Abstract
Background: We aimed to describe changes in serum thyroglobulin (Tg) and anti-Tg autoantibody shortly following high-intensity focused ultrasound (HIFU) ablation in patients with positive anti-Tg status by comparing them with patients with negative anti-Tg and to correlate them with 6-month nodule shrinkage and treatment success.
Methods: From 2015 to 2017, patients who underwent HIFU ablation of a benign thyroid nodule were analysed. Serum Tg and anti-Tg were checked on treatment day (baseline) and 4 days after treatment. Anti-Tg >99 IU/ml were considered positivity. Percentage Tg or anti-Tg change = [Level on Day-4 – baseline level]/[Baseline level] × 100 while nodule shrinkage was measured by volume reduction ratio (VRR) = [Baseline volume – volume at 6 month]/[Baseline volume] × 100. Treatment success was defined as VRR >50%.
Results: Among the 276 eligible patients, 85 (30.8%) patients were positive for anti-Tg (Group I) while the others (n = 191, 69.2%) were negative (Group II). Relative to group II, Group I had a less significant Tg rise on Day 4 (4121.78 ± 9321.90% vs. 5711.53 ± 23487.20%, p = .013). There was a fall in anti-Tg on day 4 for group I (−11.56 ± 139.69%). This percentage anti-Tg drop significantly correlated with the 6-month VRR (ρ = −0.602, p = .030) but was not a significant factor of treatment success.
Conclusions: Given the fact that the percentage anti-Tg drop correlated significantly with 6-month nodule shrinkage in group I, monitoring early anti-Tg change may help to predict the 6-month nodule shrinkage in patients with positive anti-Tg.
Introduction
Thyroid nodules are common and although most are benign and will remain relatively static over time, some can become large and cause symptoms [Citation1–3]. Surgery is normally indicated in this situation [Citation1,Citation2]. However, surgery is associated with complications, high cost and needs a general anesthesia. As a result, there has been a growing interest in developing less invasive, non-surgical technique in the treatment of benign thyroid nodules [Citation4,Citation5]. For solid nodules, radiofrequency ablation and laser ablation have been the most widely applied techniques in the ablation benign thyroid nodules [Citation6–8]. They have resulted in excellent short and medium-term outcomes [Citation6–12]. High intensity focused ultrasound (HIFU), on the other hand, is a relatively new ablation technique that utilizes focused ultrasound energy to induce ablation. It has been shown to be effective in inducing significant nodule shrinkage and alleviating nodule-related symptoms in the relatively short term [Citation13–18].
However, it is unclear whether early serum markers can help the clinicians to determine if an effective ablation has been given. Since not all nodules after ablation can shrink satisfactorily [Citation15,Citation18], a serum marker may help to identify which nodules would shrink over time. Serum thyroglobulin (Tg) is a 660 kDa dimeric protein produced by the follicular cells in the thyroid gland that rises sharply shortly after ablation. The percentage rise in Tg after ablation has been shown to correlate with the extent of tissue ablation [Citation19] but because anti-Tg autoantibody (anti-Tg) can interfere with serum Tg measurements, Tg is considered less useful in patients with positive anti-Tg [Citation20,Citation21]. To our knowledge, no studies have specifically examined the early change in serum Tg and anti-Tg after ablation in patients with positive anti-Tg status. We aimed to describe these changes following single ablation in patients with positive anti-Tg by comparing them with those with negative anti-Tg status and to correlate these changes with subsequent nodule shrinkage and treatment success.
