Abstract
Background: Programmed death-ligand 1 (PD-L1) and CD8+ tumor-infiltrating lymphocytes (TILs) were associated with non-small cell lung cancer (NSCLC). We conducted this study to evaluate the correlation between PD-L1 or CD8+ TILs expression and MWA or survival in advanced NSCLC patients treated with microwave ablation (MWA) plus chemotherapy.
Methods: Previously untreated, pathologically verified advanced NSCLC patients with adequate tissues for the analysis of PD-L1 expression and the presence of CD8+ TILs were retrospectively enrolled. None of the patients had sensitive mutations, and therefore, they were treated with MWA of the primary tumors followed by chemotherapy.
Results: A total of 51 patients were enrolled. PD-L1 expression and the presence of CD8+ TILs were identified in 31 (60.8%) and 9 (17.6%) patients, respectively. PD-L1 expression and CD8+ TILs had no correlation with baseline characteristics, the response to chemotherapy or MWA. Patients with PD-L1 expression had similar progression-free survival (PFS: 7.9 months for PD-L1-positive vs. 5.8 months for PD-L1-negative; p = .660) and overall survival (OS: 18.7 months for PD-L1-positive vs. 15.2 months for PD-L1-negative; p = .901). Patients with CD8+ TIL expression did not show superior PFS (CD8+ TIL vs. CD8– TIL, 8.0 vs. 6.2 months, p = .435) or OS (CD8+ TIL vs. CD8– TIL, 20.5 vs. 16.9 months, p = .653).
Conclusion: PD-L1 expression and the presence of CD8+ TILs could predict neither the patients’ response to chemotherapy or MWA nor survival in advanced NSCLC patients treated with MWA plus chemotherapy.
Introduction
Lung cancer is the leading cause of cancer-associated mortality and morbidity both in China and worldwide [Citation1–3]. Lung cancer can be differentiated into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC accounts for nearly 85% of cases, and 80% of these patients are diagnosed with advanced-stage cancer and thus cannot be treated by radical surgery.
For patients without kinase-sensitive mutations in epidermal growth factor receptor (EGFR) tyrosine kinase, anaplastic lymphoma kinase (ALK) or ROS1 kinase, platinum-based doublet chemotherapy remains the first-line treatment for advanced NSCLC [Citation4–5]. Previously, three retrospective studies verified that microwave ablation (MWA) at the primary tumor sites followed by chemotherapy could improve the progression-free survival (PFS) of advanced NSCLC patients [Citation6–8]. One multicenter, prospective, randomized, controlled phase III study proved that both the progression-free survival (PFS) and overall survival (OS) were prolonged for those treated with a combination of MWA and chemotherapy [Citation9].
Checkpoint molecules, especially programmed death-1 (PD-1) and its ligands PD-L1 (B7H1) and PD-L2 (B7-DC) have drawn increasing attention [Citation10–17]. PD-L1 expression is widely observed in NSCLC and its interaction with PD-1 induces immune suppression by the following mechanisms: (1) induction of apoptosis in activated T cells [Citation10]; (2) facilitation of T cell anergy and exhaustion [Citation11–13]; (3) enhancement of regulatory T cell function [Citation14]; (4) inhibition of T cell proliferation [Citation15–16]; and (5) impaired T cell activation and IL-2 production [Citation17]. The PD-1 immune checkpoint inhibitors, pembrolizumab and nivolumab, have improved the survival of patients with advanced NSCLC [Citation18–20].
PD-L1 expression is associated with the survival of both early-stage and advanced-stage NSCLC, although some of the results are inconsistent. For early-stage NSCLC, most studies have demonstrated that patients with PD-L1 overexpression had a shorter disease-free survival (DFS) and OS [Citation21–22], but others failed to show any negative association between PD-L1 expression and survival [Citation23]. Similar findings have been reported from studies with advanced-stage patients [Citation24–27].
Tumor-infiltrating lymphocytes (TILs), especially CD8+ cytotoxic T-lymphocytes (CD8+ TILs), have been associated with favorable survival in NSCLC [Citation21,Citation25,Citation27]. Moreover, PD-L1 expression was negatively associated with the presence of CD8+ TILs [Citation27].
