibroids. Int J Gynaecol Obstet. 2012;117:273–277.
- Munro MG, Critchley HO, Fraser IS. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204–2208, 2208: e1–3.
- Orsini LF, Salardi S, Pilu G, et al. Pelvic organs in premenarcheal girls: real-time ultrasonography. Radiology. 1984;153:113–116.
- Claeys J, Hellendoorn I, Hamerlynck T, et al. The risk of uterine rupture after myomectomy a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11:197–206.
- Gambacorti-Passerini Z, Gimovsky AC, Locatelli A, et al. Trial of labor after myomectomy and uterine rupture: a systematic review. Acta Obstet Gynecol Scand. 2016;95:724–734.
- Dubuisson JB, Fauconnier A, Deffarges JV, et al. Pregnancy outcome and deliveries following laparoscopic myomectomy. Hum Reprod. 2000;15:869–873.
- Bernardi TS, Radosa MP, Weisheit A, et al. Laparoscopic myomectomy: a 6-year follow-up single-center cohort analysis of fertility and obstetric outcome measures. Arch Gynecol Obstet. 2014; Jul290:87–91.
- Ofir K, Sheiner E, Levy A, et al. Uterine rupture: risk factors and pregnancy outcome. Am J Obstet Gynecol. 2003;189:1042–1046.
- Ji Y, Hu K, Zhang Y, et al. High-intensity focused ultrasound (HIFU) treatment for uterine fibroids: a meta-analysis. Arch Gynecol Obstet. 2017;296:1181–1188.
- Morita Y, Ito N, Ohashi H. Pregnancy following MR-guided focused ultrasound surgery for a uterine fibroid. Int J Gynaecol Obstet. 2007;99:56–57.
- Zou M, Chen L, Wu C, et al. Pregnancy outcomes in patients with uterine fibroids treated with ultrasound-guided high-intensity focused ultrasound. BJOG: Int J Obstet Gy. 2017;124: 30–35.
- Li JS, Wang Y, Chen JY, et al. Pregnancy outcomes in nulliparous women after ultrasound ablation of uterine fibroids: A single-central retrospective study. Sci Rep. 2017;7:3977.
- Seracchioli R, Manuzzi L, Vianello F, et al. Obstetric and delivery outcome of pregnancies achieved after laparoscopic myomectomy. Fertil Steril. 2006;86:159–165.
- Rovio PH, Heinonen PK. Pregnancy outcomes after transvaginal myomectomy by colpotomy. Eur J Obstet Gynecol Reprod Biol. 2012;161:130–133.
- Fagherazzi S, Borgato S, Bertin M, et al. Pregnancy outcome after laparoscopic myomectomy. Clinical and Experimental Obstetrics & Gynecology. 2014;41:375–379.
- Kumakiri J, Takeuchi H, Itoh S, et al. Prospective evaluation for the feasibility and safety of vaginal birth after laparoscopic myomectomy. J Minim Invasive Gynecol. 2008;15:420–424.