Methods
This retrospective analysis was approved by local institutional review board and all patients gave their informed consent before treatment. All consecutive patients who underwent HIFU ablation for a symptomatic, solid or predominantly solid (<30% cystic areas), benign thyroid nodule from November 2015 to December 2017 were analyzed. Over this period, HIFU ablation was only indicated for patients who did not wish to undergo surgery. Details on the eligibility for ablation were previously described [Citation15]. In brief, the nodule had to be benign on at least one occasion by fine needle aspiration cytology (Bethesda category II) and have a low or very-low suspicion sonographic pattern [Citation2] and its center located within the treatable depth of 7–30 mm from the skin surface. Also, the swelling (which could either be a solitary nodule or a dominant nodule in a multinodular gland) had to be causing obstructive symptoms and the longest diameter of the nodule had to be ≥20 mm but ≤60 mm on ultrasonography (US). To be eligible for the study, patients had to fulfil the following criteria: (1) Received one single ablation treatment only. (2) Results of the serum TSH, FT4, Tg, and anti-Tg autoantibody were available on the day of treatment (baseline) and day 4 after treatment (i.e. first clinic). (3) Had to have ≥6 months’ follow-up after treatment. Patients who received two treatments to the same nodule or had two nodules treated in the same session or those who did not have their Tg or anti-Tg antibody checked on day of treatment and day 4 after treatment were excluded.
To assess the early changes in serum Tg and anti-Tg levels in patients with positive anti-Tg status (Group I), we compared them with those seen in patients with negative anti-Tg (Group II). All Tg, anti-Tg and anti-thyroid peroxidase (anti-TPO) autoantibody measurements were carried out in the same laboratory. Serum Tg was determined by the same immunometric assay (the Immulite 2000, Diagnostic Products Corp. Roche, Los Angeles, CA) that was calibrated against the CRM- 457 standard. It had an upper limit of 4800 ng/mL Serum anti-Tg and anti-TPO were determined by radioimmunoassay (Bio Code, Izasa, LieÁge, Belgium). Any values >99 IU/ml were considered positive while values ≤99 IU/mL were considered negative.
The primary study endpoints were percentage serum Tg and anti-Tg changes at day 4 from baseline. The extent of serum Tg change (%) on day 4 was calculated from the formula: [Serum Tg on day 4 – serum Tg at baseline]/[Serum Tg at baseline] × 100. Similarly, the extent of anti-Tg change on Day 4 was calculated based on the formula: [Serum anti-Tg on day 4 – serum anti-Tg at baseline]/[Serum anti-Tg at baseline] × 100. Secondary endpoints were 6-month nodule shrinkage and its correlation with serum Tg or anti-Tg on day 4, correlations between change in Tg and anti-Tg and baseline characteristics, treatment parameters, and nodule shrinkage and factors associated with treatment success in patients with positive anti-Tg.
Nodule shrinkage
Each nodule was measured by USG on the day of treatment (baseline), 3 and 6 months. Nodule dimensions were measured using the LOGIQ e (GE Healthcare, Milwaukee) scanner equipped with a 10–14 MHz linear matrix transducer. Three orthogonal diameters of the index nodule (its longest diameter and two other perpendicular diameters) were measured. In general, the longest diameter was the cranio-caudal dimension (length) of the nodule while the other two perpendicular diameters were the medio-lateral (width) and antero-posterior (depth) dimensions of the nodule. All measurements were made to the nearest 0.1 mm. To estimate nodule volume, we used the formula: volume (mL) = (width (in cm) × length (in cm) × depth (in cm)) × (π/6) where π was taken as 3.14159. The volume reduction ratio (VRR) was calculated based on the formula: [Baseline volume – volume at visit]/[Baseline volume] × 100. Treatment success was defined as ≥50% volume reduction at 6-month from baseline.