Up to date, only one study explored the correlation between thermal ablation with both PD-L1 and TIL. Shi et al. [Citation28] found that RFA treatment of liver metastases increased not only T cell infiltration but also PD-L1 expression in primary human colorectal tumors. Using mouse tumor models, it was demonstrated that RFA treatment of one tumor initially enhanced a strong T cell-mediated immune response in tumor. Nevertheless, tumor could quickly overcome the immune responses by inhibiting the function of CD8+ and CD4+ T cells, driving a shift to higher Treg to Teff ratio and upregulating of PD-L1/PD-1 expression. The combined therapy of RFA and anti-PD-1 antibodies significantly enhanced T cell immune responses, resulting in stronger antitumor immunity and prolonged survival. Silvestrini et al. [Citation29] proved that immunotherapy begun before ablation can be curative and can enhance efficacy in the presence of a high tumor burden in a syngeneic model of epithelial cancer by both mechanical changes in the tumor microenvironment and inflammatory-mediated changes in immune phenotype.
However, so far, no study has explored the correlation between PD-L1 expression or CD8+ TILs and MWA or survival in advanced NSCLC patients treated with MWA plus chemotherapy. Therefore, we conducted this study to determine whether PD-L1 expression and CD8+ TILs are correlated with survival in these patients.
Materials and methods
Inclusion and exclusion criteria
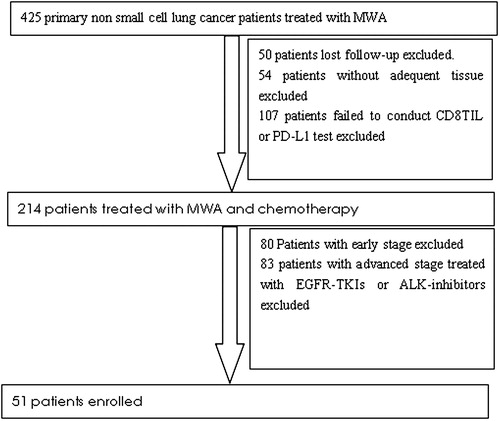
Patients meeting the following criteria were retrospectively enrolled in the study: (1) pathologically verified NSCLC; (2) clinical stage of IIIB to IV; (3) adequate tissue for IHC tests (with the tumor content no less than 80%); (4) patients without any sensitive mutations including EGFR mutations, ALK fusion or ROS1 fusion; (5) First-line treatment with third-generation chemotherapy drugs in combination with platinum; (6) MWA conducted at the primary tumor sites (all primary tumors located peripheral of the lung) and (7) Patients could be followed up.
The exclusion criteria were as follows: (1) appearance of secondary tumors during the past 5 years; (2) mixed NSCLC; (3) patients treated with targeted therapies including EGFR-tyrosine kinase inhibitors (TKIs) or ALK inhibitors; (4) previous local control treatment of the primary tumors such as by using irradiation or thermal ablations other than MWA and radioactive seed implantation; (5) uncontrolled symptomatic brain metastases and (6) a life expectancy of less than 6 months. The study was approved by the ethics committee of Shandong Provincial Hospital Affiliated to Shandong University. Written informed consent was obtained from all patients.
MWA and chemotherapy
MWA was conducted under the guidance of computed tomography (CT). The mean interval between pathology verified NSCLC and treatment was 3 days (range 1 to 7 days). For patients with a tumour diameter larger than 3.5 cm, two antennas were applied and for those no larger than 3.5 cm, one antenna was applied. Local anesthesia (lidocaine) and preemptive analgesia (morphine) were administered. Preoperative localization was confirmed with CT images and patient movement in different positions. After satisfactory anesthesia was achieved, the skin at the puncture point was cut and the microwave antenna was inserted into the target lesion following the preoperatively planned “target skin distance”. MWA was started after the cold circulating pipes and pumps connected to the MWA antennae and machine were activated. If a post-ablation ground glass opacity (GGO) 5 to 10 mm larger than the tumor was achieved, the treatment was deemed as a technical success. The process before, during and post MWA has been described in detail in our previous studies [Citation6–7].