HIFU treatment
All treatments were performed by one person (B.H.L.) with >3 years of experience using the same USG-guided HIFU device. This device comprised an energy generator, a treatment head, a skin cooling device, and a touch-screen interface for planning. The treatment head incorporated an image transducer (7.5 MHz, 128 elements, linear array) and HIFU transducer (3 MHz, single element, 60 mm in diameter). After positioning, patients were sedated with diazepam (10–15 mg) and pethidine (50–100 mg). The area over the target nodule was anesthetized with 10 ml lignocaine 1%. Under USG guidance, the treatment head was positioned until the entire index nodule was within the treatable depth. Once the nodule had been marked on the screen, the device computer (Beamotion version no. TUS 3.2.2, Theraclion, Paris, France) automatically divided the nodule into multiple ablation subunits (voxels). Each voxel measured ∼7.3 mm in thickness and 5 mm in width and received a continuous 8-s pulses of HIFU energy followed by 20–30 s of cooling time before the beam was moved to the adjacent voxel. This cycle continued until all planned voxels were ablated. To ensure safety, nearby structures like the carotid artery, trachea, and skin were marked on the treatment screen before the start of treatment. To avoid inadvertent heat injury to important surrounding structures, the device automatically selected the following safety margins: (a) 0.5 cm from the skin, (b) at least 0.5 cm from the trachea and RLN, and (c) 0.2 cm from the ipsilateral carotid artery and cancelled any voxels which were within these distances. A laser-based movement detector enabled immediate power interruption when the patient moved or swallowed during ablation. To avoid skin burn, the skin was cooled by a balloon (filled with 10 °C liquids) at the tip of the treatment head. All ablations started at 280 J/pulse and increased up to 360 J/pulse until hyperechoic marks appeared at the focal point. Both the total amount of energy delivered to the nodule (in KJ) and the ‘on-beam’ (sonification) time taken (in minutes) were automatically recorded by the device’s computer. Treatment time was taken as the time required for both planning and sonification. Oral diet was resumed immediately afterwards and patients were allowed to go home two hours after treatment.
Laboratory methods
All serum TSH and FT4 levels were carried out at the same laboratory. Plasma TSH was determined by a specific two-site immunometric assay (Eleosys 2010, Boehringer Mannheim, Germany).
Statistical analysis
Continuous variables were expressed as mean ± SD. Median and interquartile range were also presented when appropriate. Continuous variables between groups were compared using the Mann–Whitney U test. Chi-square tests were used to compare categorical variables. For correlation between two continuous variables, the Spearman’s correlation test was performed. Binary logistic regression model was performed to evaluate factors leading to treatment success. All statistical analyses were performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL). All significance tests were two-tailed and those with a p values less than .05 were considered statistically significant.
Results
Patient selection
Altogether 293 patients completed single-session HIFU ablation of a benign thyroid nodule. Fifteen patients (5.1%) received two sequential treatments because of two large nodules that needed treatment at the same time while 2 (0.7%) patients were followed up <6 months. Therefore, 276 patients were eligible for analysis. Of these, 85 (30.8%) patients were tested to be positive (>99 IU/mL) for anti-Tg (Group I) while the rest (n = 191, 69.2%) had undetectable anti-Tg (≤99 IU/mL).
Change in Tg and anti-Tg on day 4 for groups I and II
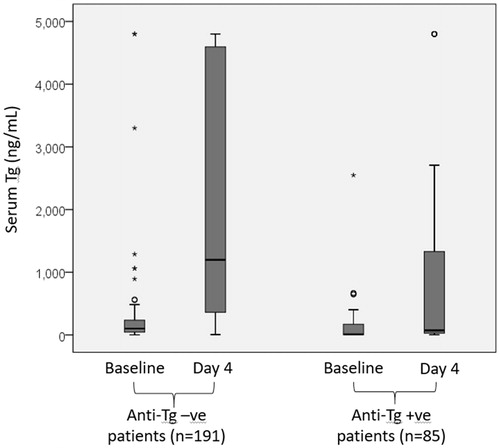
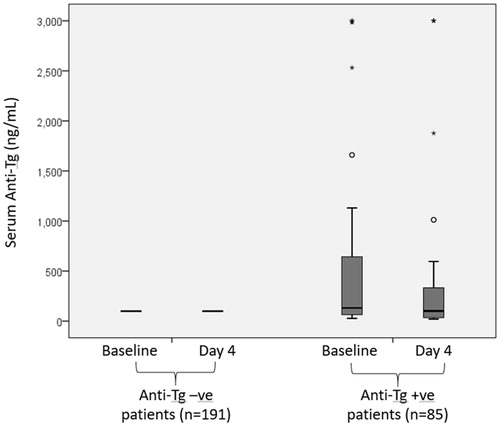
In group I, the baseline serum Tg significantly increased from 220.81 ± 808.38 ng/mL to 1025.11 ± 1716.97 ng/ml on day 4 (p < .001) and the mean percentage change was 4121.78 ± 9321.90% (median = 678.23%, IQR = 84481.25%). On the other hand, the baseline serum anti-Tg significantly dropped from 547.89 ± 865.25 IU/mL to 460.15 ± 822.70 IU/mL, p = .011) and the mean percentage change was −11.56 ± 139.69% (median = 35.00%, IQR = 999.33%). There was a significant inverse correlation between Tg rise and anti-Tg drop (ρ = −0.653, p < .001). In group II, the baseline serum Tg also significantly increased from 331.79 ± 883.59 ng/mL to 2041.50 ± 1858.53 ng/ml on day 4 (p < .001) and the mean percentage change was 5711.53 ± 23487.20% (median = 1229.45%, IQR = 156700.00%). However, the mean percentage change in anti-Tg was 0.00 ± 0.00% (median = 0.00%). shows the boxplots for serum Tg at baseline and on day 4 in groups I and II. shows the boxplots for serum anti-Tg at baseline and on day 4 in groups I and II.