Platinum-based doublet chemotherapy was conducted once the patients recovered from the MWA procedure. The average interval between MWA and chemotherapy was 7 days. Chemotherapy consisted of one of the following: pemetrexed at a dose of 500 mg/m2 on day 1, docetaxel at a dose of 75 mg/m2 on day 1, paclitaxel at a dose of 175 mg/m2 on day 1 or gemcitabine at a dose of 1000 mg/m2 on days 1 and 8. The corresponding platinum consisted of cisplatin or nedaplatin that was administered at a dose of 75 mg/m2 that was divided between days 1 and 2. Alternatively, carboplatin with the area under the curve (AUC) of 5 mg/mL per min was administered on day 1. Chemotherapy was repeated every 3 weeks for a maximum of 6 cycles. For patients who finished 6 cycles of chemotherapy, maintenance treatment was considered acceptable.
Follow-up
Enhanced CT was conducted every month for the first 3 months post-ablation and then at 3-month intervals to evaluate the response to MWA. Patients treated with chemotherapy received enhanced CT scans every six weeks. The response to MWA was classified as complete ablation or incomplete ablation according to the Chinese expert consensus on thermal ablation of primary and metastatic lung tumors [Citation30] and image-guided tumor ablation: Standardization of Terminology and Reporting Criteria: A 10-year Update [Citation31]. The chemotherapy-associated response was evaluated according to the Tumor Response Evaluation of Criteria in Solid Tumors version 1.1 (RECIST 1.1). The objective response rate was the probability of a complete response (CR) or partial response (PR), and the disease control rate was the probability of a CR, PR or stable disease (SD).
Immunohistochemical staining
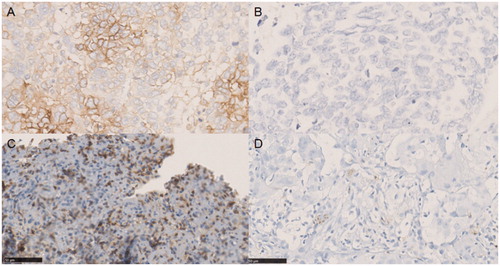
Formalin-fixed paraffin-embedded (FFPE) samples were heated at 60 °C for 1 h before being dewaxed and hydrated. Antigen retrieval was achieved with EDTA buffer (pH 9.0) for 10 min. After EDTA treatment, the slides were washed three times with PBS for 3 min each time. Then, the slides were treated with 3% hydrogen peroxide for 30 min at room temperature to block endogenous peroxidases. The slides were then incubated for 1 h at 37 °C with anti-PD-L1 rabbit monoclonal antibody (E1L3N, Cell Signaling Technology, USA) or anti-CD8 antibody (ZA-0508, Zhongshan Golden Bridge Biotechnology, China).
Slides were then washed with PBS and incubated with a Maxvision™-antibody (05269806001, Zhongshan Golden Bridge Biotechnology, China) for 15 min at 37 °C. After incubation, the slides were washed with PBS. At the same time, diaminobenzidine chromogen substrate (DAB) solution was prepared and tissues were covered for 5 min. Slides were then counterstained with hematoxylin, dehydrated and mounted.
In accordance with a previous study [Citation32], PD-L1 was assessed as negative when PD-L1 was expressed in <5% of tumor cells and defined as PD-L1-positive when expressed in ≥5% of tumor cells.
CD8+ TIL expression was defined as positive when ≥30% of stroma lymphocytes expressed CD8 ().
Statistical analyses
The continuous variables were expressed as the mean ± SD or median (range). The categorical variables were expressed as percentages. Pearson’s chi-square test or Fisher’s exact test were applied for the comparison of differences in PD-L1 expression and CD8+ TILs with clinical characteristics and treatments. PFS was calculated from the time of MWA to disease progression (including both ablative tumor sites and other tumor sites) or death. OS was calculated from the time of MWA to death or the most recent follow-up contact (censored). PFS and OS were estimated using Kaplan–Meier plots and compared using a two-sided exact log-rank test. All tests were two-sided, and a p values less than .05 was considered to be of significance. SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
Results
Patient characteristics
From January 2011 to June 2017, a total of 51 patients were enrolled (). Among them, 36 (70.6%) were male, and their mean age was 60.2 (range 36–83) years. All patients had an Eastern Cooperation Oncology Group Performance Status (ECOG PS) of 0 to 1. Adenocarcinoma (39, 76.5%) was the most common histology, and most patients presented with stage IV (44, 86.3%). The characteristics of all enrolled patients are shown in .
Table 1. The baseline characteristics of enrolled patients
Correlation between PD-L1 expression, presence of CD8+ TILs and clinicopathological characteristics
PD-L1 positive expression was observed in 31 patients (60.8%) and 20 patients (39.2%) were negative for PD-L1. PD-L1 expression failed to show a correlation with sex (p = .050), age (≥65 vs. <65, p = .927), smoking history (p = .244) or pathology (adenocarcinoma vs. non-adenocarcinoma, p = .249). No correlation between PD-L1 expression and other characteristics could be identified (). Nine (17.6%) patients showed CD8+ TIL expression. The presence of CD8+ TILs had no correlation with baseline characteristics (). PD-L1 expression also had no correlation with CD8+ TIL expression.
Table 2. Correlation between PD-L1 expression, CD8+ TIL expression and clinicopathological characteristics.
Correlation between PD-L1 expression, presence of CD8+ TILs and the response to MWA or chemotherapy
All patients were assessed for their response. Complete ablation was found in 49 patients (96.1%). CR, PR, SD and PD were observed in 0, 16, 24 and 11 patients, respectively. The ORR was 32.7% and the DCR was 81.6%. No correlation between PD-L1 expression and the response to chemotherapy was observed (PD-L1-positive vs. PD-L1-negative, ORR: 29.0% vs. 35.0%, p = .478; DCR, 90.3% vs. 60.0%, p = .093). Similarly, no correlation between CD8+ TIL expression with the ORR or DCR of chemotherapy could be identified (CD8+ TIL vs. CD8– TIL, ORR: 33.3% vs. 31.0%, p = .605; DCR, 100.0% vs. 73.8%, p = .299). No correlation between PD-L1 expression and the response to MWA was observed either (PD-L1-positive vs. PD-L1-negative, 95.0% vs. 96.8%, p = .753). Similarly, no correlation between CD8+ TIL expression and the response to MWA was detected (CD8+ TIL vs. CD8– TIL, 88.9% vs. 97.6%, p = 1.000) (). The ORR was similar with the reponse of advanced NSCLC patients treated with first-line chemotherapy. PD-L1 expression and CD8 infiltration had no effect on response to chemotherapy in advanced NSCLC patients treated with first-line chemotherapy plus microwave ablation in the primary tumor sites.
Correlation between PD-L1 expression, CD8+ TILs and survival
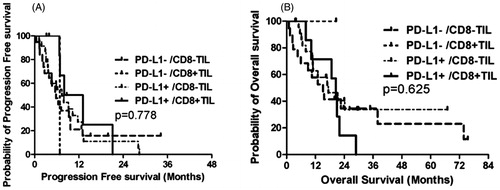
Until the last follow up on December 12, 2017, nine patients experienced disease progression in the ablative tumor sites, while 12 experienced disease progression in tumor sites other than the ablative sites. A total of 16 patients experienced tumor progression. Thirty-four patients died due to NSCLC progression or other disease. Patients with PD-L1 expression had similar PFS (PD-L1-positive vs. PD-L1-negative, 7.9 months, 95% CI: 6.3–9.4 vs. 5.8 months, 95% CI: 4.1–7.5, p = .660) (, ) and OS (PD-L1-positive vs. PD-L1-negative, 18.7 months, 95% CI: 13.9–23.4 vs. 15.2 months, 95% CI: 9.8–20.6, p = .901) (, ). Patients with CD8+ TIL expression did not show superior PFS (CD8+ TIL vs. CD8– TIL, 8.0 months, 95% CI: 5.2–10.8 vs. 6.2 months, 95% CI: 4.3–8.2, p = .435) (, ) or OS (CD8+ TIL vs. CD8– TIL, 20.5 months, 95% CI: 17.7–23.3 vs. 16.9 months, 95% CI: 10.8–23.0, p = .653) ().
Figure 3. Kaplan–Meier plots of PFS and OS. (A) PFS and PD-L1 expression; (B) OS and PD-L1 expression; (C) PFS and CD8+ TIL and (D) OS and CD8+ TIL.