Nodule shrinkage at 3 and 6 months
At baseline, the mean nodule volume was 22.61 ± 25.36 ml. At 3 months after treatment, the mean volume was reduced to 12.98 ± 22.58 ml. The mean VRR was 49.7 ± 21.9% (median = 50.2%; interquartile range (IQR) = 32.5%). One hundred and forty-two (51.4%) patients achieved treatment success at 3 months. At 6 months, the mean VRR increased to 63.8 ± 25.6% (median = 71.0%; IQR = 33.6%) and 214 (77.5%) patients achieved treatment success.
Correlation between anti-Tg percentage drop on day 4 and VRR at 6 month in group I
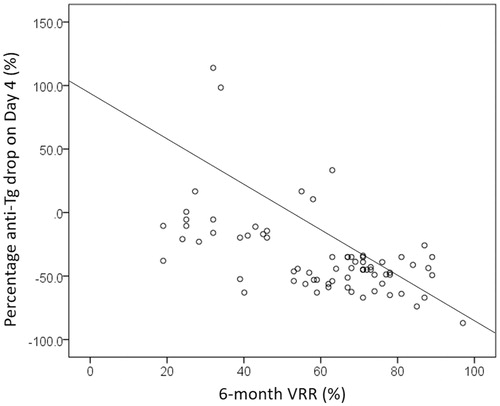
By using the median of 35.0% as the drop cut-off, those with an anti-Tg percentage drop above the median had a significantly higher chance of treatment success than those below the median (38/43 versus 29/42, p = .029) ( and ). Percentage anti-Tg drop (%) significantly correlated with the 6-month VRR (ρ = −0.602, p = .030) and the mean % drop in serum anti-Tg on day 4 was significantly more in those with treatment success (n = 69) than without treatment success (n = 16) (−44.1 versus 22.1%, p = .039). However, the extent of serum Tg rise (%) on day 4 did not significantly correlated with the 6-month VRR (ρ = −0.249, p = .413).
Figure 3. A scatterplot showing the correlation between percentage anti-Tg drop on day 4 and 6-month nodule shrinkage (VRR).
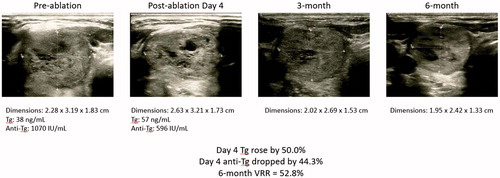
Baseline characteristics including age, sex ratio, WHO nodule grade, body mass index, serum TSH, and FT4 and initial nodule dimensions were comparable between groups I and II (). Seventy-seven (91.5%) patients in group I had concomitant elevated (>99 IU/mL) anti-TPO autoantibody while no patients in group II had concomitant elevated anti-TPO. Similarly, treatment efficacy (VRR) at 3 and 6 months as well as percentage of treatment success were similar between the two groups. The pre- and postablation thyroid function were similar between the two groups ().