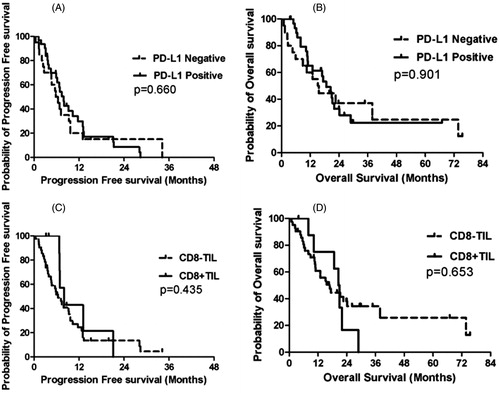
Table 3. Correlation between PD-L1 expression, CD8+ TIL expression and survival.
We divided the patients according to both PD-L1 expression and CD8+ TIL status into four groups: PD-L1-negative/CD8–TIL, PD-L1-negative/CD8+ TIL, PD-L1-positive/CD8– TIL and PD-L1-positive/CD8+ TIL. The corresponding median PFS for those four groups were 5.8, 6.8, 7.4 and 8.0 months (p = .778), and the corresponding median OS were 15.2, not reached, 17.4 and 20.5 months (p = .625), respectively ().
Discussion
We first explored the correlation of PD-L1 expression and the presence of CD8+ TILs with baseline characteristics, the response to MWA or chemotherapy and survival in advanced NSCLC patients treated with MWA plus chemotherapy. Neither PD-L1 expression nor CD8+ TIL expression were correlated with baseline characteristics, the response to MWA or chemotherapy or survival.
In our study, PD-L1 expression and CD8+ TILs were identified in 60.8 and 17.6% of patients, respectively. The rate of PD-L1 expression was in accordance with previous reports, which showed PD-L1 expression in 23.8–74.0% of NSCLC patients [Citation21–27,Citation33]. The prevalence of CD8+ TILs was lower than that in previous studies, which reported a range of 32.0% to 47.0 [Citation25,Citation27]. A possible explanation for this discrepancy could be the stage of the patients enrolled in our study. One previous study showed that patients with stage III rather than stage IV NSCLC more commonly presented with CD8+ TILs [Citation27]. However, this study enrolled only seven stage III NSCLC patients.
Patients with the following characteristics more commonly had PD-L1-positive tumors: Male, smoking history, squamous cell carcinoma, poorly differentiated cancer, vascular invasion, advanced stage, wide-type EGFR mutation status and lack of CD8+ TIL expression [Citation21,Citation23–27,Citation33]. However, we did not find any characteristics that were associated with PD-L1 expression or CD8+ TILs. Two previous studies showed that patients with PD-L1 negative tumors and CD8+ TILs responded favorably to chemotherapy [Citation27,Citation33]. However, other studies, including our own, did not confirm this correlation [Citation21,Citation24–26].
In the studies that reported a correlation between PD-L1 expression and the response to chemotherapy, PD-L1 expression was not correlated with the response to MWA. MWA is a localized treatment that induces tumor necrosis by heat injury and has a minor influence on immunity [Citation34,Citation35]. However, chemotherapy induces tumor necrosis through cytotoxic effects and has a major influence on immunity.
Several studies showed that patients with PD-L1 negative tumors or CD8+ TILs had superior PFS or even OS [Citation23–25,Citation27,Citation33], especially when presenting with CD8+ TILs in the absence of PD-L1 expression. Rashed et al. [Citation27] showed that PD-L1 overexpression is an unfavorable prognostic marker, while a high CD8 + TIL count is a good prognostic marker in advanced NSCLC. Tokito et al. [Citation25] demonstrated that CD8+ TIL density was an independent and significant predictive factor for PFS and OS, whereas PD-L1 expression was not correlated with PFS and OS. Sub-analysis revealed that the PD-L1+/CD8+ TIL-low group had the shortest PFS (8.6 months) and OS (13.9 months) and that the PD-L1–/CD8+ TIL-high group had the longest survival prognosis (median PFS and OS were not reached). Sorensen et al. [Citation26] reported that PD-L1 expression is not a strong prognostic marker in patients with advanced NSCLC treated with chemotherapy. The median PFS of PD-L1-positive patients and PD-L1-negative patients was 7.9 and 5.8 months, respectively, and the corresponding OS was 18.7 and 15.2 months, respectively. The median PFS of patients with or without CD8+ TILs was 8.0 and 6.2 months, respectively, and the corresponding OS was 20.5 and 16.9 months, respectively. These differences were not significant.
Conclusion
PD-L1 expression and CD8+ TILs were common in advanced NSCLC patients. PD-L1 expression and CD8+ TILs had no correlation with baseline characteristics, response to MWA or chemotherapy or survival in advanced NSCLC patients treated with MWA plus chemotherapy. However, the small sample size of our study limited the conclusions, and our results must be verified in a prospective study with large sample size.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551.
- Wei Z, Ye X, Yang X, et al. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38:135–142.
- Wei Z, Ye X, Yang X, et al. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol. 2015;32:464.
- Zhong L, Sun S, Shi J, et al. Clinical analysis on 113 patients with lung cancer treated by percutaneous CT-guided microwave ablation. J Thorac Dis. 2017;9:590–597.
- Wei Z, Yang X, Ye X, et al. Microwave ablation in combination with chemotherapy versus chemotherapy in advanced non small cell lung cancer. A prospective, randomized, phase III clinical trial. J Clin Oncol. 2017;35:abstracts 9048.
- Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1improves myeloid dendritic cell-mediated antitumorimmunity. Nat Med. 2003;9:562–567.
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044.
- Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687.
- Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–6912.
- Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029.
- Sheppard KA, Fitz LJ, Lee JM, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41.
- Patsoukis N, Sari D, Boussiotis VA. PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressingCdc25A. Cell Cycle. 2012;11:4305–4309.
- Charlton JJ, Chatzidakis I, Tsoukatou D, et al. Programmed death-1 shapes memory phenotype CD8 T cell subsets in a cell-intrinsic manner. J Immunol. 2013;190:6104–6114.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550.
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35:3924–3933.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833.
- Teng F, Meng X, Wang X, et al. Expressions of CD8 + TILs, PD-L1 and Foxp3 + TILs in stage I NSCLC guidingadjuvant chemotherapy decisions. Oncotarget. 2016;7:64318–64329.
- Song Z, Yu X, Zhang Y. Altered expression of programmed death-ligand 1 after neo-adjuvantchemotherapy in patients with lung squamous cell carcinoma. Lung Cancer. 2016;99:166–171.
- Sun JM, Zhou W, Choi YL, et al. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016;11:1003–1011.
- Takada K, Toyokawa G, Okamoto T, et al. A comprehensive analysis of programmed cell death ligand-1 expression with the clone SP142 antibody in non-small-cell lung cancer patients. Clin Lung Cancer. 1997;18:95–82.
- Tokito T, Azuma K, Kawahara A, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14.
- Sorensen SF, Zhou W, Dolled-Filhart M, et al. PD-L1 expression and survival among patients with advanced non-small cell lung cancer treated with chemotherapy. Transl Oncol. 2016;9:64–69.
- Rashed HE, Abdelrahman AE, Abdelgawad M, et al. Prognostic significance of programmed cell death ligand 1 (PD-L1), CD8+ tumor-infiltrating lymphocytes and p53 in Non-Small Cell Lung Cancer: An immunohistochemical study. Turk Patoloji Derg. 2017;1:211–222.
- Shi L, Chen L, Wu C, et al. PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin Cancer Res. 2016;22:1173–1184.
- Silvestrini MT, Ingham ES, Mahakian LM, et al. Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols. JCI Insight. 2017;2:e90521.
- Ye X, Fan W, Wang H, et al. Expert consensus workshop report: Guidelines for thermal ablation of primary and metastatic lung tumors. J Can Res Ther. 2018;14:730–744.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. J Vasc Interv Radiol. 2014;25:1706–1705.
- Jiang L, Su X, Zhang T, et al. PD-L1 expression and its relationship with oncogenic drivers in non-small cell lung cancer (NSCLC). Oncotarget. 2017;8:26845–26857.
- Zhang P, Ma Y, Lv C, et al. Upregulation of programmed cell death Ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 2016;107:1563–1571.
- Yang X, Ye X, Huang G, et al. Repeated percutaneous microwave ablation for local recurrence of inoperable stage I nonsmall cell lung cancer. J Cancer Res Ther. 2017;13:683–688.
- Zhou Y, Xu X, Ding J, et al. Dynamic changes of T-cell subsets and their relation with tumor recurrence after microwave ablation in patients with hepatocellular carcinoma. J Cancer Res Ther. 2018;14:40–45.