Table 1. A comparison of baseline characteristics between patients with positive (> 99 IU/mL) anti-thyroglobulin antibodies (Group I) and patients with negative (≤ 99 IU/mL) antithyroglobulin antibodies (Group II).
Table 2. A comparison of thyroid function, thyroglobulin and anti-thyroglobulin auto-antibody between patients with positive (> 99 IU/mL) anti-thyroglobulin antibodies (Group I) and with negative (≤99 IU/mL) anti-thyroglobulin antibodies (Group II).
Correlations between baseline Tg and anti-Tg and patient characteristics, treatment parameters and VRR in group I
A higher serum Tg level at baseline was significantly associated with a larger sized nodule (ρ = 0.405, p = .007), a larger volume nodule (ρ = 0.526, p = .070), more energy delivered (ρ = 0.310, p = .009), higher mean power per voxel (ρ = 0.253, p = .036) but lower 6-month VRR (ρ = −0.551, p = .002) (). Interestingly, a higher baseline serum anti-Tg was also significantly associated with larger sized nodule (ρ = 0.435, p = .030), a larger volume nodule (ρ = 0.528, p = .007), higher mean power per voxel (ρ = 0.334, p = .029 and lower 6-month VRR (ρ=−0.660, p = .014). It was also significantly associated with a lower serum TSH (ρ = −0.392, p = .006).
Table 3. Bivariate Pearson’s correlations between change in serum thyroglobulin (Tg) and anti-Tg and baseline characteristics, treatment parameters and extent of nodule shrinkage for patients with positive anti-Tg (i.e. Group I only).
Factors associated with treatment success (VRR ≥50% at 6 months) in group I
By binary logistic regression, only baseline nodule diameter (OR = 0.025, 95% CI = 0.058–0.699, p = .025) and baseline nodule volume (OR = 0.790, 95% CI = 0.655–0.952, p = .013) were determinants of treatment success. Other clinical factors like total energy given (OR = 0.893, 95% CI = 0.792–1.007, p = .166), total ‘on-beam’ time (OR = 0.985, 95% CI = 0.955–1.016, p = .985), power per voxel (OR = 0.938, 95% CI = 0.913–1.017, p = .613), baseline Tg (OR = 0.998, 95% CI = 0.995–1.001, p = .281), Tg on day 4 (OR = 0.999, 95% CI = 0.999–1.000, p = .053), extent of Tg rise on day 4 (OR = 1.000, 95% CI = 1.000–1.000, p = .352) and extent anti-Tg drop on day 4 (OR = 0.975, 95%CI = 0.948–1.004, p = .093) did not turn out to be significantly associated with treatment success at 6 months.
Discussion
Although thermal ablation could be an alternative to surgery in the treatment of symptomatic benign thyroid nodules, they are effectively very different in nature. Thermal ablation like RFA and LA causes physical shrinkage of thyroid nodules via thermal energy while surgery entails the removal of the entire involved lobe [Citation22–24]. Furthermore, because patients who seek one particular treatment are unlikely to choose the other treatment, a direct comparison without selection biases is difficult. To date, three studies have compared outcomes of RFA with open thyroidectomy [Citation25–27] while only two have compared outcomes of HIFU with open thyroidectomy [Citation23,Citation24]. However, none of them were prospective randomized studies.
To our knowledge, this was the first study to specifically examine the early changes of serum Tg and anti-Tg after HIFU ablation of benign thyroid nodules in patients having pre-existing anti-Tg (i.e. positive anti-Tg status). The reasons for examining this specific patient group are, first, about one-third of patients (like the present cohort) undergoing thermal ablation of thyroid nodules have positive anti-Tg status and because anti-Tg cross-reacts with Tg, serum Tg becomes a less useful marker. Second, with the reciprocal relationship between Tg and anti-Tg levels after thermal ablation [Citation27,Citation28], we hypothesized that a drop in anti-Tg (because of Tg rise) could be a novel way of measuring effective ablation in patients with positive anti-Tg status.
Similar to group II, group I patients experienced a dramatic rise in serum Tg but the mean percentage rise on day 4 was somewhat limited in comparison (5711.53 ± 23487.20% versus 4121.78 ± 9321.90%, p = .013). Presumably, this was because the baseline serum Tg was significantly higher in group II than I (331.79 versus 220.81 ng/mL, p < .001) and so, even with a same extent of ablation, the postablation level would be less. Second, the presence of anti-Tg cross-reacted and falsely lowered Tg measurement and its percentage rise. As predicted, the percentage rise in serum Tg on day 4 did not significantly correlated to 6-month VRR (ρ = −0.249, p = .413) and so, it was not a good marker for predicting eventual nodule shrinkage.
On the other hand, as hypothesized, group I patients had a significant drop in anti-Tg on day 4 after ablation (from 547.89 ± 865.25 IU/mL to 460.15 ± 822.70 IU/mL) and the percentage drop in anti-Tg on day 4 significantly correlated with 6-month VRR (ρ = −0.602, p = .030). Furthermore, those with a percentage drop ≥35.0% had a significantly higher chance of treatment success than those <35.0% (38/43 versus 29/42, p = .029). Therefore, there was a significant relationship between the percentage drop in anti-Tg and the extent of nodule shrinkage afterwards. Although this drop in anti-Tg was reported previously [Citation27,Citation28], its relationship with nodule shrinkage has yet to be described.
However, relative to other significant factors, the percentage drop in anti-Tg did not turn out to be a significant factor (OR = 0.975, 95% CI = 0.948–1.004, p = .093) and so, it was probably a minor predictor when compared to nodule diameter (OR = 0.025, 95% CI = 0.058–0.699, p = .025) and nodule volume (OR = 0.790, 95% CI = 0.655–0.952, p = .013).
Another finding worth highlighting was that a majority (77/85 or 90.6%) of group I patients had concomitant raised anti-TPO level (i.e. ≥99 IU/mL) before ablation, suggesting that perhaps, thyroiditis was very prevalent in this patient subgroup. Given that the 6-month VRR was similar between the two groups (p = .246), this would suggest that underlying or surrounding thyroiditis does not directly impair the efficacy of HIFU ablation.
Perhaps, it should be highlighted that our findings are probably applicable to other forms of thermal ablation techniques to the thyroid gland and not confined to HIFU alone. One point of note is that although Tg represents one of the largest known self-antigens involved in autoimmunity (i.e. able to trigger and perpetuating autoimmune thyroid diseases), the rise in serum Tg after ablation is relatively short-lived [Citation29]. The same could be said for anti-Tg because of its close relationship with Tg. Therefore, at this time, nether one is probably to be involved in immune-modulation of nodule shrinkage. To date, the nodule shrinkage is believed to due to the thermal effect of HIFU and not its mechanical effect [Citation30].
We would like to acknowledge several shortcomings with the study. First, our study was a moderately-sized study and so, some of the non-significant findings such as the lack of significant correlation between extent of serum Tg rise and subsequent nodule shrinkage might have been due to inadequate power of the study. Second, since there was an upper limit for the Tg assay (>4800 ng/mL), it was difficult to obtain an accurate postablation measurement when the baseline level was already close to the upper limit of the assay. Third, it was impossible to evaluate the change in anti-Tg levels in group II because they all began with an undetectable anti-Tg level.
Conclusion
For patients with a positive anti-Tg status, the extent of Tg rise shortly after HIFU ablation was significantly less than that in those with a negative anti-Tg status. At the same time, there was a corresponding drop in anti-Tg level after HIFU ablation and this drop correlated significantly with the eventual nodule shrinkage at 6 months. Therefore, monitoring the early changes in anti-Tg level might be a novel method of knowing whether an effective ablation have been given in patients with a positive anti-Tg status.
Acknowledgments
BHH Lang/YC Woo/KWH Chiu were involved in the review of literature, acquisition of data and drafting and completing the manuscript. BHH Lang/YC Woo/KWH Chiu were also involved in the review of literature and drafting the manuscript. BHH Lang/YC Woo/KWH Chiu conceived the study, participated in the co-ordination and the acquisition of data and helped to draft the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gharib H, Papini E, Garber JR, et al. AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the diagnosis and management of thyroid nodules - 2016 Update. Endocr Pract. 2016;22:1–39.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133.
- Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926–935.
- Gharib H, Hegedüs L, Pacella CM, et al. Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. 2013;98:3949–3957.
- Sung JY, Baek JH, Kim KS, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology. 2013;269:293–300.
- Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20:11–22.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44:14–36.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33:1–919.
- Pacella CM, Mauri G, Cesareo R, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia. 2017;15:1–5.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100:3903–3910.
- Kim JH, Baek JH, Lim HK, et al. Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19:632–655.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19:167–174.
- Korkusuz H, Sennert M, Fehre N, et al. Local thyroid tissue ablation by high-intensity focused ultrasound: effects on thyroid function and first human feasibility study with hot and cold thyroid nodules. Int J Hyperthermia. 2014;30:480–485.
- Kovatcheva RD, Vlahov JD, Stoinov JI, et al. Benign solid thyroid nodules: US-guided high-intensity focused ultrasound ablation-initial clinical outcomes. Radiology. 2015;276:597–605.
- Lang BH, Woo YC, Wong CK. High-intensity focused ultrasound for treatment of symptomatic benign thyroid nodules: a prospective study. Radiology. 2017;284:897–906.
- Trimboli P, Bini F, Marinozzi F, Baek JH, Giovanella L. High-intensity focused ultrasound (HIFU) therapy for benign thyroid nodules without anesthesia or sedation. Endocrine. 2018;61:210–215.
- Lang BHH, Woo YC, Chiu KW. Two-year efficacy of single-session high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Eur Radiol. 2018. DOI:10.1007/s00330-018-5579-8.
- Lang BH, Woo YC, Chiu KW. Role of second high intensity focused ultrasound (HIFU) treatment for unsatisfactory benign thyroid nodules after first treatment. Eur Rad. 2018. DOI:10.1007/s00330-018-5671-0.
- Lang BHH, Woo YC, Chiu KWH. The percentage of serum thyroglobulin rise in the first-week did not predict the eventual success of high-intensity focussed ablation (HIFU) for benign thyroid nodules. Int J Hyperthermia. 2017;33:1–887.
- Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:1121–1127.
- Spencer C, LoPresti J, Fatemi S. How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancer, in the absence or presence of thyroglobulin autoantibodies. Curr Opin Endocrinol Diabetes Obes. 2014;21:394–404.
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:1.
- Lang BHH, Wong CKH, Ma EPM. Single-session high intensity focussed ablation (HIFU) versus open cervical hemithyroidectomy for benign thyroid nodule: analysis on early efficacy, safety and voice quality. Int J Hyperthermia. 2017;33:868–874.
- Lang BH, Wong CK, Ma EP, et al. A propensity-matched analysis of clinical outcomes between open thyroid lobectomy and high intensity focused ultrasound (HIFU) ablation in benign thyroid nodules. Surgery. 2019; (in press)
- Yue WW, Li XL, Xu HX, et al. Quality of life and cost-effectiveness of radiofrequency ablation versus open surgery for benign thyroid nodules: a retrospective cohort study. Sci Rep. 2016;6:37838.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36:1321–1325.
- Cakir B, Topaloglu O, Gul K, et al. Effects of percutaneous laser ablation treatment in benign solitary thyroid nodules on nodule volume, thyroglobulin and anti-thyroglobulin levels, and cytopathology of nodule in 1 yr follow-up. J Endocrinol Invest. 2006;29:876–884.
- Heck K, Happel C, Grünwald F, et al. Percutaneous microwave ablation of thyroid nodules: effects on thyroid function and antibodies. Int J Hyperthermia. 2015;31:560–567.
- Korkusuz H, Sennert M, Fehre N, et al. Localized thyroid tissue ablation by high intensity focused ultrasound: volume reduction, effects on thyroid function and immune response. Rofo. 2015;187:1011–1015.
- Lang BH, Wu ALH. The efficacy and safety of high-intensity focused ultrasound ablation of benign thyroid nodules. Ultrasonography. 2018;37:89–97.